Abstract
Galanin type 1 receptor knockout mice show altered responses to high-fat diet and glucose challenge. PHYSIOL BEHAV 00(0) 000-000, 200X. Galanin, a brain and pancreatic peptide with three receptor subtypes (GALR1,GALR2,GALR3), is hypothesized to participate in energy homeostasis and glucoregulation. Hypothalamic galanin expression is induced by dietary fat, and intra-hypothalamic galanin administration has orexigenic/anabolic properties. Systemic galanin infusion alters glucoregulation in non-human species, partly through direct actions on pancreatic islets. However, the physiologic significance of endogenous galanin-GALR signaling is unclear. The present studies tested the hypotheses that GALR1 deficiency alters food intake and feed efficiency following switches to high-fat diet and that GALR1 deficiency alters whole-body glucose homeostasis. Adult, male GALR1 knockout (−/−), heterozygote (+/−), and C57BL/6J control (+/+) mice were studied. GALR1 deficiency impaired adaptation to a 3-day high-fat diet challenge, leading to increased food intake, feed efficiency and weight gain. However, during the following 2 weeks, GALR1 knockout mice decreased intake, consuming less daily energy than while maintained on low-fat diet and also than heterozygote littermates. Chow-maintained GALR1 knockout mice showed relative hyperglycemia in fed and d-glucose (i.p. 1.5 g/kg)-challenged states. GALR1 knockout mice showed normal food intake, feed efficiency and weight accrual on low-fat diets, normal fasted glucose levels, and normal glucose sensitivity to porcine insulin (i.p. 1 IU/kg) in vivo. The results support the hypotheses that galanin-GALR1 systems help adapt food intake and metabolism to changes in dietary fat and modulate glucose disposition in mice.
Keywords: Obesity, high-fat diet, dietary fat, glucose homeostasis, glucoregulation, galanin receptor subtype 1, food intake, feeding, feed efficiency, metabolism, body weight, intraperitoneal glucose tolerance test, insulin sensitivity test, macronutrient composition, peptide, mouse, mice
Introduction
Isolated from pig intestine by Tatemoto, Mutt and colleagues in 1983, galanin is a 29 (30 in humans) amino acid residue peptide named for the N-terminal glycine and C-terminal alanine in porcine galanin [1]. Galanin-like immunoreactivity and mRNA are found throughout the CNS of several species, including human, non-human primate, rat and mouse [2], with particularly high expression in hypothalamus [3-13]. Increasing evidence suggests that galanin is involved in energy homeostasis. For example, hypothalamic galanin-expressing neurons carry the long form of the leptin receptor, and neuropeptide Y (NPY)-expressing neurons synapse on galanin immunoreactive neurons [14]. Furthermore, central insulin administration suppresses galanin expression in the CNS [15].
Leibowitz and colleagues based on experiments in rat have speculated that hypothalamic galanin, via a positive feedback mechanism, increases food intake and promotes adiposity when palatable, high-fat diets are available [16, 17]. That is, consumption of diets high in fat (>30-35% kcal) stimulates the synthesis and secretion of galanin from the anterior paraventricular nucleus of the hypothalamus (PVN) [16, 18-21]. High fat meals rapidly (30-60 min) increase (2-4 fold) PVN galanin mRNA expression, with the degree of galanin induction correlating directly with the amount of fat, rather than protein or carbohydrate, eaten [16, 22]. Intraperitoneal injection of Intralipid at a dose (20% v/v, 5 ml) that rapidly increases circulating triglycerides and free fatty acids to high-fat meal-like levels [23], also increases PVN galanin mRNA expression, suggesting that dietary fat-induced PVN galanin induction may be post-absorptively mediated.
Although the functional significance of dietary-fat induced PVN galanin induction remains unknown, a “positive feedback” orexigenic role has been proposed based on actions of central galanin administration. Galanin robustly increases food intake for 30-120 min when given intracerebroventricularly (i.c.v.) or targeting the PVN of satiated rats [24]. Orexigenic actions of intracranial galanin administration are blocked by intra-hypothalamic administration of M40, a subtype non-selective galanin receptor antagonist [25, 26]. Galanin has been suggested to promote intake of high-fat or highly preferred diets preferentially (see [17]).
Central galanin administration also has anabolic-like metabolic effects. Third ventricle galanin administration reduced whole-body energy expenditure and decreased firing rates of sympathetic nerves that innervate brown adipose tissue [16, 27, 28]. Intra-hypothalamic galanin administration also shifted skeletal muscle energy substrate utilization towards carbohydrate and away from fat and resulted in increased lipoprotein lipase activity in adipose tissue, suggesting increased fat deposition. Chronic intra-hypothalamic galanin administration promoted adiposity, increasing white fat mass in rodents, but only in those with access to high-fat diets [29].
Still, functional studies have not provided clear evidence of the overall, physiologic role of galanin in the control of food intake, feed efficiency or body weight. For example, intracranial administration of the galanin receptor antagonist M40 did not reduce spontaneous intake of chow, high fat diet or cookie mash in a series of collaborative studies by the Crawley/Bartfai laboratories [26]. On the other hand, one laboratory did observe that intra-PVN M40 reduced high-fat diet intake dose-dependently in fat-preferring rats [30], and another found that intra-PVN administration of two other GALR antagonists, C7 and galantide, reduced fat preference in Brattleboro rats, which overexpress galanin [31]. Repeated intra-PVN infusion of antisense oligonucleotides against galanin mRNA also reduced high fat diet intake ∼65% within the first 3 hours of the dark cycle [18]. In addition to these discrepant findings, peripheral administration of galnon, a systemically acting subtype non-selective galanin receptor agonist, suppressed feeding [32], unlike intracranial galanin infusion and pointing to the possible importance of peripheral targets such as pancreatic insulin secretion in the overall effects of galanin [16, 30, 33]. Indeed, galanin inhibits glucose-induced pancreatic insulin secretion upon peripheral administration in rodents [1, 34].
