Abstract
High titer antibodies to type 1 interferons have been recently reported as being highly specific for patients with autoimmune polyglandular syndrome type 1 (APS1) in Finnish and Norwegian patients with mutations in the AIRE gene. Those studies employed a complex neutralization assay to define the type 1 interferon autoantibodies. Here we have established a competitive europium time resolved fluorescence assay for IFN-a autoantibodies and measured sera from subjects with APS1, first degree relatives of APS1 patients, patients with Addison’s disease or Type 1 diabetes. The europium-based immunoassay utilizes plate bound human IFN-a incubated with sera with or without competition with fluid phase IFN-a, followed by anti-IgG biotinylated antibody and detection with streptavidin-europium. The index of IFN-a Ab was calculated as (CPS (Counts per second) without competition-CPS with competition)/(CPS positive standard sera without competition-CPS positive standard sera with competition). Results are reported for raw CPS and indices and are compared across the different subjects.
Results
For normal controls (n=100) CPS without competition were 31,237±17,328 CPS while after subtracting the competition value, the results were −6,563±10,303 CPS. The initial APS1 patient (used to create the index as 1.0) gave 394,063 CPS without competition and a delta of 363,662±31,587 CPS with competition. Scatchard plot analysis of this patient sample revealed a high avidity for IFN-a (Kd of 0.5 nM). The CPS, delta, and index for 6/7 APS1 patients was strongly positive and 3 standard deviations or more above that of the normal controls. Using a cut–off of 2 standard deviations above normal controls, relatives of APS1 patients were negative for type I interferon autoantibodies as were 71 patients with Addison’s disease (non-APS1) and 141 Type 1 diabetes patients. This simple high throughput competitive europium time resolved fluorescence assay had a sensitivity of =86% or greater and a specificity of > 99.5%.
Keywords: APS1, AIRE, IFN-α, Autoantibodies
Introduction
Autoimmune polyglandular syndrome type 1 (APS1) also known as APECED (Autoimmune polyendocrinopathy Mucocutaneous Candidiasis Ectodermal Dystrophy) is an autosomal recessive disorder characterized by chronic mucocutaneous candidiasis, autoimmune hypoparathyroidism (hypoparathyroidism), primary adrenal insufficiency (Addison’s Disease), and a variety of other organ-specific autoimmune features that are variable between patients [1]. The defective gene in this disorder was identified in a positional cloning effort and is termed AIRE (for AutoImmune REgulator) [2, 3] A major mechanism by which AIRE appears to prevent autoimmunity is through promoting thymic expression of self-antigens which helps promote the deletion of autoreactive T cells that develop in the thymus [4–6]. Besides helping with understanding the mechanism of the autoimmunity in APS1 patients, the identification of the AIRE gene has also afforded the opportunity to use genetic testing on these patients and their relatives. Because the APS1 syndrome arises from a loss of function in the AIRE gene, mutations can be scattered throughout the entire gene and over 40 mutations in the gene have been described in APS1 subjects [7–9]. Therefore, identification of the causative mutation in an individual patient may require the labor intensive process of sequencing the entire gene.
Recently, Meager et al. [10] found high titer neutralizing IgG autoantibodies reactive to most IFN-a subtypes in 76 Scandanavian APS1 patients with high specificity for those patients with known AIRE mutations. This raised the possibility that the assay could be used to screen for APS1, however, the sensitivity and specificity of the autoantibodies are unknown in APS1 patient collections outside of Scandanavia [10,11]. Therefore, we have established an independent and relatively simple assay for INF-a autoantibodies, using a time-resolved immunofluorometric assay (IFMA) approach. Here we report that our independent assay for INF-a autoantibodies is robust and shows high specificity and sensitivity for APS1 in a non-Scandanavian collection of APS1 patients, thus supporting the utility of the assay in this clinical syndrome.
