Abstract
Measures of arousal were used to study effects of estradiol and food restriction, and their potential interactions, in ovariectomized female C57Bl/6 mice. It was hypothesized based on a proposed theoretical equation (Pfaff, 2006) that each treatment would increase arousal-related behaviors and that their combination would further increase arousal behavior. Following baseline testing, animals (n = 28) were divided into 3 groups that, in different experimental phases, received either estradiol (in subcutaneous capsules), restricted diet (a liquid diet providing 60% of daily caloric requirements) or a combination of those two. An automated arousal behavior monitoring system was used to measure home cage voluntary motor activity and sensory responsiveness, these being components of a new operational definition of ‘generalized arousal’. Key findings: (1) During the light, all treatments reduced voluntary activity. (2) In the dark, estrogens increased, while estrogens in combination with restricted diet decreased, horizontal activity. (3) In the dark, restricted diet alone had little effect on voluntary activity, but reduced it when combined with estrogen treatment. (4) All treatments reduced responses to the olfactory stimulus. The dependence of results on time of day was unexpected. Further, different patterns of results for the three treatments suggest that estrogens and food restriction did not have equivalent or additive effects on arousal. While contrary to the main prediction, these findings are discussed in terms of the animals’ adaptive preparations for reproduction (Schneider, 2006).
Keywords: CNS arousal, estrogens, restricted diet, motor activity, sensory responsiveness, risk assessment
While CNS arousal is not a unitary phenomenon, a comprehensive recent review of the literature (Pfaff, 2006a) yielded a quantitative hypothesis that there is a ‘generalized arousal’ term supplementing various specific forms of arousal (sex, hunger, etc.) in an equation that calculates overall arousal state in any animal. In this theoretical approach, arousal is considered to be necessary for motivated behavior: an animal can be aroused without motivation, but can not show motivated responses without being aroused. The current experiments were based on the notion that the concept of CNS arousal underlies (i) some of the estrogenic effects on mating behaviors that involve vigorous motion, (ii) some of the searching, foraging behaviors caused by hunger. Estrogen treatments and restricted diet conditions that were chosen do stimulate mating behavior and hunger, respectively (Shelley et al, in prep); and these two motivational systems were chosen because of their known relations to each other (Wade and Schneider, 1992).
A large body of work has delineated hormonal, neural and genomic mechanisms producing the primary female-typical behavior lordosis (reviewed in Pfaff, 2006b). However, behavioral states and acts leading to females’ mating behavior, namely, sexual arousal and courtship responses, have received somewhat less attention. Dating back to the work of Frank Beach (Beach, 1976) and others (Edwards and Pfeifle, 1983), it was understood that female rat courtship behaviors consist of extremely unusual patterns of rapid locomotion and are accompanied by a high degree of axial muscle tension. These aroused, rapid movements are exquisitely dependent on estrogens followed by progesterone, and have the effect of pacing copulatory encounters (Erskine, 1989; Paredes and Vazquez, 1999; Pfaus et al., 1999). Rapid, aroused courtship behaviors were not often observed in female mice until ‘semi-natural environments’ were employed (Garey, et al., 2002). The neural pathways involved in females’ courtship behaviors have been studied, from olfactory mechanisms (Coria-Avila, et al., 2005), through preoptic area regions (Kato and Sakuma, 2000; Kondo et al., 1997) to the midbrain (Edwards and Pfeifle, 1983). Neurochemical mechanisms have also been explored (Pfaus et al., 2004).
These courtship behaviors involve active responses and energy expenditure by the female and therefore would require CNS arousal. Mammalian reproductive processes are so sensitive to the availability of appropriate metabolic fuels (Wade and Schneider, 1992; Wade and Jones, 2004) that reproduction may be deferred, especially by the female, when supplies are low. Neural and genomic mechanisms underlying hypothalamic control of food intake in hungry rats and mice are being worked out (Segal, et al., 2005; Zigman and Elmquist, 2003). For example, hypothalamic neurons expressing the gene for hypocretin/orexin represent mechanisms directly relating arousal and hunger (Lecea, et al., 2002; Mieda, et al., 2004); this gene product shows how hunger could lead to arousal.
Both the rapid, excited behaviors of courtship and the motivation to seek food by foraging involve arousal – the activation of behavior (Pfaff, 2006b). Thus, the current experiment examined the effects of estrogens and diet restrictions on physical, quantitative measures of arousal in female mice.
Recently, CNS arousal received a much-needed precise operational definition. A more aroused animal (i) emits more voluntary motor activity, and (ii) is more alert and responsive to sensory stimuli in all modalities. This definition leads to quantitative, physical measures (Pfaff, 2006b, and see Methods) that we index by measuring movements of mice in ‘high tech’ home cages. Indeed, the arousal concept is directly relevant to the mechanisms of sexual courtship behaviors. On the sensory side, greater olfactory responsiveness would facilitate partner preferences reported by Coria-Avila et al. (2005). On the motor side, female mice and rats exhibiting solicitation and approach behaviors are emitting more voluntary motor activity. In fact, preoptic neurons important for courtship overlap with the preoptic locomotor zone (Sinnamon, 1993), and estrogen implantation in this area increases locomotion (Fahrbach, et al., 1985). These preoptic neurons project to the midbrain locomotor zone (Takeo and Sakuma, 1995), toward which axons found important for active female approaches are coursing (Edwards and Pfeifle, 1983).
Because estrogens lead to high degrees of axial muscle tension in female rats and mice, and because hunger due to a restricted diet should lead to active searching for food, it was hypothesized that both treatments would lead to increased measures of CNS arousal using the ‘high throughput’ assay described in detail in Methods. And according to the simplest theoretical equation for CNS arousal (Garey et al, 2003; Pfaff, 2006b) their effects should be additive.