The receptor subtype that mediates galanin actions on energy homeostasis also is uncertain. Three mammalian class A (rhodopsin-like), seven transmembrane domain GPCR receptor targets for galanin have been cloned - GALR1, GALR2, GALR3. The subtypes differ in amino acid sequence (35-40% identity), pharmacology, second messenger systems and distribution [35]. GALR1 and GALR3 receptors via Gi proteins inhibit adenylate cyclase, reduce cAMP-responsive element binding (CREB) protein phosphorylation, and hyperpolarize via opening of ATP-sensitive K+ channels and inwardly rectifying K+ channels [36]. GALR2 receptors via Gq/11 stimulate 1,4,5-triphosphate phosphatidylinositol (IP3) formation via phospholipase C and activate mitogen-activated protein kinase. Of the galanin receptor subtypes, GALR1 receptors are synthesized and expressed in brain at highest levels. In the PVN, the GALR1 subtype predominates, accounting for approximately 90% of all 125I-galanin binding sites [37], but the residual 10% consists of either GALR2 or GALR3 receptors [38-40].
Because of the reviewed actions of pharmacological galanin administration on food intake, metabolism, body weight and glucoregulation and of the sensitivity of PVN galanin to dietary fat intake, galanin signaling systems may be potential therapeutic targets for weight management or glucose homeostasis. In the absence of subtype-selective GALR antagonists, we have begun to study the effects of GALR1 receptor null mutations on feeding and energy homeostasis in mice. The present studies tested the hypotheses that GALR1 deficiency differentially altered food intake and feed efficiency following switches to purified high-fat, as opposed to low-fat, diet and that GALR1 deficiency altered whole-body glucose homeostasis.
Methods
Subjects
Male offspring from breeding of adult GALR1 heterozygote (+/−) mutant mice [41], backcrossed for at least 6 generations onto a C57BL/6J background, and C57BL/6J mice were subjects. Mice were housed in a 12:12 light cycle room (light on 0600), humidity- (60%) and temperature-controlled (22°C) vivarium with continuous access to corn-based, low-to-moderate-fat (28% kcal from fat) breeder chow (metabolizable energy 3.52 kcal/g; Harlan Teklad #7004) and water ad libitum unless stated otherwise. Procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication number 85-23, revised 1996) and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Feeding studies
Age-matched, adult (7 months of age at study onset), individually-housed male GALR1 knockout (−/−; n=5) and heterozygote (+/−, n=7) mutant mice that were reared on breeder chow and their wild type (wt, n=5) C57BL/6J controls were subjects. After obtaining baseline estimates of daily chow intake for 1 week, subjects were switched to a purified, corn starch/sucrose-based extruded pellet low-fat diet (10% kcal from fat, 70% carbohydrate, 3.85 kcal/g, Research Diets, D12450B) for 10 days followed successively by a 3-day challenge with a purified high-fat lard-based diet (60% kcal from fat, 20% carbohydrate, 5.24 kcal/g, Research Diets, D12492) that was otherwise matched in nutrient composition to the purified low-fat diet. Intake and body weight accrual on the high-fat diet were then determined 3 times per week for a further 2 weeks beyond the acute challenge period. Food, water, and body weight measurements were taken 3 hr prior to the onset of dark cycle to 0.01 g precision. Feed efficiency was calculated as body weight gained per unit energy intake (mg/kcal).
Glucose homeostasis
Beginning at 7 months of age, breeder chow-maintained GALR1 knockout and wildtype control mice were subjected to an intraperitoneal (i.p.) glucose tolerance test (1.5 g/kg in sterile water, 10 ml/kg) after a 16 hr fast. One week later, GALR1 knockout, heterozygote and wildtype control mice received a porcine insulin sensitivity test (i.p., 1 IU/kg in isotonic saline, 10 ml/kg) 1 hr after removal of food to control recent feeding. In both tests, glucose was measured in nicked tail blood by a glucometer (Ascensia ELITE, Bayer Health Care, Morristown, NJ) at baseline (∼5 hr into light cycle) and 15, 30, 60 and 120 min following experimental treatment.
Statistical analysis
Food intake, feed efficiency and body weight were analyzed by factorial analyses of variance (ANOVAs). Glucose levels were analyzed by two-way split-plot ANOVAs with Time or Energy State (fed vs. fasted) as a within-subject factor and Genotype a between-subjects factor. Significant omnibus tests involving Genotype were interpreted by post hoc Fisher's least significant difference (LSD) tests. Comparisons between only two groups were evaluated by Student's t-test.
Results
GALR1 feeding studies
At study onset, adult, chow diet-raised GALR1 knockout (−/−) or heterozygote (+/−) mutant mice did not significantly differ from C57BL/6J wild-type controls (+/+) in body weight (M±SEM: +/+: 29.18± 0.34, +/−: 29.87±0.77, −/− : 30.70±0.76 g) or in daily breeder chow intake (data not shown). Similarly, no genotype effects on energy intake, the rate of body weight gain, or feed efficiency were seen when mice were switched to (1st 3 days) (not shown) or maintained on (10 days) the purified low-fat diet (see Figure 1). All groups were in near neutral energy balance when maintained on the low-fat diet (Figure 1), reflected in minimal weight change.