Methods
Patients
We studied seven subjects who met clinical criteria for APS1 as defined by the presence of at least two of the clinical triad of chronic mucocutaneous candidiasis, Addison’s disease, and hypoparathyroidism and/or a defined mutation(s) in the AIRE gene. The patients had serum collected at ages 2 to 32 and had a mean age of 14.9 years. Three of them were from Italy and were homozygous for the R275X mutation. Two were homozygous for the 1094-1106del mutation in Exon 8 and were from the United States. One patient was from Iran and was homozygous for the Y85C (A374G) mutation. One APS1 patient, also from the United States, was presumed APS1 based on clinical history (candidiasis and suffering hypoparathyroidism from infancy); however, we have not identified a mutation in the AIRE gene in this patient (data not shown). Six non-APS1 patients including relatives of APS1 subjects and patients with immunodeficiency were also studied.
Seventy-one Addison’s disease patients with or without diabetes and 141 patients with Type 1 diabetes at diagnosis were also screened for IFNa Ab’s. The diagnosis of Addison’s disease was made on clinical grounds, by typical symptoms of adrenal insufficiency such as fatigue, weight loss and salt craving with laboratory confirmation of adrenal insufficiency. Addison’s disease patients were tested and were identified 21-hydroxylase and/or adrenal cortex autoantibodies (21OHAb and ACA, respectively) positive. Type 1 diabetes mellitus patients had symptoms of diabetes plus casual plasma glucose concentration more than 200mg/dl or their FPG = 126mg/dl or 2h postload glucose = 200mg/dl; and were anti-islet autoantibody positive (antibody against insulin, GAD65 or tyrosine phosphatases IA-2 or IA-2β). One hundred normal controls who were negative for antibodies to insulin, GAD65, IA-2, 21-hydroxylase and the celiac disease autoantibody, tissue transglutaminase (age 9.8 years to 49.7 years old) were also tested. All research patients and normal controls gave informed consent in conjunction with an institutional review board approved protocol at the University of California -San Francisco or the University of Colorado.
Competitive europium interferon alpha antibody assay (CE-IFN-a Ab)
Figure 1 illustrates the general scheme of the competitive europium-IFN-α assay. Corning highbinding clear 96-well plates (costar 3590) were coated with 100 μl of human IFN-a protein (ABcam: ab9661) in PBS buffer overnight at 4° at a working concentration of 2 μg/ml. The next day, the plate was washed 3 times with washing buffer and then blocked with 3% HSA (human serum album, Sigma A-1653) for 2 hours at room temperature on a plate shaker. Each sample was run both with and without competition and performed in duplicate. For the non-competitive assay, serum samples (5μl) were diluted with 45 μl of assay buffer. For the competition assay, 5 μl of serum was diluted with 45 μl assay buffer which contained IFN-a protein at a final concentration of 8 μg/ml. The samples were incubated for 1 hour at room temperature. They were then transferred to the prepared plates and incubated for 2 hours on a shaker at room temperature. Plates were washed and then 100 μl of biotinylated anti-human IgG (BD: 555785) diluted 1:10000 with assay buffer was added to each well and reacted for 30 minutes. Europium labeled streptavidin (DELFIA: 1244-360, diluted 1:2000 in assay buffer) was then added. After washing with 300μl washing buffer for 3 times, 200 μl of enhancement solution (DELFIA: 1244-105) was added to plates and incubated for 25 min to achieve complete reaction. Finally, fluorescence was detected with a Wallac fluorimeter (Victor V 1420 Multilabel counter, Turku, Finland). The index of IFN-a antibodies was calculated as: Index= (CPS (Counts per second) sample without competition-CPS sample with competition)/(CPS positive standard sera without competition-CPS positive standard sera with competition). The intra- and inter-assay coefficient of variation was 10.53% (n=10) and 8.69% (n=11), respectively.
Figure 1.
