Abstract
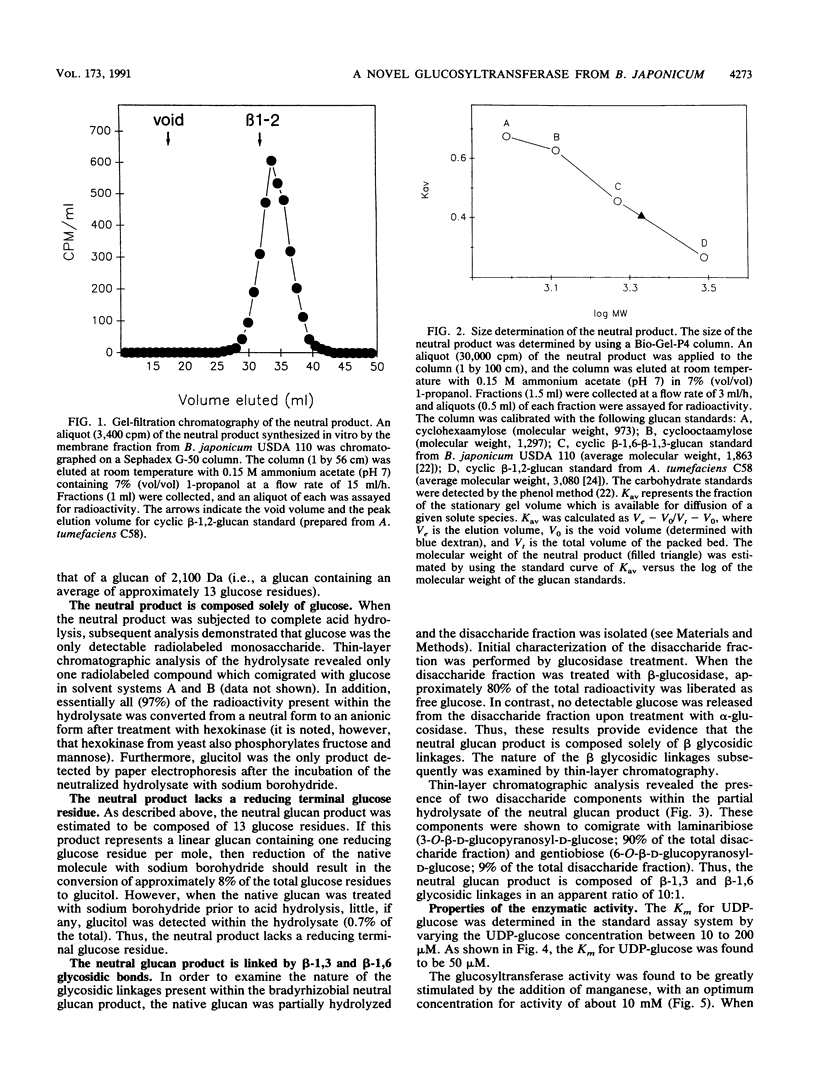
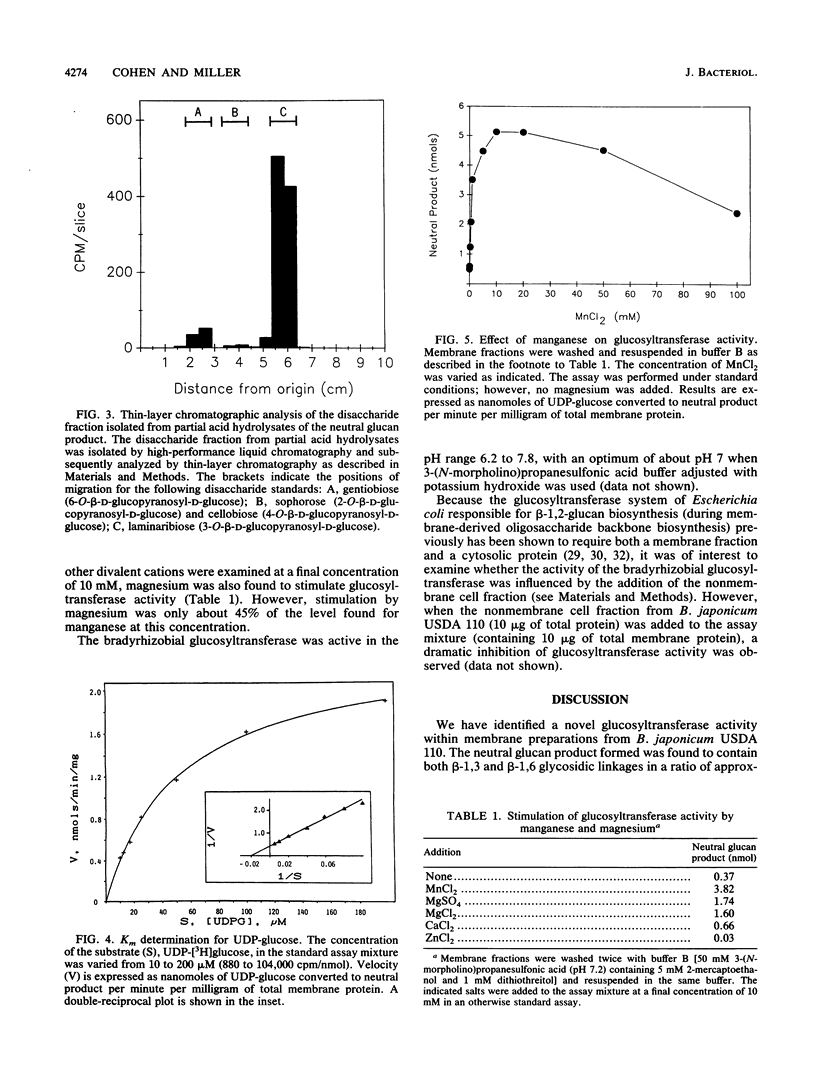
Bacteria within the family Rhizobiaceae are distinguished by their ability to infect higher plants. The cell envelope carbohydrates of these bacteria are believed to be involved in the plant infection process. One class of cell envelope carbohydrate, the cyclic beta-1,2-glucans, is synthesized by species within two genera of this family, Agrobacterium and Rhizobium. In contrast, species of the genus Bradyrhizobium, a third genus within this family, appear to lack the capacity for cyclic beta-1,2-glucan biosynthesis. Instead, these bacteria synthesize cyclic glucans containing beta-1,6 and beta-1,3 glycosidic linkages (K.J. Miller, R.S. Gore, R. Johnson, A.J. Benesi, and V.N. Reinhold, J. Bacteriol. 172:136-142, 1990). We now report the initial characterization of a novel membrane-bound glucosyltransferase activity from Bradyrhizobium japonicum USDA 110. Analysis of the product of this glucosyltransferase activity revealed the following: the presence of beta-1,3 and beta-1,6 glycosidic linkages, an average molecular weight of 2,100, and no detectable reducing terminal residues. The glucosyltransferase activity was found to have an apparent Km of 50 microM for for UDP-glucose, and activity was stimulated optimally by Mn2+ ions. On the basis of the structural properties of the in vitro glucan product, it is possible that this membrane-bound glucosyltransferase activity may be responsible for the biosynthesis of cyclic beta-1,6-beta-1,3-glucans by this organism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cangelosi G. A., Martinetti G., Leigh J. A., Lee C. C., Thienes C., Theines C., Nester E. W. Role for [corrected] Agrobacterium tumefaciens ChvA protein in export of beta-1,2-glucan. J Bacteriol. 1989 Mar;171(3):1609–1615. doi: 10.1128/jb.171.3.1609-1615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangelosi G. A., Martinetti G., Nester E. W. Osmosensitivity phenotypes of Agrobacterium tumefaciens mutants that lack periplasmic beta-1,2-glucan. J Bacteriol. 1990 Apr;172(4):2172–2174. doi: 10.1128/jb.172.4.2172-2174.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEDONDER R. A., HASSID W. Z. THE ENZYMATIC SYNTHESIS OF A (BETA-I,2-)-LINKED GLUCAN BY AN EXTRACT OF RHIZOBIUM JAPONICUM. Biochim Biophys Acta. 1964 Aug 19;90:239–248. doi: 10.1016/0304-4165(64)90187-4. [DOI] [PubMed] [Google Scholar]