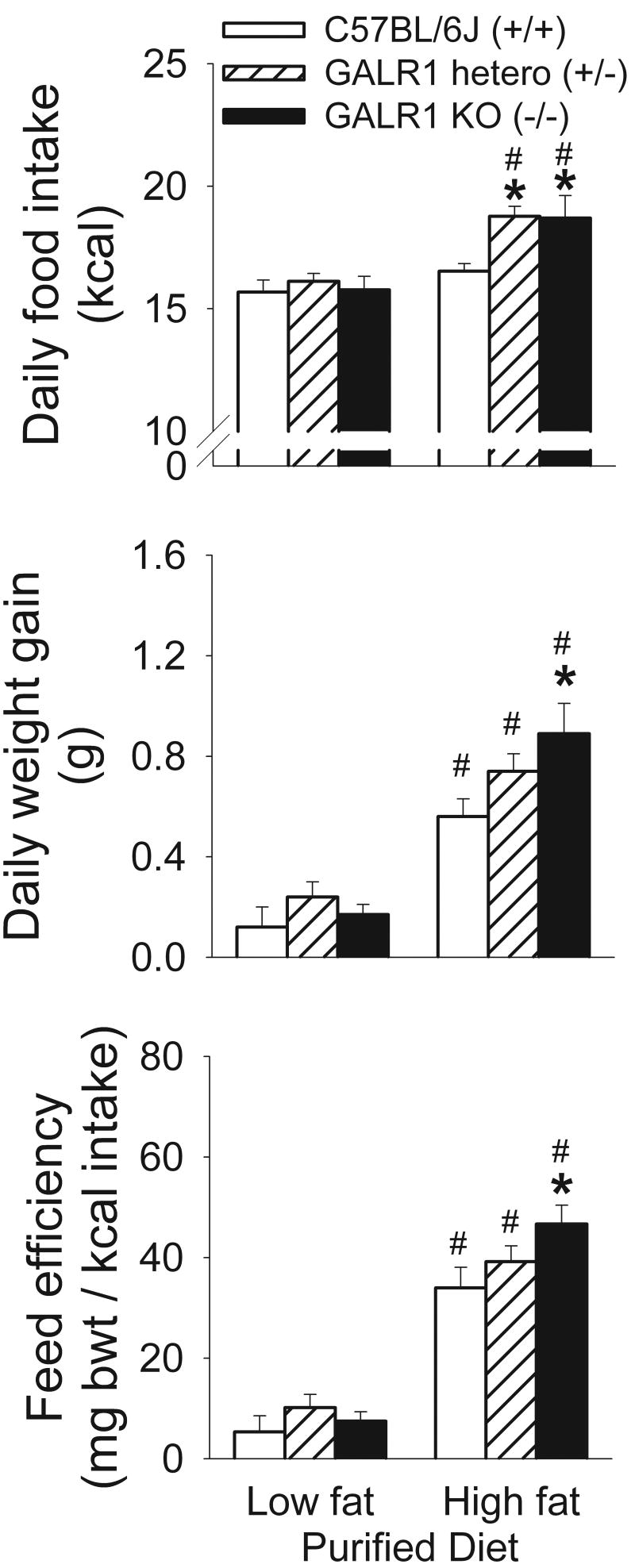
Figure 1.
Adaptation to high-fat /high-energy diets in age-matched, adult, male GALR1 knockout (−/−; KO, n=5), GALR1 heterozygote (+/−; hetero, n=7), and wildtype (+/+, C57BL/6J, n=5) mice. Data reflect M±SEM (top) daily food intake, (middle) daily weight gain, and (right) feed efficiency in mice while maintained for 10 days on purified low-fat/low-energy control diet or during a 3-day “challenge” of being switched to high-fat/high-energy diet. *p<0.05 vs. respective wild type control, #p<0.05 vs. respective low-fat diet condition (Fisher's LSD tests).
However, as shown in Figure 1, GALR1 deficiency acutely impaired the ability to adapt to a high fat/high energy diet. The impaired adaptation was reflected in substantially increased daily energy intake of high-fat diet fed GALR1 heterozygote and knockout mice when switched to the high-fat diet as compared to intake of wt mice as well as to the prior low-fat diet intake of mutant mice (Figure 1). Wild type mice significantly reduced the weight of food they consumed daily by almost one gram when switched from low-fat to high-fat diet (M±SEM, 4.07 ± 0.13 vs. 3.15 ± 0.06 g, p<0.02), approximately accommodating the increased energy density of the high-fat diet. In contrast, heterozygote (M±SEM, 4.19 ± 0.08 vs. 3.58 ± 0.08 g, p=0.15, ns) and knockout mice (M±SEM, 4.10 ± 0.14 vs. 3.57 ± 0.17 g, p=0.77, ns) showed statistically nonsignificant reductions of about one-half gram in the quantity of food consumed. Thus, GALR1 knockout and heterozygote mice exhibited “passive” energy hyperphagia when switched to a high-fat/high-energy diet, because they did not reduce their intake to the same degree as C57BL/6J wildtype mice.
Perhaps reflecting the energy hyperphagia, GALR1 knockout (−/−) mice also gained weight faster than wildtype mice after being switched to high-fat/high-energy diet, with heterozygotes intermediate (see Figure 1). Impaired metabolic adaptation to the high-fat diet also might contribute to the excess, acute weight gain of GALR1 knockout mice. Although all groups exhibited increased feed efficiency in response to the high-fat diet, the GALR1 knockout mice were most thrifty with the energy they ingested (Figure 1).
However, in the 2 week period after the acute challenge period, GALR1 knockout mice consumed significantly less (∼2 kcal/day) high-fat diet than their heterozygote littermates (see Table 1). These findings reflected that GALR1 knockout mice significantly reduced their daily high-fat diet intake significantly more than heterozygote and wt control mice from the acute switch to maintenance high-fat diet periods (M±SEM, knockout: 4.37±0.63, heterozygote: 2.51±0.63, wt: 1.53±0.21 kcal/day reductions, p=0.05 vs. heterozygote, p<0.003 vs. wt). Consequently, GALR1 knockout mice consumed significantly less daily energy in the maintenance phase (post 3-day challenge period) of high-fat diet feeding than they consumed during the maintenance phase of low-fat diet feeding, effects not reliably seen in GALR1 heterozygote or wt mice (see Table 1) Perhaps accordingly, GALR1 knockout mice also tended to gain less weight per day (0.19±0.04 vs. 0.30±0.03 g, p=0.06) and to have lower food efficiencies than heterozygote mice (13.0±2.2 vs. 18.2±1.6 mg body weight/kcal, p=0.08) across the 2-week high-fat diet maintenance phase, with wt mice intermediate (0.25±0.06 g gain/day, 16.7±3.7 mg body weight/kcal).