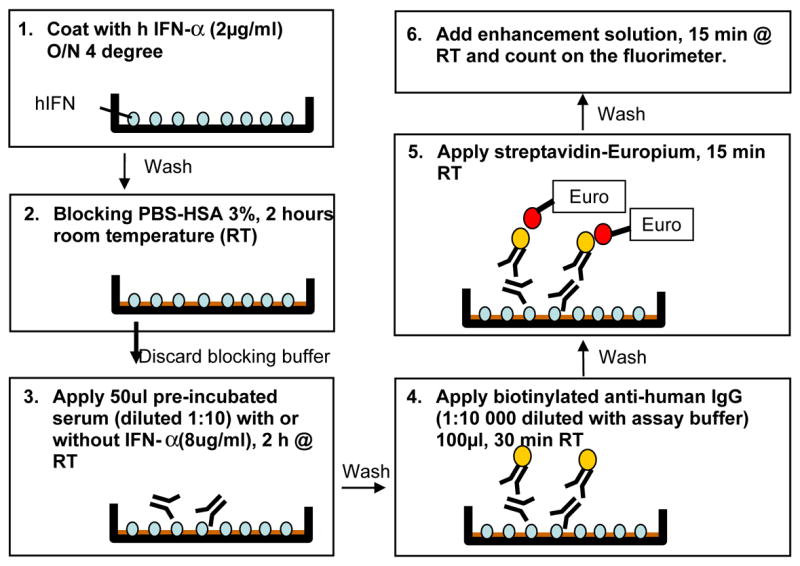
Schematic of the immunofluorometric IFN-a autoantibody assay.
Statistics
The index is presented as a mean value of the duplicate measurement. The frequency differences between groups were tested by Fisher’s exact tests. P values (two sides) less than 0.05 were regarded as significant. The capacity and affinity of IFN-a Ab’s were calculated by Scatchard plot analysis with Prism software.
Results
An europium-based immunoassay completely differentiated APS1 patients from non-APS1 patients, Addison’s Disease and Type 1 diabetes patients
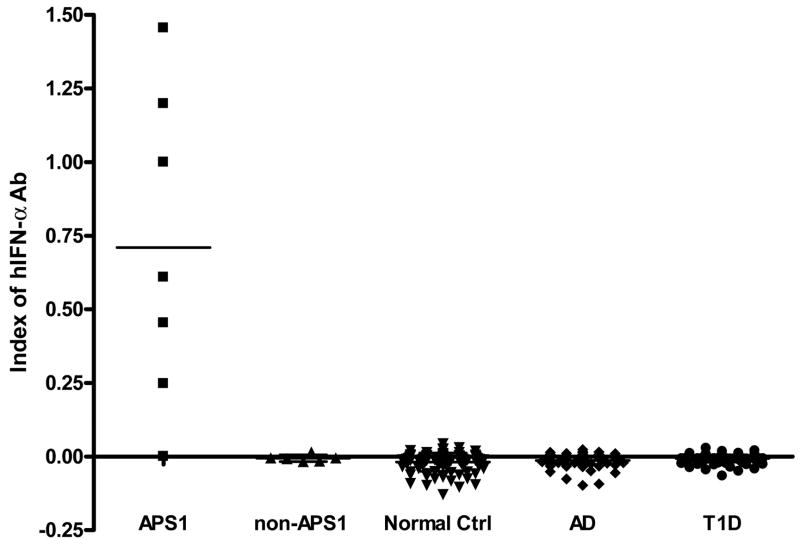
We developed a novel europium-based immunoassay for the detection of IFN-α autoantibodies. In this assay, normal controls had a mean CPS of 31,237±17328 (range: 8,806–89,279) and after subtracting the values with competition (delta) the mean CPS of normal controls was −6,563±10,303 (range= −43,203–14,523 CPS). The initial patient with APS1 studied (sera used to create an index of 1) gave 394,063 CPS without competition and a delta of 363,662±31,587 CPS with competition (394,063-30,402=363,662 CPS). The indexes of normal controls were low: −0.0189±0.0303 (mean ±SD). Figure 2 plots the index with 6/7 APS1 patients strongly positive (index from 0.25 to 1.46). The IFN-α autoantibody negative APS1 patient was presumed as APS1 by clinical symptoms, however, we could not identify a mutation in the AIRE gene in this patient. All non-APS1 patients had a low index similar to normal controls. We also tested 71 non-APS1 Addison’s disease patients and 141 Type 1 diabetes patients. Using a cut-off of two SD above mean for normal controls, none of the Addison’s disease patients with or without diabetes, or patients with Type 1 diabetes alone were IFN-a autoantibody positive.