- Douglas C. J., Staneloni R. J., Rubin R. A., Nester E. W. Identification and genetic analysis of an Agrobacterium tumefaciens chromosomal virulence region. J Bacteriol. 1985 Mar;161(3):850–860. doi: 10.1128/jb.161.3.850-860.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dylan T., Helinski D. R., Ditta G. S. Hypoosmotic adaptation in Rhizobium meliloti requires beta-(1----2)-glucan. J Bacteriol. 1990 Mar;172(3):1400–1408. doi: 10.1128/jb.172.3.1400-1408.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dylan T., Ielpi L., Stanfield S., Kashyap L., Douglas C., Yanofsky M., Nester E., Helinski D. R., Ditta G. Rhizobium meliloti genes required for nodule development are related to chromosomal virulence genes in Agrobacterium tumefaciens. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4403–4407. doi: 10.1073/pnas.83.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dylan T., Nagpal P., Helinski D. R., Ditta G. S. Symbiotic pseudorevertants of Rhizobium meliloti ndv mutants. J Bacteriol. 1990 Mar;172(3):1409–1417. doi: 10.1128/jb.172.3.1409-1417.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson L. J., Stacey G. Signal exchange in plant-microbe interactions. Microbiol Rev. 1986 Jun;50(2):193–225. doi: 10.1128/mr.50.2.193-225.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ielpi L., Dylan T., Ditta G. S., Helinski D. R., Stanfield S. W. The ndvB locus of Rhizobium meliloti encodes a 319-kDa protein involved in the production of beta-(1----2)-glucan. J Biol Chem. 1990 Feb 15;265(5):2843–2851. [PubMed] [Google Scholar]
- Lerouge P., Roche P., Faucher C., Maillet F., Truchet G., Promé J. C., Dénarié J. Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature. 1990 Apr 19;344(6268):781–784. doi: 10.1038/344781a0. [DOI] [PubMed] [Google Scholar]
- Long S. R. Rhizobium-legume nodulation: life together in the underground. Cell. 1989 Jan 27;56(2):203–214. doi: 10.1016/0092-8674(89)90893-3. [DOI] [PubMed] [Google Scholar]