Table 1.
Effects of GALR1 null gene mutation on (M±SEM) daily energy intake (kcal/day) during the maintenance phase of purified diet feeding in mice
| Mice | Low-fat diet
(10% kcal fat) |
High-fat diet
(60% kcal fat) |
Difference |
|---|---|---|---|
| GALR1 knockout (−/−) | 16.41 ± 0.34 | 14.33 ± 0.71* | −2.08 ± 0.96*,# |
| GALR1 heterozygote (+/−) | 16.87 ± 0.15 | 16.38 ± 0.26 | −0.49 ± 0.24 |
| Wildtype C57BL/6J (−/−) | 15.52 ± 0.45 | 15.00 ± 0.14 | −0.52 ± 0.36 |
p<0.05 vs. heterozygote controls
p<0.05 vs. 0
Glucose homeostasis
GALR1 knockout mice showed significantly increased circulating glucose levels in a fed, but not fasted, state, indicated in a Genotype X Energy State interaction, F(2,14)=4.26, p<0.05. As shown in Figure 2A, post hoc tests revealed that GALR1 knockout mice had elevated fed glucose levels as compared to both heterozygote and wildtype control mice. As expected, glucose levels overall were substantially higher in the fed than fasted state (F[1,14]=491.7, p<0.0001).
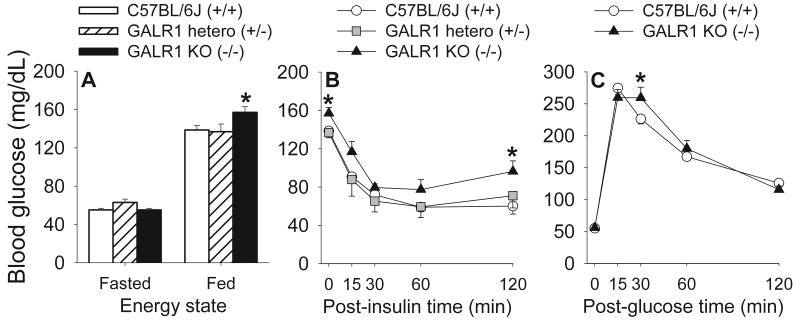
Figure 2.
Effects of GALR1 deficiency on whole-body glucose homeostasis. Panels show tail nick blood glucose levels of mice in (A) 16-hr fasted or fed states, (B) the 2 hr following bolus administration of porcine insulin (i.p., 1 IU/kg) (“insulin sensitivity test”), or (C) the 2 hr following bolus administration of d-glucose (i.p., 1.5 g/kg) (“glucose tolerance test”). Subjects were age-matched, adult, male GALR1 knockout (−/−; KO, n=5), GALR1 heterozygote (+/−; hetero, n=7), and wildtype (+/+; C57BL/6J, n=5) mice. Data are expressed as M and SEM. *p<0.05 vs. all groups in the same energy state or time point (Fisher's LSD tests).
Although insulin significantly reduced circulating glucose levels (Time: F[5,56]=95.63, p<0.0001), which recovered with time (quadratic contrast: F[1,14]=320.3, p<0.0001), insulin actions did not differ reliably according to genotype (see Figure 2B; Genotype: F[2,14]=1.66; Genotype X Time: F[8,56]=.95, ps>0.2). Accordingly, the insulin-induced integrated reduction from baseline in circulating glucose levels did not differ across genotypes (M±SEM: GALR1 knockout: 3894±296, heterozygote: 4404±350 wt: 4041±342 mg*min/dl). By 2 hr post-insulin administration GALR1 knockout mice had regained significant relative hyperglycemia, as observed in the baseline fed condition (see Figure 2B).
As expected, circulating glucose levels rapidly increased and then fell following the intraperitoneal glucose load in fasted mice (F[4,32]=38.61; quadratic contrast F[1,8]=136.71, p<0.0001). However, glucose disappearance differed transiently, but significantly, in relation to genotype (Genotype X Time: F[4,32]=2.76, p<0.05). GALR1 knockout mice showed brief glucose intolerance at +30 min. At that timepoint, post hoc contrasts showed that glucose levels were significantly elevated relative to those of wt mice, because, unlike in wt mice, glucose levels did not fall from 15 to 30 min in GALR1 knockout mice (see Figure 2C).
Discussion
The present studies show that GAR1 knockout (−/−) mutant mice acutely underadjusted food intake and feed efficiency to a high-fat/high-energy diet challenge and also show relative hyperglycemia in fed and glucose-challenged states. In contrast, GALR1 knockout mice showed normal food intake, feed efficiency and weight accrual on low-to-moderate fat/energy diets, normal fasted glucose levels, and normal glucose sensitivity to insulin injection in vivo.
The current findings support the hypothesis that endogenous galanin-GALR1 systems have a special role in adapting food intake and/or metabolism to acute changes in dietary fat, perhaps reflecting the actions of dietary fat to induce PVN galanin expression. The absence of an effect of GALR1 deficiency on body weight and food intake of mice maintained on the purified low- (10% kcal) and low-to-moderate fat breeder chow (28% kcal) diets is consistent with the phenotype reported for galanin null mutant mice [42]. The results also are consistent with reviewed literature which suggests a differential regulatory role for galanin vis-à-vis high-fat diets (>30-35% kcal), whether due to their macronutrient composition, energy density or preferedness. Unexpectedly, however, GALR1 deletion facilitated energy intake and body weight gain during the subacute, 3-day period after being switched from low-fat to high-fat diet. These effects suggest that, overall, GALR1 receptors oppose positive energy balance (or help maintain neutral energy balance) during acute dietary perturbations to energy homeostasis. However, the functional significance of GALR1 receptors for energy homeostasis in animals chronically maintained on high-fat/energy “Western” diets or of other galanin receptor subtypes could be different. Consistent with the former possibility, GALR1 null mutant mice in the present study consumed significantly less (∼2 kcal/day) high-fat diet than their heterozygote littermates and also than their own prior low-fat diet intake during the 2-week maintenance phase of high-fat diet feeding that followed the acute challenge period. Thus, chronic studies which examine the effects of GALR1 deletion on long-term, rather than short-term switches to, high-fat diet intake also appear warranted in order to examine this potentially different aspect of GALR1 action on high-fat diet intake.