Figure 2.
Index of IFN-a Ab’s in different patients groups. Anti-IFN a autoantibodies showed significant specificity in APS1 patients. Serum was diluted in 1:10 and measured by europium-based fluorescence. Each point presents the mean index of the duplicated well for that individual sample. The bar indicates the mean value for each cohort. APS1, n=7; non-APS1, n=6; normal controls, n=100; Addison’s Disease, n =71; Type 1 diabetes, n=141.
Specificity including all non-APS1 individuals analyzed was greater than 99.5%
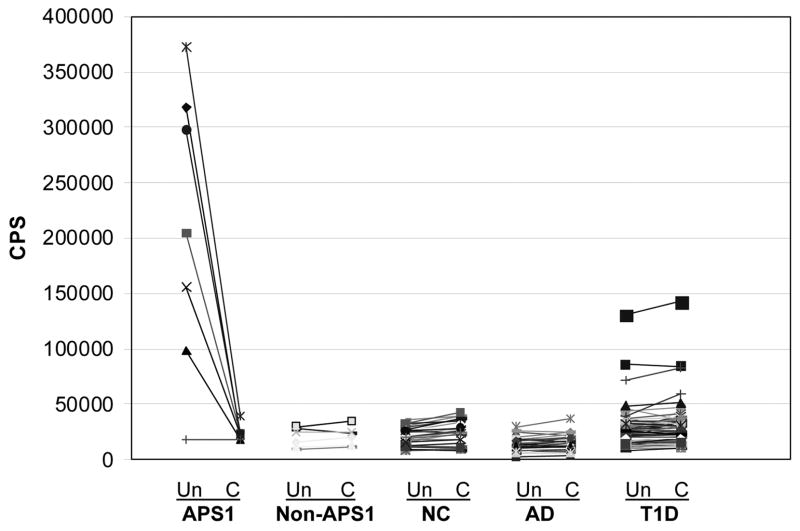
To compare the specificity between the competitive assay of IFN-α antibodies and a more classic non-competitive assay, we analyzed the results without competition. The mean+2SD without competition of 100 normal controls (65,894 CPS) was set as a cutoff value. All positive APS1 patients remained positive but 3 Type 1 diabetes patients were judged as “positive” because their CPS were higher than 65,894 CPS (Figure 3). If the cutoff was set as at the 100th percentile of normal controls (93,838 CPS) or mean +3SD (83,223 CPS) of normal controls, 1/141 type 1 patients or 2/141 type 1 patients were positive, respectively. This result was confirmed by several repeat experiments, however, when the competition assay was simultaneously performed, the index of IFN-a antibodies fell below 2 SD above normal, indicating non-specific binding. In order to confirm this non-specificity, these samples were found to have similarly high CPS when IFN-a protein was increased to 64μg/ml for competition. Therefore, competition improves specificity as would be expected for this assay where subsets of human sera can bind non-specifically to the plate.
Figure 3.
IFN- a Ab assay results with and without competition for the five different patient groups. Shown are CPS results for individual samples with and without competition for IFN-α(8 μg/ml). Un: without competition assay results; C: competition assay results; NC: normal controls.