- Miller K. J., Gore R. S., Johnson R., Benesi A. J., Reinhold V. N. Cell-associated oligosaccharides of Bradyrhizobium spp. J Bacteriol. 1990 Jan;172(1):136–142. doi: 10.1128/jb.172.1.136-142.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. J., Kennedy E. P., Reinhold V. N. Osmotic adaptation by gram-negative bacteria: possible role for periplasmic oligosaccharides. Science. 1986 Jan 3;231(4733):48–51. doi: 10.1126/science.3941890. [DOI] [PubMed] [Google Scholar]
- Miller K. J., Reinhold V. N., Weissborn A. C., Kennedy E. P. Cyclic glucans produced by Agrobacterium tumefaciens are substituted with sn-1-phosphoglycerol residues. Biochim Biophys Acta. 1987 Jul 10;901(1):112–118. doi: 10.1016/0005-2736(87)90262-8. [DOI] [PubMed] [Google Scholar]
- Puvanesarajah V., Schell F. M., Stacey G., Douglas C. J., Nester E. W. Role for 2-linked-beta-D-glucan in the virulence of Agrobacterium tumefaciens. J Bacteriol. 1985 Oct;164(1):102–106. doi: 10.1128/jb.164.1.102-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandermann H., Jr, Dekker R. F. Beta-1,2-glucosyl transfer by membrane preparations from Acetobacter xylinum. FEBS Lett. 1979 Nov 1;107(1):237–240. doi: 10.1016/0014-5793(79)80504-9. [DOI] [PubMed] [Google Scholar]
- Stanfield S. W., Ielpi L., O'Brochta D., Helinski D. R., Ditta G. S. The ndvA gene product of Rhizobium meliloti is required for beta-(1----2)glucan production and has homology to the ATP-binding export protein HlyB. J Bacteriol. 1988 Aug;170(8):3523–3530. doi: 10.1128/jb.170.8.3523-3530.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therisod H., Kennedy E. P. The function of acyl carrier protein in the synthesis of membrane-derived oligosaccharides does not require its phosphopantetheine prosthetic group. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8235–8238. doi: 10.1073/pnas.84.23.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therisod H., Weissborn A. C., Kennedy E. P. An essential function for acyl carrier protein in the biosynthesis of membrane-derived oligosaccharides of Escherichia coli. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7236–7240. doi: 10.1073/pnas.83.19.7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully R. E., Keister D. L., Gross K. C. Fractionation of the beta-Linked Glucans of Bradyrhizobium japonicum and Their Response to Osmotic Potential. Appl Environ Microbiol. 1990 Jun;56(6):1518–1522. doi: 10.1128/aem.56.6.1518-1522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissborn A. C., Kennedy E. P. Biosynthesis of membrane-derived oligosaccharides. Novel glucosyltransferase system from Escherichia coli for the elongation of beta 1----2-linked polyglucose chains. J Biol Chem. 1984 Oct 25;259(20):12644–12651. [PubMed] [Google Scholar]