Consistent with the reported phenotype of galanin knockout mice, GALR1 knockout mice showed mild, transient glucose intolerance during a glucose tolerance test [42]. Galanin peptide knockout mice previously showed mild, but significant, relative hyperglycemia 10 and 20, but not 50, min following an intraveneous glucose challenge (1 g/kg). In the present study, GALR1 knockout mice were relatively hyperglycemic 30 min, but not 60 min, following an intraperitoneal glucose challenge (1.5 g/kg). Perhaps accordingly, GALR1 knockout mice had reliably elevated glucose levels in fed, but not fasted, states. Glucose intolerance did not result from impaired insulin sensitivity, because GALR1 knockout mice exhibited normal integrated glucose reductions to insulin. The latter finding is also consistent with the observation that glucose intolerance of galanin peptide knockout mice was not due to insulin resistance [42].
The finding that both galanin and GALR1 deficiency impair glucose clearance in vivo is somewhat unexpected given that systemic galanin infusion inhibited glucose-induced insulin secretion in several non-human species [1, 43-45] including mouse. Galanin also directly inhibited glucose-stimulated insulin secretion in vitro from isolated pancreatic tissue of mouse, rat, and pig [34, 46-51]. However, in humans, intravenous galanin infusion did not inhibit glucose-induced insulin secretion or glucose clearance, even at biologically significant doses that elicit substantial growth hormone release [52-55]. Furthermore, galanin receptor antagonists, including galantide, M35, and C-terminally truncated galanin analogs did not alter glucose-stimulated insulin secretion from non-human pancreatic tissue in vitro at doses that blocked exogenous galanin's inhibition of insulin release [34, 56, 57]. These contrary results led to uncertainty about the generality and physiologic significance of reported insulinostatic properties of pharmacological galanin in mouse [44, 51, 58], rat [49, 50], dog [43], and pig [50]. The results obtained with galanin and GALR1 knockout mice suggest that galanin-GALR1 systems do not have an essential, overall inhibitory, physiological action on glucose disposition in vivo, because both mutations resulted in transient glucose intolerance under experimental conditions of a glucose tolerance test.
However, the present results should not be interpreted to mean that pancreatic galanin-GALR1 systems may not exert direct physiologically-relevant inhibitory actions on insulin release under some conditions. Galanin has been proposed to mediate insulinostatic actions of the sympathetic nervous system on islet function, reflecting its distribution in autonomic nerve terminals of the endocrine pancreas [42, 43, 45, 59]. Consistent with this hypothesis, galanin knockout mice lack the putatively sympathetic inhibition of insulin release that is otherwise elicited by intravenous, glucoprivic 2-deoxyglycose administration [42]. However, the net effect of galanin or GALR1 deficiency was to impair glucose clearance following bolus glucose challenges. Thus, other, disposal-impairing glucoregulatory actions of galanin-GALR1 deficiency on pancreatic islet function [42] or brain may be for responsible for the transient glucose intolerance of these null mutant mice.
To address the site of action of observed effects on feeding and glucose homeostasis, the contribution of central (e.g., hypothalamic) versus peripheral (e.g., pancreatic) galanin signaling is being addressed by site-specific reinstatement of GALR1 expression through lentiviral vector-mediated expression and by local administration of novel subtype-selective galanin receptor ligands [60, 61]. Combining these molecular and pharmacological tools with the recently available GALR null mutant mice may help mechanistically clarify the implicated role of galanin systems in ingestive and metabolic responses to dietary fat and in glucose disposition.
Acknowledgments
This study was supported by NIMH grant R01MH63080-05 and MH074055-01 to T. Bartfai. This is manuscript number 18461 from The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tatemoto K, Rokaeus A, Jornvall H, McDonald TJ, Mutt V. Galanin - a novel biologically active peptide from porcine intestine. FEBS Lett. 1983;164:124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]
- 2.Kohler C, Chan-Palay V. Galanin receptors in the post-mortem human brain. Regional distribution of 125I-galanin binding sites using the method of in vitro receptor autoradiography. Neurosci Lett. 1990;120:179–182. doi: 10.1016/0304-3940(90)90032-5. [DOI] [PubMed] [Google Scholar]
- 3.Benzing WC, Kordower JH, Mufson EJ. Galanin immunoreactivity within the primate basal forebrain: evolutionary change between monkeys and apes. J Comp Neurol. 1993;336:31–39. doi: 10.1002/cne.903360103. [DOI] [PubMed] [Google Scholar]