IFN-a antibodies have high affinity and low capacity in APS1 serum
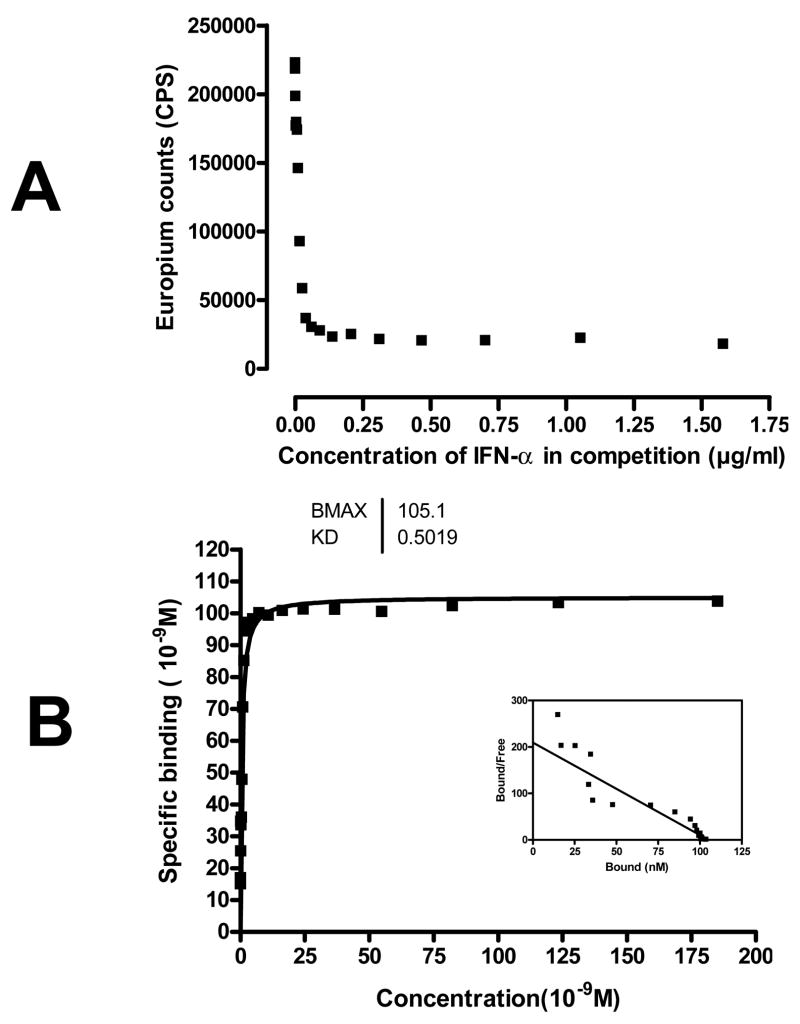
To analyze the affinity and capacity of the autoantibodies, we tested serial concentrations of IFN-a protein in a competition format using the initial patient. Scatchard plot analysis revealed IFN-a antibodies from an individual APS1 patient had a Kd of 0.5 nM and a capacity of 105 nM. (Figure 4). Other positive samples showed similar capacities and affinities (data not shown).
Figure 4.
A) Competition assay of inhibition by different concentrations of fluid phase IFN-a protein (ranging from 0.0016 to 1.6 μg/ml) in the initial APS1 patient sample. Results are average CPS of duplicated wells. B) Scatchard plot of IFN-a protein binding to antibodies from the initial APS1 patient. The Scatchard analysis was performed with competition concentrations of IFN-a protein ranging from 0.0016 to 1.6 μg/ml. Results are the average of duplicate wells.
Discussion
Our results have confirmed the high prevalence of autoantibodies to IFN-a in patients with clinically defined APS1. In addition, we have developed an assay that is relatively simple to employ and could be implemented by many independent laboratories. Our data support that this assay has high specificity (greater than 99%) and is in agreement with that of previous studies in that the antibodies to type 1 interferon are prevalent in the APS1 patients [10, 11].