Thus, these experiments test the hypothesis that there is a component of motivation, known as general arousal, that is common to the motivation to engage in all behaviors, behaviors such as eating, having sex, defending territory, etc. This hypothesis predicts that the stimuli that increase sex behavior and eating (estrogen and food deprivation respectively) would both increase measures of general arousal even though one stimulus increases sex behavior and decreases food intake, whereas the other stimulus increases food intake and decreases sex behavior, and that the combination would enhance arousal additively. That is, we have started with the simplest hypothesis. The alternative hypothesis is that motivations for separate behaviors do not share a component of general arousal. This hypothesis predicts that estrogenic and food deprivation effects on arousal-related assays would not interact at all.
Methods
Animals
Adult female C57BL/6 mice, obtained from Jackson Laboratory (Bar Harbor, Maine) were ovariectomized by the supplier prior to delivery at 3–4 months of age. N’s are in Table 1. At the start of the experiment, body weights ranged from 17.6g–28.1g. Each subject’s initial body weight was used for group assignments, which resulted in three balanced groups described below. Mice were housed individually in plastic shoebox cages and maintained on a 12:12 light/dark cycle with lights off at 1300 h. Water was provided ad libitum, but mice were fed a commercial liquid diet according to the methods described below. Stud mice (Jackson Laboratory) used for sexual behavior tests were members of a stud colony maintained by the lab. A record of sexual interactions for each stud male was routinely recorded to ensure that studs received a similar amount sexual experience prior to interactions with experimental females. All procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and a protocol approved by The Rockefeller University’s IACUC.
TABLE 1.
Experimental Design.
| Group | Phase 1 | Phase 2 | Phase 3 |
|---|---|---|---|
| A (n = 9) | Control diet (Baseline) | Control diet/E2 (Sexual drive only) | Restricted diet/Oil (Hunger drive only) |
| B (n = 9) | Control diet (Baseline) | Restricted diet/Oil (Hunger drive only) | Control diet/E2 (Sexual drive only) |
| C (n = 10) | Control diet (Baseline) | Restricted diet/E2 (Sexual/Hunger drives) | Control diet/Oil (Neither Sexual/Hunger) |
Experimental Design
The experiments were conducted in three phases (see Table 1). Each three week phase consisted of a 2 wk period, which allowed animals to recover from minor capsule implantation surgery and to adjust to the liquid diet regime. When animals were feeding at the target diet amount, home cage activity was collected for 3 days. The schedule for the third week was as follows: A battery of sensory responsiveness tests were given on Day 1 and Day 2 at 4–5 hr into the light cycle, a quinine adulterated food 24 hr feeding challenge was administered on Day 3, no tests were given during Day 4, and on Day 5 a sexual behavior test was administered, beginning at lights out (feeding and sexual behavior data to be reported in subsequent ms, II).
During the preliminary baseline phase all subjects received no hormone treatment and were fed the controlled liquid diet regime. During the second and third phases, a cross-over design was used to control for effects of the administration order of hormone and diet treatments; such that, during Phase 2 one group received an estradiol implant but remained on the controlled diet (n = 9), the second group received no estradiol treatment but was fed a restricted diet (n = 9), and the third group received both the estradiol treatment and the restricted diet (n = 10). During the third phase, hormone and diet treatments for the first two groups were reversed and the third group returned to the baseline treatment of no estradiol treatment and the controlled diet. The experiment was conducted twice using two squads of animals that were each divided proportionately into the three treatment groups. All other conditions were held constant across the two experimental runs.
Hormone Treatment
Animals were surgically implanted with a hormone treatment capsule that provided subcutaneous exposure to estradiol lasting for a period of more than 5 weeks. It was constructed with Silastic tubing (Dow Corning Co., Midland, MI, dimensions: 1.57 mm ID X 2.4 mm OD X 2.0 cm in length), sealed at each end with medical grade silicon, and filled with either estradiol benzoate in sesame oil (50μg/0.05 ml) or 0.05 ml sesame oil only. Animals were briefly anesthetized by inhalation of Isoflurane (Abbott Laboratories, North Chicago, IL) while capsules were implanted subcutaneously just caudal to the nape of the neck though a small incision which was closed with a wound clip.
Diet Regimes
Liquid diets were chosen for this experiment to allow convenient, accurate measurements of daily consumption, strict control over diet volume available per day, and during the feeding challenge, the addition of a bitter adulterant. Diet was mixed according to the supplier’s instructions and given in feeders made of standard water bottle stoppers with sipper tubes in plastic 20 ml Dilu-Vials (Fisher Scientific Co., Pittsburg, PA). Each daily feeder was weighed before and after placement on the cage top or as feeding tests required.
The control diet was Microstabilized Liquid Rodent Diet (Purina Mills TestDiet, Richmond IN, # LD101). This diet provides 1 kcal/gram available metabolic fuel. Preliminary tests revealed that when this diet was fed ad libitum to mice, the mice gained significantly greater weight than mice fed ad libitum on lab chow (data not shown). Consequently, 10 g of this prepared diet was provided in the special feeders daily. During Phase 1, when all animals were treated similarly and offered 10 g liquid diet, this feeding regime resulted at the end of the 19 day period in an average post-diet weight of 104.1 % of the pre-diet weight (n = 28). Average daily volume consumed was also calculated for each animal. The median average daily volume of diet consumed was 9.4 g diet/day, (n =28, range: 8.2g–9.9g). Therefore, although the diet volume was controlled, animals’ daily consumption was not restricted by the controlled feeding.