- 4.Kordower JH, Le HK, Mufson EJ. Galanin immunoreactivity in the primate central nervous system. J Comp Neurol. 1992;319:479–500. doi: 10.1002/cne.903190403. [DOI] [PubMed] [Google Scholar]
- 5.Kordower JH, Mufson EJ. Galanin-like immunoreactivity within the primate basal forebrain: differential staining patterns between humans and monkeys. J Comp Neurol. 1990;294:281–292. doi: 10.1002/cne.902940211. [DOI] [PubMed] [Google Scholar]
- 6.Melander T, Hokfelt T, Rokaeus A, Cuello AC, Oertel WH, Verhofstad A, Goldstein M. Coexistence of galanin-like immunoreactivity with catecholamines, 5-hydroxytryptamine, GABA and neuropeptides in the rat CNS. J Neurosci. 1986;6:3640–3654. doi: 10.1523/JNEUROSCI.06-12-03640.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobowitz DM, Kresse A, Skofitsch G. Galanin in the brain: chemoarchitectonics and brain cartography--a historical review. Peptides. 2004;25:433–464. doi: 10.1016/j.peptides.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Skofitsch G, Jacobowitz DM. Quantitative distribution of galanin-like immunoreactivity in the rat central nervous system. Peptides. 1986;7:609–613. doi: 10.1016/0196-9781(86)90035-5. [DOI] [PubMed] [Google Scholar]
- 9.Perez SE, Wynick D, Steiner RA, Mufson EJ. Distribution of galaninergic immunoreactivity in the brain of the mouse. J Comp Neurol. 2001;434:158–185. doi: 10.1002/cne.1171. [DOI] [PubMed] [Google Scholar]
- 10.Ryan MC, Loiacono RE, Gundlach AL. Galanin messenger RNA during postnatal development of the rat brain: expression patterns in Purkinje cells differentiate anterior and posterior lobes of cerebellum. Neuroscience. 1997;78:1113–1127. doi: 10.1016/s0306-4522(96)00652-5. [DOI] [PubMed] [Google Scholar]
- 11.Gundlach AL, Wisden W, Morris BJ, Hunt SP. Localization of preprogalanin mRNA in rat brain: in situ hybridization study with a synthetic oligonucleotide probe. Neurosci Lett. 1990;114:241–247. doi: 10.1016/0304-3940(90)90570-y. [DOI] [PubMed] [Google Scholar]
- 12.Ryan MC, Gundlach AL. Localization of preprogalanin messenger RNA in rat brain: identification of transcripts in a subpopulation of cerebellar Purkinje cells. Neuroscience. 1996;70:709–728. doi: 10.1016/s0306-4522(96)83009-0. [DOI] [PubMed] [Google Scholar]
- 13.Cheung CC, Hohmann JG, Clifton DK, Steiner RA. Distribution of galanin messenger RNA-expressing cells in murine brain and their regulation by leptin in regions of the hypothalamus. Neuroscience. 2001;103:423–432. doi: 10.1016/s0306-4522(01)00012-4. [DOI] [PubMed] [Google Scholar]
- 14.Hakansson ML, Brown H, Ghilardi N, Skoda RC, Meister B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci. 1998;18:559–572. doi: 10.1523/JNEUROSCI.18-01-00559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Leibowitz KL. Central insulin inhibits hypothalamic galanin and neuropeptide Y gene expression and peptide release in intact rats. Brain Res. 1997;777:231–236. doi: 10.1016/s0006-8993(97)00963-3. [DOI] [PubMed] [Google Scholar]
- 16.Leibowitz SF. Regulation and effects of hypothalamic galanin: relation to dietary fat, alcohol ingestion, circulating lipids and energy homeostasis. Neuropeptides. 2005;39:327–332. doi: 10.1016/j.npep.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 17.Kyrkouli SE, Strubbe JH, Scheurink AJ. Galanin in the PVN increases nutrient intake and changes peripheral hormone levels in the rat. Physiol Behav. 2006;89:103–109. doi: 10.1016/j.physbeh.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Akabayashi A, Koenig JI, Watanabe Y, Alexander JT, Leibowitz SF. Galanin-containing neurons in the paraventricular nucleus: a neurochemical marker for fat ingestion and body weight gain. Proc Natl Acad Sci U S A. 1994;91:10375–10379. doi: 10.1073/pnas.91.22.10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dourmashkin JT, Chang GQ, Gayles EC, Hill JO, Fried SK, Julien C, Leibowitz SF. Different forms of obesity as a function of diet composition. Int J Obes (Lond) 2005;29:1368–1378. doi: 10.1038/sj.ijo.0803017. [DOI] [PubMed] [Google Scholar]
- 20.Kyrkouli SE, Stanley BG, Hutchinson R, Seirafi RD, Leibowitz SF. Peptideamine interactions in the hypothalamic paraventricular nucleus: analysis of galanin and neuropeptide Y in relation to feeding. Brain Res. 1990;521:185–191. doi: 10.1016/0006-8993(90)91541-n. [DOI] [PubMed] [Google Scholar]
- 21.Leibowitz SF. Differential functions of hypothalamic galanin cell grows in the regulation of eating and body weight. Ann N Y Acad Sci. 1998;863:206–220. doi: 10.1111/j.1749-6632.1998.tb10696.x. [DOI] [PubMed] [Google Scholar]
- 22.Leibowitz SF, Dourmashkin JT, Chang GQ, Hill JO, Gayles EC, Fried SK, Wang J. Acute high-fat diet paradigms link galanin to triglycerides and their transport and metabolism in muscle. Brain Res. 2004;1008:168–178. doi: 10.1016/j.brainres.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 23.Chang GQ, Karatayev O, Davydova Z, Leibowitz SF. Circulating triglycerides impact on orexigenic peptides and neuronal activity in hypothalamus. Endocrinology. 2004;145:3904–3912. doi: 10.1210/en.2003-1582. [DOI] [PubMed] [Google Scholar]