Our specific immunofluorometric assay (IFMA) has several potential advantages that are worth discussing when compared to the other assays used to detect type 1 interferon autoantibodies in APS1 (Table 1). First, is the relative ease and simplicity of the europium based immunoassay. The previous studies that have looked for type 1 interferon autoantibodies in APS1 patients utilized both an ELISA-based assay and a separate neutralizing assay that requires a cell culture system to employ [10, 11]. In these studies, the neutralization assays showed better sensitivity and specificity than the standard ELISA without competition; however, the neutralization assay is more labor intensive because of the need for cell culture and viral infection to measure neutralization. In addition, the ELISA assay that was utilized in these studies (which involved an alkaline phosphatase-dependent optical density readout) was not completely specific or sensitive for APS1 patients. In a study by Wolff and colleagues, an ELISA assay for IFN-ω autoantibodies was positive in 17/19 APS1 patients and for IFN-α2 autoantibodies was positive in 23/29 APS1 patients.
Table 1.
Comparison of type 1 interferon antibody assays in APS1 subjects
| Assay | Advantages | Disadvantages |
|---|---|---|
| ELISA | Ease of use | Decreased sensitivity and specificity when compared to the two other assay methods |
| Neutralization assay | High specificity and sensitivity | Need for cell culture and viral infection to perform assay |
| IFMA | Ease of use, high specificity and sensitivity | Need for a fluorimeter |
In our competitive based ELISA, 6/6 APS1 patients with confirmed AIRE mutations were positive for autoantibodies to IFN-α (IFNα1). A second important feature of our assay is its high specificity. In a study by Meager et al. [12], the ELISA-based assay for IFN-α was positive in 1/70 normal control subjects (1.4%), while in our study 0/100 normal control subjects were positive. Finally, we found that competition in our assay could be added to achieve >99% specificity. Using competition we did not detect a positive signal in any of the 100 normal controls, 71 Addison’s disease, or 141 Type 1 diabetes samples. The possible improved sensitivity and specificity of our assay is likely to be due to the europium-based fluorescence assay (or IFMA) that we used in this study to detect antibody binding. Previous studies have shown that this technique can enhance both specificity and sensitivity when compared head to head with other detection methods like that in ELISA’s or radioimmunoassays [13, 14]. This is due to a variety of factors and includes the large Stokes’ shift and long decay time of fluorescence that is associated with lanthanide chelates like europium and the effective labeling of detection reagents with europium. Further study using head to head comparisons, however, will be needed to determine the exact efficacy of our assay when compared to the previous published assays for type 1 interferon antibodies in APS1 subjects. A final point to comment on is the nature of the antigen that we used in our immunoassay. There are over 12 different subtypes of IFN-α and in our assay we utilized a recombinant form of IFN-α1. The previous published studies with APS1 subjects tested for IFN-α subtype 2 on ELISA and IFN-α subtypes 2, 4, 5, 6, 7, 8, 10, 14, 16, and 17 in neutralization assays [10, 11]. Thus, our assay results could again differ due to the slightly different nature of the antigens used in the assays. Taken together, given the relative ease of use of our assay and its high specificity for APS1 subjects, it may have certain advantages over the other published assays for type 1 interferon autoantibodies.
Given the high specificity of the assay in several independent studies, anti-IFN-α antibody testing should aid in the confirmation of the diagnosis of APS1. The diagnosis of APS1 requires a high-index of clinical suspicion and long-term follow-up, as components of the disorder develop over time in the affected individual [1]. Therefore, in order to make the diagnosis of APS1 at any point in time, the clinician must be aware of the many manifestations of the disorder. Subjects who meet the clinical criteria for the disorder can have mutational analysis of the AIRE gene performed, however, this is labor intensive as there are many potential mutations in a given patient or family and exhaustive sequencing of the entire gene may be required. The use of an assay to detect antibodies to IFN-a may provide a quick and reliable way to screen for APS1 in subjects with autoimmunity. In a similar study on a large collection of Scandinavian APS1 patients, it was determined that anti-type 1 interferon antibodies likely develop at an early age and persist for an extended period of time [10]. In agreement with these findings, we found evidence of these antibodies being present in a two year old with APS1 and also in a 32 year old adult who had the syndrome for over 20 years with a diagnosis at age 12. To date, the presence of IFN-α antibodies has not been reported on North American, Iranian, or Italian APS1 patients and here we also demonstrate the presence of these antibodies in samples from these APS1 patient populations. None of our subjects with Addison’s Disease or Type 1 diabetes in the absence of APS1 were positive for this autoantibody, indicating that even in subjects with “related” autoimmunity these antibodies are negative. Taken together, our results suggest that the IFN-α antibody assay is useful for helping confirm the diagnosis of APS1.