The restricted diet (Purina Mills TestDiet, Richmond IN, “Restricted Microstabilized Liquid Rodent Diet”) was specially formulated for this experiment to provide the same 1 kcal/gram metabolic fuel ratio as the control diet, but was modified so that when animals were fed 60% of the control daily volume (6 g/day), they would still receive equivalent amounts of nutrients important to maintain proper health. Thus, quantities of specific nutrients in the formula were increased while fat, protein and carbohydrates quantities were held constant. We were striving for a nutritionally adequate, but calorically restricted liquid diet. Preliminary tests with the restricted diet revealed that a drastic reduction of the amount of diet available to the mice (from 10.0 g/day to 6.0 g/day) was not well tolerated, particularly when animals were given more than 1 day’s allotment. Animals invariably converted to a binge and fast pattern. For example, 12 g diet for 2 days was eaten in the first day, leaving none for the second day and forcing a fast when no more food was provided. To overcome this feeding pattern, starting with 9.5 g on the first day of the restricted diet feeding regime, animals were given 0.5 g less, every two days progressively until the target volume of 6.0 g/day was reached 3 days before testing began and was maintained throughout the testing week. The average daily diet consumption during test week was 5.7 g (n = 28, range: 5.3–6.0g) and this restricted feeding regime resulted at the end of the test week in an average post-diet weight of 85.5% that of the pre-diet weight (n = 28).
Arousal assay
Assay equipment
Arousal behavior was measured using an automated infra-red beam break behavior monitoring system integrated with a stimulus delivery system surrounding a home cage. One computer controlled 16 units that were run in parallel. The infra-red photobeams covered the cage area in a 1 cm three dimensional grid (AccuScan Instruments, Columbus, OH). Disruption of a beam resulted in an activity count, which was recorded in a PC using Versamax software (AccuScan Instruments). The hardware that controlled each stimulus delivery was LabLinc V (Coulbourn Instruments, Allentown, PA), a modular instrument system that consisted of an isolated power base with stimulus synthesis, control, and computer interface modules. Instruments were controlled by Graphic State software (Coulbourn Instruments). An experiment was conceived as a series of states that were specified by a stimulus configuration in the subject’s environment such that the exit out of each state and movement into the next state was directed according to a set of time and animal response requirements.
Motor activity
Home cage was assessed using the behavior monitoring equipment which collected data as number of beam breaks, in 1 hour bins for each 24 hour period and each light and dark cycle. Movement measurements include Vertical Activity, Horizontal Activity, and Total Distance—an accumulation of horizontal movements made in a continuous path. Measurements of motor activity were also collected during each of the sensory alertness tests in response to sensory stimuli.
Sensory responsiveness
Sensory responsiveness tests were administered during the light cycle during the resting state of each mouse to facilitate administration of sensory stimuli while animals were quiescent. This resting state was operationally defined as the absence of ambulatory movement, i.e. no Total Distance moved for 5 min. This assessment criterion was written into the stimulus delivery computer program. Each mouse was given a mandatory 10 minutes wait after equipment start-up before the program entered the state during which it is vigilant for the criterion that signals the animal’s resting state. When the criterion was met, the first stimulus was delivered. After the first stimulus, the assessment criterion for the next resting state configuration began at the end of the animal’s response activity, but was required to be at least 10 minutes.
Animals were exposed to three stimuli in the following order: tactile, vestibular, and olfactory. The 10 second tactile stimulus was provided by air streams supplied by a distal compressed air tank (@15 psi), released through jets in each corner of the cage in such a manner that the air gently lifted the animal’s hair regardless of its position in the cage. The 15 second vestibular stimulus consisted of the circular rotation of the cage about its vertical axis by an orbital shaker (Barnstead International, Dubuque, IA) at 90 RPM. Lastly, the olfactory stimulus was a 10 second release of air from a compressed air tank through a jar containing 3 ml of almond extract (McCormick and Co., Inc, Hunt Valley, MD) and into the cage through 2 jets placed near the floor in the center of each of the long edges of the cage. Sensory response data measurements included Vertical Activity, Horizontal Activity and Total Distance moved for each response session after stimulus delivery.
Data Analysis
All statistical analyses were non-parametric. These were chosen for the following reasons. Both parametric and non-parametric statistics have requirements for homogeneity of variance, but for data sets, some of whose distributions do not follow the normal curve, non-parametric statistics are superior because their underlying derivations and assumptions do not depend on a normal distribution whereas parametric statistics’ do. Furthermore, non-parametric statistics provide a more conservative approach, such that incorrect reporting of a group difference is much less likely.
There were no statistically significant differences across phases or experimental runs between groups of animals receiving the same treatment, so data from the same treatment groups were pooled for statistical analyses. Initial informal insights into interactions between estrogen and food restriction effects were facilitated by constructing spreadsheets that analyze ordinal data, based on non-parametric multivariate u-statistics {Wittkowski, 2004}. For Figures 1 and 2, individual treatment scores, following estrogen (n=18), food restriction (n=18) or both (n=10), were compared to their baseline (no treatment) scores, then analyzed by group using the distribution-free Wilcoxon Matched-Pair Signed-Rank test with the theoretical median set at zero. Using this statistical method, no change in behavior between the baseline and treatment phases would result in group scores not significant from zero, while scores significantly different from zero represent both the magnitude and direction of the treatment-related change in behavior. Significance levels were: * P < 0.05, ** P < 0.01, and *** P < 0.001.