- 24.Kyrkouli SE, Stanley BG, Leibowitz SF. Galanin: stimulation of feeding induced by medial hypothalamic injection of this novel peptide. Eur J Pharmacol. 1986;122:159–160. doi: 10.1016/0014-2999(86)90175-5. [DOI] [PubMed] [Google Scholar]
- 25.Corwin RL, Robinson JK, Crawley JN. Galanin antagonists block galanin-induced feeding in the hypothalamus and amygdala of the rat. Eur J Neurosci. 1993;5:1528–1533. doi: 10.1111/j.1460-9568.1993.tb00221.x. [DOI] [PubMed] [Google Scholar]
- 26.Crawley JN, Robinson JK, Langel U, Bartfai T. Galanin receptor antagonists M40 and C7 block galanin-induced feeding. Brain Res. 1993;600:268–272. doi: 10.1016/0006-8993(93)91382-3. [DOI] [PubMed] [Google Scholar]
- 27.Nagase H, Bray GA, York DA. Effect of galanin and enterostatin on sympathetic nerve activity to interscapular brown adipose tissue. Brain Res. 1996;709:44–50. doi: 10.1016/0006-8993(95)01292-3. [DOI] [PubMed] [Google Scholar]
- 28.Nagase H, Nakajima A, Sekihara H, York DA, Bray GA. Regulation of feeding behavior, gastric emptying, and sympathetic nerve activity to interscapular brown adipose tissue by galanin and enterostatin: the involvement of vagal-central nervous system interactions. J Gastroenterol. 2002;37 14:118–127. doi: 10.1007/BF03326430. [DOI] [PubMed] [Google Scholar]
- 29.Yun R, Dourmashkin JT, Hill J, Gayles EC, Fried SK, Leibowitz SF. PVN galanin increases fat storage and promotes obesity by causing muscle to utilize carbohydrate more than fat. Peptides. 2005;26:2265–2273. doi: 10.1016/j.peptides.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Leibowitz SF, Kim T. Impact of a galanin antagonist on exogenous galanin and natural patterns of fat ingestion. Brain Res. 1992;599:148–152. doi: 10.1016/0006-8993(92)90863-5. [DOI] [PubMed] [Google Scholar]
- 31.Odorizzi M, Fernette B, Angel E, Burlet C, Tankosic P, Burlet A. Galanin receptor antagonists decrease fat preference in Brattleboro rat. Neuropharmacology. 2002;42:134–141. doi: 10.1016/s0028-3908(01)00115-0. [DOI] [PubMed] [Google Scholar]
- 32.Abramov U, Floren A, Echevarria DJ, Brewer A, Manuzon H, Robinson JK, Bartfai T, Vasar E, Langel U. Regulation of feeding by galnon. Neuropeptides. 2004;38:55–61. doi: 10.1016/j.npep.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Crawley JN. The role of galanin in feeding behavior. Neuropeptides. 1999;33:369–375. doi: 10.1054/npep.1999.0049. [DOI] [PubMed] [Google Scholar]
- 34.Gregersen S, Lindskog S, Land T, Langel U, Bartfai T, Ahren B. Blockade of galanin-induced inhibition of insulin secretion from isolated mouse islets by the non-methionine containing antagonist M35. Eur J Pharmacol. 1993;232:35–39. doi: 10.1016/0014-2999(93)90725-w. [DOI] [PubMed] [Google Scholar]
- 35.Gundlach AL, Burazin TC, Larm JA. Distribution, regulation and role of hypothalamic galanin systems: renewed interest in a pleiotropic peptide family. Clin Exp Pharmacol Physiol. 2001;28:100–105. doi: 10.1046/j.1440-1681.2001.03411.x. [DOI] [PubMed] [Google Scholar]
- 36.Branchek TA, Smith KE, Gerald C, Walker MW. Galanin receptor subtypes. Trends Pharmacol Sci. 2000;21:109–117. doi: 10.1016/s0165-6147(00)01446-2. [DOI] [PubMed] [Google Scholar]
- 37.Lu X, Mazarati A, Sanna P, Shinmei S, Bartfai T. Distribution and differential regulation of galanin receptor subtypes in rat brain: effects of seizure activity. Neuropeptides. 2005;39:147–152. doi: 10.1016/j.npep.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Smith KE, Walker MW, Artymyshyn R, Bard J, Borowsky B, Tamm JA, Yao WJ, Vaysse PJ, Branchek TA, Gerald C, Jones KA. Cloned human and rat galanin GALR3 receptors. Pharmacology and activation of G-protein inwardly rectifying K+ channels. J Biol Chem. 1998;273:23321–23326. doi: 10.1074/jbc.273.36.23321. [DOI] [PubMed] [Google Scholar]
- 39.Kolakowski LF, Jr, O'Neill GP, Howard AD, Broussard SR, Sullivan KA, Feighner SD, Sawzdargo M, Nguyen T, Kargman S, Shiao LL, Hreniuk DL, Tan CP, Evans J, Abramovitz M, Chateauneuf A, Coulombe N, Ng G, Johnson MP, Tharian A, Khoshbouei H, George SR, Smith RG, O'Dowd BF. Molecular characterization and expression of cloned human galanin receptors GALR2 and GALR3. J Neurochem. 1998;71:2239–2251. doi: 10.1046/j.1471-4159.1998.71062239.x. [DOI] [PubMed] [Google Scholar]
- 40.Mennicken F, Hoffert C, Pelletier M, Ahmad S, O'Donnell D. Restricted distribution of galanin receptor 3 (GalR3) mRNA in the adult rat central nervous system. J Chem Neuroanat. 2002;24:257–268. doi: 10.1016/s0891-0618(02)00068-6. [DOI] [PubMed] [Google Scholar]
- 41.Fetissov SO, Jacoby AS, Brumovsky PR, Shine J, Iismaa TP, Hokfelt T. Altered hippocampal expression of neuropeptides in seizure-prone GALR1 knockout mice. Epilepsia. 2003;44:1022–1033. doi: 10.1046/j.1528-1157.2003.51402.x. [DOI] [PubMed] [Google Scholar]