Interestingly, one of our APS1 patients did not test positive for IFN-α antibodies. This patient had chronic mucocutaneous candidiasis and hypoparathyroidism diagnosed in infancy and thus meets clinical criteria for APS1. However, we were also unable to identify a mutation in this patient’s AIRE gene on either allele after exhaustive sequencing of all coding exons and 2000 bp upstream of the first exon (data not shown). Thus, it is currently unclear to us if this patient has APS1 or a distinct disorder from APS1 that has overlapping clinical features.
What do these autoantibodies tell us about the underlying pathophysiology of APS1? The AIRE gene has been shown to be important in driving the “ectopic” expression of self-antigens within the thymus [4]. Many self- antigens, such as insulin, whose expression is restricted to limited tissues (like the pancreatic islets in the case of insulin), have also been demonstrated to be expressed in the thymus and this expression is likely important for the development of central tolerance to self [15–17]. The presence of antibodies to IFN-a in almost all patients so far reported with APS1, despite the extreme variability of their clinical phenotype suggests that for some reason the lack of interferon autoantibodies is critically dependent on AIRE. Interestingly, to date, the only other clinical syndrome associated with these autoantibodies appears to be patients with thymoma and myasthenia gravis [10, 12, 18, 19]. Perhaps it is possible that these antibodies emerge as a failure of selection against T cells with specificity for type 1 interferons given the clear link to the thymus in the two clinical syndromes (APS1 and thymoma/myasthenia gravis). Given the broad expression pattern of type 1 interferons in many cell popluations, however, it is unclear why thymic expression would be necessary to induce tolerance to them. The clinical contribution to the APS1 syndrome by these autoantibodies is also unclear. One interesting possibility is the potential link of these autoantibodies to the propensity to develop susceptibility to candida infections in these patients as suggested by Meager et al. [10]. Clearly additional work is needed to unravel how these interesting antibodies arise and also to determine what, if any, contribution they have to the pathophysiology of APS1.
In conclusion, we have developed a novel and highly sensitive and specific assay for IFN-a antibodies that employs a competitive step to improve the test accuracy. This assay is admirably suited to clinically to screen patients for APS1 and an evaluation of large series of patients with APS1 from multiple nations still needs to be performed to refine the sensitivity and specificity of the test, Not only does our data in conjunction with recent studies show that measurement of autoantibodies to type 1 interferons can be useful in confirming the diagnosis of APS1, a rare disorder, but in a much broader content, our data shows the potential for the broad application of such competitive assays in clinical medicine.