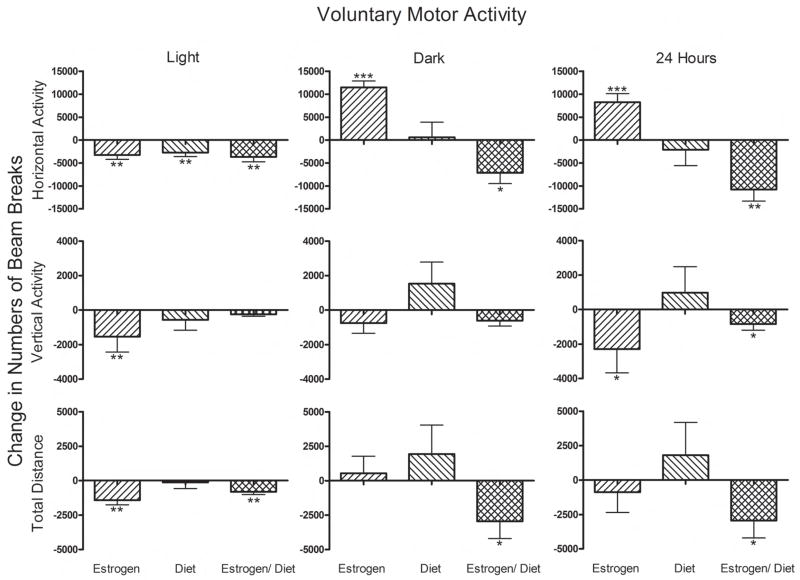
Figure 1.
Effects of Estrogen, Restricted Diet and the combination of those two treatments on voluntary motor activity in the custom-designed home cages set up for this arousal assay. The Changes in Numbers of Beam Breaks were calculated by subtracting each mouse’s activity in the absence of any treatment from that mouse’s activity under the influence of the experimental treatment. * p<0.05; ** p<0.01.
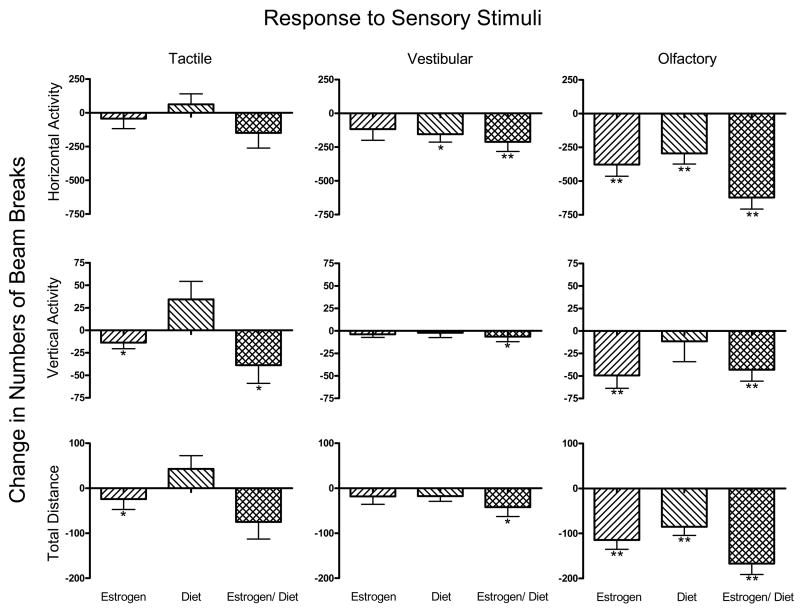
Figure 2.
Effects of Estrogen, Restricted Diet and the combination of those two treatments on the mouse’s responses to Tactile, Vestibular and Olfactory stimuli. Methods of calculation and statistics as in Figure 1.
Results
Voluntary motor activity
For voluntary motor activity the effects of estrogen, restricted diet, and combination treatments depended on the light/dark cycle. These are reported in Figure 1. In the light, Horizontal Activity was reduced by all three treatments (P < 0.01 each). Estrogen reduced motor activity for all three measures (P < 0.01 each). In fact, Total Distance was reduced by both estrogen and the estrogen/restricted diet treatments (P < 0.01 each).
However, in the dark, estrogen treatment alone increased Horizontal Activity (P < 0.001) and this increase overshadowed the light-associated decrease, resulting in an overall increase in activity across 24 hours (P < 0.001).
Irrespective of the light condition, the estrogen plus restricted diet treatment reduced all forms of motor activity (p<0.01), such that Horizontal Activity, Vertical Activity and Total Distance all were reduced when measured across the 24 hour period (P < 0.05).
Sensory responsiveness
The olfactory stimulus elicited significant changes in activity responses most consistently. In response to olfactory stimulation, estrogen reduced all three behavioral measures, (each P< 0.01). Restricted diet, likewise, reduced responses to the olfactory stimulus (Horizontal Activity and Total Distance, P < 0.01), but had no effect on olfactory-stimulated Vertical Activity. Estrogen treatment also reduced the response to tactile stimulation (Vertical Activity and Total Distance, P < 0.05) and restricted diet alone reduced the vestibular stimulus (Horizontal Activity response, P < 0.05).
Significant reductions were seen due to the combined treatments in 7 of the 9 measures, including all three stimulus modalities and all three types of response measures. The combined treatment decreased all three behavioral measures in response to the vestibular (P < 0.05) and olfactory stimuli (P < 0.01), but only Vertical Activity responses to the cutaneous stimulus (P < 0.05).
Interactions between estrogen and hunger effects
Interactions were not necessarily simple or additive. In the dark, estrogens increased, while estrogens in combination with restricted diet decreased Horizontal Activity (Figure 1). With respect to the effect of estrogens on Vertical Activity in the light, the addition of a restricted diet abolished the estrogen effect (Figure 1). On the other hand, with respect to Total Distance in the dark, the combination of the two treatments brought out an effect that was not apparent with either treatment alone (Figure 1); and in Figure 2 the numerical values of changes in response magnitude tended to be larger with the combined treatment than with either treatment alone.
Discussion
The primary finding of this study was that the original prediction of simple additivity was not met; estradiol and food restriction did not have the expected additive effects on arousal measures. Instead, we must offer an alternative hypothesis that relates hunger to the nutritional requirements for reproduction. This alternative hypothesis will be presented in the next section.