- 42.Ahren B, Pacini G, Wynick D, Wierup N, Sundler F. Loss-of-function mutation of the galanin gene is associated with perturbed islet function in mice. Endocrinology. 2004;145:3190–3196. doi: 10.1210/en.2003-1700. [DOI] [PubMed] [Google Scholar]
- 43.Dunning BE, Ahren B, Veith RC, Bottcher G, Sundler F, Taborsky GJ., Jr Galanin: a novel pancreatic neuropeptide. Am J Physiol. 1986;251:E127–E133. doi: 10.1152/ajpendo.1986.251.1.E127. [DOI] [PubMed] [Google Scholar]
- 44.Lindskog S, Ahren B. Galanin: effects on basal and stimulated insulin and glucagon secretion in the mouse. Acta Physiol Scand. 1987;129:305–309. doi: 10.1111/j.1748-1716.1987.tb08073.x. [DOI] [PubMed] [Google Scholar]
- 45.Ahren B, Lindskog S. Galanin and the regulation of islet hormone secretion. Int J Pancreatol. 1992;11:147–160. doi: 10.1007/BF02924180. [DOI] [PubMed] [Google Scholar]
- 46.Ahren B, Arkhammar P, Berggren PO, Nilsson T. Galanin inhibits glucose-stimulated insulin release by a mechanism involving hyperpolarization and lowering of cytoplasmic free Ca2+ concentration. Biochem Biophys Res Commun. 1986;140:1059–1063. doi: 10.1016/0006-291x(86)90742-4. [DOI] [PubMed] [Google Scholar]
- 47.Silvestre RA, Miralles P, Monge L, Moreno P, Villanueva ML, Marco J. Effects of galanin on hormone secretion from the in situ perfused rat pancreas and on glucose production in rat hepatocytes in vitro. Endocrinology. 1987;121:378–383. doi: 10.1210/endo-121-1-378. [DOI] [PubMed] [Google Scholar]
- 48.Miralles P, Peiro E, Silvestre RA, Villanueva ML, Marco J. Effects of galanin on islet cell secretory responses to VIP, GIP, 8-CCK, and glucagon by the perfused rat pancreas. Metabolism. 1988;37:766–770. doi: 10.1016/0026-0495(88)90012-1. [DOI] [PubMed] [Google Scholar]
- 49.Lindskog S, Ahren B. Effects of galanin on insulin and glucagon secretion in the rat. Int J Pancreatol. 1989;4:335–344. doi: 10.1007/BF02938468. [DOI] [PubMed] [Google Scholar]
- 50.Lindskog S, Dunning BE, Martensson H, Ar'Rajab A, Taborsky GJ, Jr, Ahren B. Galanin of the homologous species inhibits insulin secretion in the rat and in the pig. Acta Physiol Scand. 1990;139:591–596. doi: 10.1111/j.1748-1716.1990.tb08963.x. [DOI] [PubMed] [Google Scholar]
- 51.Lindskog S, Ahren B. Effects of galanin and norepinephrine on insulin secretion in the mouse. Pancreas. 1992;7:636–641. doi: 10.1097/00006676-199211000-00003. [DOI] [PubMed] [Google Scholar]
- 52.Gilbey SG, Stephenson J, O'Halloran DJ, Burrin JM, Bloom SR. High-dose porcine galanin infusion and effect on intravenous glucose tolerance in humans. Diabetes. 1989;38:1114–1116. doi: 10.2337/diab.38.9.1114. [DOI] [PubMed] [Google Scholar]
- 53.Ahren B. Effects of galanin and calcitonin gene-related peptide on insulin and glucagon secretion in man. Acta Endocrinol (Copenh) 1990;123:591–597. [PubMed] [Google Scholar]
- 54.Carey DG, Iismaa TP, Ho KY, Rajkovic LA, Kelly J, Kraegen EW, Ferguson J, Inglis AS, Shine J, Chisholm DJ. Potent effects of human galanin in man: growth hormone secretion and vagal blockade. J Clin Endocrinol Metab. 1993;77:90–93. doi: 10.1210/jcem.77.1.7686918. [DOI] [PubMed] [Google Scholar]
- 55.Holst JJ, Bersani M, Hvidberg A, Knigge U, Christiansen E, Madsbad S, Harling H, Kofod H. On the effects of human galanin in man. Diabetologia. 1993;36:653–657. doi: 10.1007/BF00404076. [DOI] [PubMed] [Google Scholar]
- 56.Lindskog S, Ahren B, Land T, Langel U, Bartfai T. The novel high-affinity antagonist, galantide, blocks the galanin-mediated inhibition of glucose-induced insulin secretion. Eur J Pharmacol. 1992;210:183–188. doi: 10.1016/0014-2999(92)90669-u. [DOI] [PubMed] [Google Scholar]
- 57.Yanaihara N, Mochizuki T, Takatsuka N, Iguchi K, Sato K, Kakuyama H, Li M, Yanaihara C. Galanin analogues: agonist and antagonist. Regul Pept. 1993;46:93–101. doi: 10.1016/0167-0115(93)90018-4. [DOI] [PubMed] [Google Scholar]
- 58.Lindskog S, Ahren B. Galanin and pancreastatin inhibit stimulated insulin secretion in the mouse: comparison of effects. Horm Res. 1988;29:237–240. doi: 10.1159/000181010. [DOI] [PubMed] [Google Scholar]
- 59.Lindskog S, Ahren B, Dunning BE, Sundler F. Galanin-immunoreactive nerves in the mouse and rat pancreas. Cell Tissue Res. 1991;264:363–368. doi: 10.1007/BF00313975. [DOI] [PubMed] [Google Scholar]
- 60.Swanson CJ, Blackburn TP, Zhang X, Zheng K, Xu ZQ, Hokfelt T, Wolinsky TD, Konkel MJ, Chen H, Zhong H, Walker MW, Craig DA, Gerald CP, Branchek TA. Anxiolytic- and antidepressant-like profiles of the galanin-3 receptor (Gal3) antagonists SNAP 37889 and SNAP 398299. Proc Natl Acad Sci U S A. 2005;102:17489–17494. doi: 10.1073/pnas.0508970102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barr AM, Kinney JW, Hill MN, Lu X, Biros S, Rebek J, Jr, Bartfai T. A novel, systemically active, selective galanin receptor type-3 ligand exhibits antidepressant-like activity in preclinical tests. Neurosci Lett. 2006;405:111–115. doi: 10.1016/j.neulet.2006.06.033. [DOI] [PubMed] [Google Scholar]