- York W. S., McNeil M., Darvill A. G., Albersheim P. Beta-2-linked glucans secreted by fast-growing species of Rhizobium. J Bacteriol. 1980 Apr;142(1):243–248. doi: 10.1128/jb.142.1.243-248.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zevenhuizen L. P. Cellular glycogen, beta-1,2,-glucan, poly beta-hydroxybutyric acid and extracellular polysaccharides in fast-growing species of Rhizobium. Antonie Van Leeuwenhoek. 1981;47(6):481–497. doi: 10.1007/BF00443236. [DOI] [PubMed] [Google Scholar]
- Zevenhuizen L. P., Scholten-Koerselman H. J. Surface carbohydrates of Rhizobium. I. Beta-1, 2-glucans. Antonie Van Leeuwenhoek. 1979;45(2):165–175. doi: 10.1007/BF00418581. [DOI] [PubMed] [Google Scholar]
- Zorreguieta A., Cavaignac S., Geremia R. A., Ugalde R. A. Osmotic regulation of beta(1-2) glucan synthesis in members of the family Rhizobiaceae. J Bacteriol. 1990 Aug;172(8):4701–4704. doi: 10.1128/jb.172.8.4701-4704.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorreguieta A., Geremia R. A., Cavaignac S., Cangelosi G. A., Nester E. W., Ugalde R. A. Identification of the product of an Agrobacterium tumefaciens chromosomal virulence gene. Mol Plant Microbe Interact. 1988 Mar;1(3):121–127. doi: 10.1094/mpmi-1-121. [DOI] [PubMed] [Google Scholar]
- Zorreguieta A., Tolmasky M. E., Staneloni R. J. The enzymatic synthesis of beta 1-2 glucans. Arch Biochem Biophys. 1985 May 1;238(2):368–372. doi: 10.1016/0003-9861(85)90176-6. [DOI] [PubMed] [Google Scholar]
- Zorreguieta A., Ugalde R. A. Formation in Rhizobium and Agrobacterium spp. of a 235-kilodalton protein intermediate in beta-D(1-2) glucan synthesis. J Bacteriol. 1986 Sep;167(3):947–951. doi: 10.1128/jb.167.3.947-951.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Iannino N. I., Ugalde R. A. Biochemical characterization of avirulent Agrobacterium tumefaciens chvA mutants: synthesis and excretion of beta-(1-2)glucan. J Bacteriol. 1989 May;171(5):2842–2849. doi: 10.1128/jb.171.5.2842-2849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]