Because of previous research on the behavioral effects of estrogens and of restricted diet quoted in the Introduction and because of the theoretical equation (Garey et al., 2003; Pfaff, 2006b) derived from a principal components analysis of five experiments using arousal-related tests in female mice, it was hypothesized that arousal measures would be increased by estrogen treatment and restricted diet. This hypothesis was denied by the Results, which showed a preponderance of decreased arousal-related behaviors. Instead, we are led to an alternative hypothesis that the relations between estrogen-sensitive and hunger-sensitive arousal measures are dictated by the physiological needs for proper nutrition in preparation for the energetic expenditures required by reproduction.
Interpretative issues
Interpretative issues include questions about sex hormone effects on arousal states, and the relation of nutrition to reproduction.
Nutrition and reproduction
Regarding restricted diet and estrogenic interactions with each other, the initial hypothesis treated them as co-equal influences on arousal state regardless of their particular biological roles. But we now favor an alternative hypothesis that the relations between estrogen-sensitive and hunger-sensitive arousal measures are dictated by the physiological needs for proper nutrition in preparation for the energetic expenditures required by reproduction. That is, these data fit the notion that those mechanisms that inhibit food intake evolved to make animals more interested in reproduction, whereas those mechanisms that increase hunger and food intake tend to delay reproductive interests until such time food is procured and stored in the home or as body fat (Schneider, 2006). This is an issue of motivational priorities that have evolved in a manner that is biologically adaptive.
A large literature shows that nutrition and reproduction in the female have important physiological relations to each other (reviewed in Schneider and Watts, 2002). For example, estradiol treatment in OVX rats increases neuronal activation in response to feeding and cyclic estradiol treatment restores patterns of spontaneous feeding and sexual receptivity (Asarian and Geary, 2002). The present study demonstrated that the relative influences of estrogen, restricted diet, and their interaction, on locomotor activity and sensory responsiveness depended on the activity measure, the behavioral measurement, and the stimulus presented. Thus, it may be that the present results, rather than being interpreted in light of the theory of CNS arousal, should instead be thought of in terms of how mechanisms that respond to hunger are integrated with those that control reproduction (Schneider and Watts, 2002). The present results included examples in which restricted diet could abolish or even reverse an estrogen effect. These results are more consistent with the Schneider and Wade papers indicating an antagonistic relation between hunger and reproduction than with an equation in which all possible sources of arousal add to each other.
Estrogens and arousal
Estrogens modulate arousal, as demonstrated by the Garey et al (2003) study which gave impetus to this investigation. In those experiments, effects on sensory responsiveness and motor activity depended on the ER-α gene, but not the ER-β gene. Estrogen facilitation of running wheel activity (Gentry and Wade, 1975) also depends on ER-α but not ER-β (Ogawa et al. 2003). More generally, the array of arousal-related phenomena that is modulated by estrogens provides indirect evidence of the importance of estrogens to elementary arousal. In women, fMRI analysis has shown that the menstrual cycle modulates arousal circuitry (Goldstein, et al., 2005), and affects cognition (Resnick, et al., 1998; Swerdlow, et al., 1997), ingestive behavior (Fricke, et al., 2006), and sexual arousal (Diamond, et al., 1972; Stanislaw and Rice, 1988). These are supported by a growing body of studies in research animals regarding mood (i.e. anxiety/fear) (Mora, et al., 1996), cognition (Fugger, et al., 2000; Koch, 1998), feeding (Gale and Sclafani, 1977; Geary, et al., 2001; Li, et al., 1994; Petersen, 1976) and sexual motivation (Edwards and Pfeifle, 1983; Pfaus, et al., 2004). It must be admitted that for purposes of experimental convenience we chose a steady state estrogen treatment in this study, which does not mimic the fluctuations of a normal estrous cycle.
Responses to olfactory stimulation are highly relevant to the motivated behaviors associated with ingestion and reproduction, such that some of the same metabolic sensory signals and hormonal modulators affect both systems (McClintock, 1971; Schneider and Watts, 2002). An important arousal-related neuropeptide, Orexin-A, influences arousal in general (Mochizuki, et al., 2004), and modulates excitability in olfactory bulb mitral cells (Hardy et al., 2005). Not all of the modulatory influences of orexin were excitatory, thus supporting the possibility that under some conditions increased arousal may lead to a smaller response (Easton et al., 2006).
Technical considerations
Technical considerations include questions about the animals’ health, circadian issues, time since ovariectomy, estradiol dose, choice of motor activity measures, and order effects.
Animal health
The experimental treatments generally reduced activity. However, it is unlikely that our treatments rendered the mice motorically impaired. Mice were observed and weighed daily during the two week period of treatment acclimation each phase. During this pre-test period, body weight and general appearance and movement were normal. Additionally, after locomotor activity and sensory responsiveness measures, mice were given additional tests including a feeding challenge with quinine adulterated food and a sexual behavior test (reported separately). During those tests, again, there was no evidence of motor problems. This was particularly evident during the scoring of the sexual testing videotapes. Thus, we believe that the mice were healthy in all respects.
One might argue that the restricted diet resulted in too little metabolic fuel to support normal activity, and thus, might have affected the responsiveness of the animals during testing. We strove to avoid this problem with the use of the specially formulated diet which contained additional nutrients without additional calories, so that when fed at 60% of the control diet, mice were underfed but not undernourished. Secondly, the restricted diet was instigated gradually to avoid binge/fast behavior, a beneficial strategy as shown by longevity studies based on the use of restricted diets (Pugh et al., 1999). The approach to diet restriction was successful, because there was no difference in the home cage behavior between the estrogen, restricted diet and no treatment groups. Again, the normal sexual behaviors referred to above in diet-restricted mice suggest that they were not weakened by a lack of metabolic fuel.
Circadian issues
Measurements of motor activity during the dark brought out phenomena which were reduced or absent during the light part of the daily cycle. Sensory responses were measured during the light in order to achieve a very stable baseline of ‘zero movement’ for precision. That is, during the light part of the cycle one could usefully employ the requirement of 5 minutes without movement. A useful extension of this type of work would be to do these experiments in exactly the same way, but during the lights-off period when the animals are more active (Albers, et al., 1981; Caldwell and Albers, 2004).
Estrogen deprivation
In this protocol, estrogen insensitivity may have been a concern (Beach and Orndoff, 1974). The experimental design focused on the question of whether individual mice responded with the same level of activity across all measures and treatments with the goal of revealing the underlying arousal state. Hence, the protocol required that we first test mice without treatment of estradiol and restricted diet before instigating the treatment regime in Phases 2 and 3. This being the case, initially mice were without estrogen replacement from the time of ovariectomy until the second or third round of treatments and testing—a range of 55–89 days for the three groups. Although previous research (Parsons et al., 1979) has shown that estrogen replacement is not as effective behaviorally if the animal has been estrogen-deprived for an extended period, perhaps because of declining co-activator concentrations, the animals were given at least 2 weeks of estradiol before home cage activity measures and sensory responsiveness testing in order to counter this possible effect. Furthermore, there were not significant differences in the estradiol effect between Phase 2 and 3 of testing.
Estradiol dose
Was the correct estrogen dose used? Regarding estradiol replacement dose, studies of lordosis have tended to show a monotonically increasing dose-response curve all the way from zero to supra-physiological levels. However, with running wheel activity, there is evidence of an inverted U shaped dose/response curve; (Ribeiro et al., unpublished). In the current work, the dose was not too low to have a behavioral effect, because during additional sexual behavior tests to be reported in a later paper (in preparation) it was effective for facilitating mating behavior. However, based on running wheel studies with mice, it may have been too high, enough to reduce locomotor responses. Therefore, studies such as reported here should be explored with a wider range of estrogen doses.
Motor activity measures
In this experiment, estrogen treatment increased Horizontal Activity, but had no effect on Total Distance moved, except a reduction during the light period. Most of the striking effects of estradiol on locomotion have used running wheels (Gentry and Wade, 1975). Estrogens have been shown to increase running wheel activity in rats (Fahrbach et al., 1985; Gerall, et al., 1973) and, in mice, the estrogenic effect depends on estrogen binding to the gene product from the ERα gene, but not the ERβ gene (Ogawa et al., 2003). Rapid forward movement as in the running wheels may resemble the rapid forward movement observed during female mouse courtship behavior (Garey et al., 2002), but also cause proprioceptive signals that are totally different than ordinary home cage locomotion. Whether that difference is important for interpretation of the results reported here is not yet clear. As well, we must consider the alternative hypothesis that the movements we measure have not been acted upon in the normal way by natural selection and therefore do not apply easily to the ‘real lives’ of animals in the wild.
Order effects
It is unlikely that there were significant order effects in these experiments, because estrogen and restricted diet treatments were counterbalanced for order, and because of the negative results in internal post-hoc statistical analyses. Within the sensory tests, stimulus presentation was not an issue, as the largest sensory responses were to olfactory stimulation, despite the fact that the order of sensory modality presentation was biased against olfaction, which came last. Finally, preliminary data (unpublished) regarding retesting three times in the arousal assay showed very few significant differences between the first and third round scores for either home cage activity or sensory responsiveness, and no obvious patterns of changes with retesting.
With this paper (I) having concentrated on measures of arousal, the next paper in preparation (II) will center on mechanisms of food intake, sex behavior and fear.
Acknowledgments
We acknowledge Elliot Albers (Georgia State University) for the useful suggestions on the circadian aspects of these experiments, Amy Easton for her work developing the arousal assay apparatuses, and Sherlyne Gilles and Allison Koslow for their work on the preliminary studies of the effects of restricted feeding and repeat testing. We also are grateful to three anonymous reviewers for their thoughtful points. This research was supported by NIH grants HD-05751 (DWP) and MH-015125 (DNS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
List of References
- Albers HE, Gerall AA, Axelson JF. Effect of reproductive state on circadian periodicity in the rat. Physiol Behav. 1981;26(1):21–5. doi: 10.1016/0031-9384(81)90073-1. [DOI] [PubMed] [Google Scholar]
- Ammassari-Teule M, Restivo L, Pietteur V, Passino E. Learning about the context in genetically-defined mice. Behav Brain Res. 2001;125:195–204. doi: 10.1016/s0166-4328(01)00301-1. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- Beach FA, Orndoff RK. Variation in the responsiveness of female rats to ovarian hormones as a function of preceding hormonal deprivation. Horm Behav. 1974;5:201–205. doi: 10.1016/0018-506x(74)90028-2. [DOI] [PubMed] [Google Scholar]
- Beach FA. Sexual attractivitiy, proceptivity, and receptivity in female mammals. Horm Behav. 1976;7:105–138. doi: 10.1016/0018-506x(76)90008-8. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Albers HE. Effect of photoperiod on vasopressin-induced aggression in Syrian hamsters. Horm Behav. 2004;46(4):444–449. doi: 10.1016/j.yhbeh.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Coria-Avila GA, Quimet AJ, Pacheco P, Manzo J, Pfaus JG. Olfactory conditioned partner preference in the female rat. Behav Neurosci. 2005;119(3):716–725. doi: 10.1037/0735-7044.119.3.716. [DOI] [PubMed] [Google Scholar]
- Diamond M, Diamond AL, Mast M. Visual sensitivity and sexual arousal levels during the menstrual cycle. J Nerv Ment Dis. 1972;155(3):170–176. doi: 10.1097/00005053-197209000-00003. [DOI] [PubMed] [Google Scholar]
- Easton A, Dwyer E, Pfaff DW. Estradiol and orexin-2 saporin action on multiple forms of behavioral arousal in female mice. Behav Neurosci. 2006;120(1):1–9. doi: 10.1037/0735-7044.120.1.1. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Pfeifle JK. Hormonal control of receptivity, proceptivity and sexual motivation. Physiol Behav. 1983;30(3):437–443. doi: 10.1016/0031-9384(83)90150-6. [DOI] [PubMed] [Google Scholar]
- Erskine MS. Solicitation behavior in the estrous female rat: A review. Horm Behav. 1989;23:473–502. doi: 10.1016/0018-506x(89)90037-8. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Meisel RL, Pfaff DW. Preoptic implants of estradiol increase wheel running but not the open field activity of female rats. Physiol Behav. 1985;35(6):985–992. doi: 10.1016/0031-9384(85)90270-7. [DOI] [PubMed] [Google Scholar]
- Fricke O, Lehmkuhl G, Pfaff DW. Cybernetic principles in the systematic concept of hypothalamic feeding control. Eur J Endocrinol. 2006;154(2):167–173. doi: 10.1530/eje.1.02081. [DOI] [PubMed] [Google Scholar]
- Fugger HN, Foster TC, Gustafsson JA, Rissman EF. Novel effects of estadiol and estrogen α and β on cognitive function. Brain Research Interactive. 2000;883:258–264. doi: 10.1016/s0006-8993(00)02993-0. [DOI] [PubMed] [Google Scholar]
- Gale SK, Sclafani A. Ovariectomy-induced changes in food motivation in the rat. Horm Behav. 1977;9:120–129. doi: 10.1016/0018-506x(77)90079-4. [DOI] [PubMed] [Google Scholar]
- Garey J, Kow L-M, Huynh W, Ogawa S, Pfaff DW. Temporal and spatial quantitation of nesting and mating behaviors among mice housed in a semi-natural environment. Horm Behav. 2002;42:294–306. doi: 10.1006/hbeh.2002.1823. [DOI] [PubMed] [Google Scholar]
- Garey J, Goodwillie A, Frohlich J, Morgan M, Gustafsson J-A, Smithies O, Korach K, Ogawa S, Pfaff D. Genetic contributions to generalized arousal of brain and behavior. Proceedings of the National Academy of Sciences, U SA. 2003;100(19):11019–22. doi: 10.1073/pnas.1633773100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation if ERα null mice. Endocrinology. 2001;142(11):4751–4757. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- Gentry RT, Wade GN. Sex differences in sensitivity of food intake, body weight, and running wheel activity to ovarian hormones. J Comp Physiol Psychol. 1975;90(8):747–754. doi: 10.1037/h0077246. [DOI] [PubMed] [Google Scholar]
- Gerall AA, Napoli AM, Cooper UC. Daily and hourly estrous running in intact, spayed, and estrone implanted rats. Physiol Behav. 1973;10:225–229. doi: 10.1016/0031-9384(73)90302-8. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, Makris N. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci. 2005;25(40):9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy AB, Aioun J, Baly C, Julliard KA, Caillol M, Salesse R, Duchamp-Viret P. Orexin A modulates mitral cell activity in the rat olfactory bulb: Patch-clamp study on slices and immunocytochemical localization of orexin receptors. Endocrinology. 2005;146(9):4042–4053. doi: 10.1210/en.2005-0020. [DOI] [PubMed] [Google Scholar]
- Kato A, Sakuma Y. Neuronal activity in female rat preoptic area associated with sexually motivated behavior. Brain Res. 2000;862:90–102. doi: 10.1016/s0006-8993(00)02076-x. [DOI] [PubMed] [Google Scholar]
- Koch M. Sensorimotor gating changes across the estrous cycle in female rats. Physiol Behav. 1998;64(5):625–628. doi: 10.1016/s0031-9384(98)00098-5. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Sachs BD, Sakuma Y. Importance of the medial amygdala in rat penile erection evoked by remote stimuli from estrous females. Behav Brain Res. 1997;88:153–160. doi: 10.1016/s0166-4328(97)02287-0. [DOI] [PubMed] [Google Scholar]
- Lecea Ld, Sutcliffe GJ, Fabre V. Hypocretins/orexins as integrators of physiological information: lessons from mutant animals. Neuropeptides. 2002;36(2–3):85–95. doi: 10.1054/npep.2002.0892. [DOI] [PubMed] [Google Scholar]
- Li H, Wade G, Blaustein J. Manipulations of metabolic fuel availability alter estrous behavior and neural estrogen receptor immunoreactivity in Syrian hamsters. Endocrinology. 1994;135(1):240–247. doi: 10.1210/endo.135.1.8013358. [DOI] [PubMed] [Google Scholar]
- McClintock MK. Menstrual synchrony and suppression. Nature. 1971;229:244–245. doi: 10.1038/229244a0. [DOI] [PubMed] [Google Scholar]
- Mieda M, Williams SC, Sinton CM, Richardson JA, Sakurai T, Yanagisawa M. Orexin Neurons Function in an Efferent Pathway of a Food-Entrainable Circadian Oscillator in Eliciting Food-Anticipatory Activity and Wakefulness. J Neurosci. 2004;24(46):10493–10501. doi: 10.1523/JNEUROSCI.3171-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T, Crocker A, McCormack S, Yanagisawa M, Sakurai T, Scammell TE. Behavioral state instability in orexin knock-out mice. J Neurosci. 2004;24(28):6291–6300. doi: 10.1523/JNEUROSCI.0586-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora S, Dussaubat N, Diaz-Veliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996;21(7):609–620. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Gustafsson JA, Korach KS, Pfaff DW. Estrogen increases locomotor activity in mice through estrogen receptor α: Specificity for the type of activity. Endocrinology. 2003;144(1):230–239. doi: 10.1210/en.2002-220519. [DOI] [PubMed] [Google Scholar]
- Paredes PG, Vazquez B. What do female rats like about sex? Paced mating. Behav Brain Res. 1999;105:117–127. doi: 10.1016/s0166-4328(99)00087-x. [DOI] [PubMed] [Google Scholar]
- Parsons B, MacLusky NJ, Krieger MS, McEwen BS, Pfaff DW. The effects of long-term estrogen exposure on the induction of sexual behavior and measurements of brain estrogen and progestin receptors in the female rat. Horm Behav. 1979;13(3):301–13. doi: 10.1016/0018-506x(79)90047-3. [DOI] [PubMed] [Google Scholar]
- Petersen S. The temporal pattern of feeding over the oestrous cycle of the mouse. Animal Behavior. 1976;24:939–955. doi: 10.1016/s0003-3472(76)80023-1. [DOI] [PubMed] [Google Scholar]
- Pfaff DW. Brain Arousal and Information Theory. Harvard University Press; Cambridge: 2006a. [Google Scholar]
- Pfaff DW, editor. Knobil and Neill’s The Physiology of Reproduction. 3 Elsevier/Academic Press; San Diego: 2006b. [Google Scholar]
- Pfaus JG, Shadiack A, Van Soest T, Tse M, Molinoff P. Selective facilitation of sexual solicitation in the female rat by a melanocortin receptor agonist. PNAS. 2004;101(27):10201–10204. doi: 10.1073/pnas.0400491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaus JG, Smith WJ, Coopersmith CB. Appetitive and consummatory sexual behaviors of female rats in bilevel chambers: I. A correlational and factor analysis and the effects of ovarian hormones. Horm Behav. 1999;35(3):224–240. doi: 10.1006/hbeh.1999.1516. [DOI] [PubMed] [Google Scholar]
- Pugh TD, Klopp RG, Weindruch R. Controlling caloric consumption: Protocols for rodents and rhesus monkeys. Neurobiol Aging. 1999;20:157–165. doi: 10.1016/s0197-4580(99)00043-3. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Maki PM, Golski S, Kraut MA, Zoderman AB. Effects of estrogen replacement therapy on PET cerebral blood flow and neuropsychological performance. Horm Behav. 1998;34:171–182. doi: 10.1006/hbeh.1998.1476. [DOI] [PubMed] [Google Scholar]
- Schneider JE. Metabolic and hormonal control of the desire for food and sex: implications for obesity and eating disorders. Hormones and Behavior. 2006;50:562–571. doi: 10.1016/j.yhbeh.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Schneider JE, Watts AG. Energy balance, ingestive behavior, and reproductive success. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 1. Academic Press; San Diego: 2002. pp. 435–524. [Google Scholar]
- Segal JP, Stallings NR, Lee CE, Zhao L, Socci N, Viale A, Harris TM, Soares MB, Childs G, Elmquist JK, Parker KL, Friedman JM. Use of laser-capture microdissection for the identification of marker genes for the ventromedial hypothalamicnucleus. J Neurosci. 2005;25(16):4181–4188. doi: 10.1523/JNEUROSCI.0158-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley DN, Meisel RL. The effects of mating stimulation on c-Fos immunoreactivity in the female hamster medial amydgala are region and context specific. Horm Behav. 2005;47(2):212–222. doi: 10.1016/j.yhbeh.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Sinnamon HM. Preoptic and hypothalamic neurons and the initiation of locomotion in the anesthetized rat. Prog Neurobiol. 1993;41(3):323–344. doi: 10.1016/0301-0082(93)90003-b. [DOI] [PubMed] [Google Scholar]
- Spearow JL, Doemeny P, Sera R, Leffler R, Barkley M. Genetic Variation in Susceptibility to Endocrine Disruption by Estrogen in Mice. Science. 1999;285(5431):1259–1261. doi: 10.1126/science.285.5431.1259. [DOI] [PubMed] [Google Scholar]
- Stanislaw H, Rice FJ. Correlation between sexual desire and menstrual cycle characteristics. Archives of Sexual Behavior. 1988;17(6):499–508. doi: 10.1007/BF01542338. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Hartman PL, Auerbach PP. Changes in sensorimotor inhibition across the menstrual cycle: Implications for neuropsychiatric disorders. Biological Psychiatry. 1997;41(4):452–460. doi: 10.1016/S0006-3223(96)00065-0. [DOI] [PubMed] [Google Scholar]
- Takeo T, Sakuma Y. Diametrically opposite effects of estrogen on the excitability of female rat medial and lateral preoptic neurons with axons to the midbrain locomotor region. Neurosci Res. 1995;22(1):73–80. doi: 10.1016/0168-0102(95)00885-w. [DOI] [PubMed] [Google Scholar]
- Wade GN, Jones JE. Neuroendocrinology of nutritional infertility. Am J Physiol Regul Integr Comp Physiol. 2004;287(6):R1277–1296. doi: 10.1152/ajpregu.00475.2004. [DOI] [PubMed] [Google Scholar]
- Wade GN, Schneider JE. Metabolic fuels and reproduction in female mammals. Neurosci Biobehav Rev. 1992;16(2):235–72. doi: 10.1016/s0149-7634(05)80183-6. [DOI] [PubMed] [Google Scholar]
- Wade GN, Schneider JE, Li HY. Control of fertility by metabolic cues. American Journal of Physiology. 1996;270:E1–E19. doi: 10.1152/ajpendo.1996.270.1.E1. [DOI] [PubMed] [Google Scholar]
- Wittkowski KM, Lee E, Nussbaum R, Chamian FN, Krueger JG. Combining several ordinal measures in clinical trials. Stat Med. 2004;23:1579–1592. doi: 10.1002/sim.1778. [DOI] [PubMed] [Google Scholar]
- Zigman JM, Elmquist JK. Minireview: From anorexia to obesity--The yin and yang of body weight control. Endocrinology. 2003;144(9):3749–3756. doi: 10.1210/en.2003-0241. [DOI] [PubMed] [Google Scholar]