Acknowledgments
This work was supported by grants from NIH (DK59958, EY016408, AI95380), the Pew Scholars Program in the Biomedical Sciences, and the Sandler Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perheentupa J. Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy. J Clin Endocrinol Metab. 2006;91:2843–2850. doi: 10.1210/jc.2005-2611. [DOI] [PubMed] [Google Scholar]
- 2.Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJ, Lalioti MD, Mullis PE, Antonarakis SE, Kawasaki K, Asakawa S, Ito F, Shimizu N. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–8. [Google Scholar]
- 3.An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. The Finnish-German APECED Consortium. Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy. Nat Genet. 1997;17:399–403. [Google Scholar]
- 4.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 5.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–4. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 6.Su MA, Anderson MS. Aire: an update. Current Opinion in Immunology. 2004;16:746–752. doi: 10.1016/j.coi.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Scott HS, Heino M, Peterson P, Mittaz L, Lalioti MD, Betterle C, Cohen A, Seri M, Lerone M, Romeo G, Collin P, Salo M, Metcalfe R, Weetman A, Papasavvas MP, Rossier C, Nagamine K, Kudoh J, Shimizu N, Krohn KJ, Antonarakis SE. Common mutations in autoimmune polyendocrinopathy-candidiasis- ectodermal dystrophy patients of different origins. Mol Endocrinol. 1998;12:1112–9. doi: 10.1210/mend.12.8.0143. [DOI] [PubMed] [Google Scholar]
- 8.Bjorses P, Halonen M, Palvimo JJ, Kolmer M, Aaltonen J, Ellonen P, Perheentupa J, Ulmanen I, Peltonen L. Mutations in the AIRE gene: effects on subcellular location and transactivation function of the autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy protein. Am J Hum Genet. 2000;66:378–92. doi: 10.1086/302765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson P, Peltonen L. Autoimmune polyendocrinopathy syndrome type 1 (APS1) and AIRE gene: new views on molecular basis of autoimmunity. J Autoimmun. 2005;25(Suppl):49–55. doi: 10.1016/j.jaut.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 10.Meager A, Visvalingam K, Peterson P, Moll K, Murumagi A, Krohn K, Eskelin P, Perheentupa J, Husebye E, Kadota Y, Willcox N. Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med. 2006;3:e289. doi: 10.1371/journal.pmed.0030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolff AS, Erichsen MM, Meager A, Magitta NF, Myhre AG, Bollerslev J, Fougner KJ, Lima K, Knappskog PM, Husebye ES. Autoimmune polyendocrine syndrome type 1 in Norway: phenotypic variation, autoantibodies, and novel mutations in the autoimmune regulator gene. J Clin Endocrinol Metab. 2007;92:595–603. doi: 10.1210/jc.2006-1873. [DOI] [PubMed] [Google Scholar]
- 12.Meager A, Wadhwa M, Dilger P, Bird C, Thorpe R, Newsom-Davis J, Willcox N. Anti-cytokine autoantibodies in autoimmunity: preponderance of neutralizing autoantibodies against interferon-alpha, interferon-omega and interleukin-12 in patients with thymoma and/or myasthenia gravis. Clin Exp Immunol. 2003;132:128–36. doi: 10.1046/j.1365-2249.2003.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madersbacher S, Shu-Chen T, Schwarz S, Dirnhofer S, Wick G, Berger P. Time-resolved immunofluorometry and other frequently used immunoassay types for follicle-stimulating hormone compared by using identical monoclonal antibodies. Clin Chem. 1993;39:1435–1439. [PubMed] [Google Scholar]
- 14.Madersbacher S, Berger P. Antibodies and Immunoassays. Methods. 2000;21:41–50. doi: 10.1006/meth.2000.0973. [DOI] [PubMed] [Google Scholar]
- 15.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary t hymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–9. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 16.Hanahan D. Peripheral-antigen-expressing cells in thymic medulla: factors in self-tolerance and autoimmunity. Curr Opin Immunol. 1998;10:656–62. doi: 10.1016/s0952-7915(98)80085-x. [DOI] [PubMed] [Google Scholar]
- 17.DeVoss J, Hou Y, Johannes K, Lu W, Liou GI, Rinn J, Chang H, Caspi R, Fong L, Anderson MS. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J Exp Med. 2006;203:2727–2735. doi: 10.1084/jem.20061864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiono H, Wong YL, Matthews I, Liu JL, Zhang W, Sims G, Meager A, Beeson D, Vincent A, Willcox N. Spontaneous production of anti-IFN-alpha and anti-IL-12 autoantibodies by thymoma cells from myasthenia gravis patients suggests autoimmunization in the tumor. Int Immunol. 2003;15:903–13. doi: 10.1093/intimm/dxg088. [DOI] [PubMed] [Google Scholar]
- 19.Bello-Rivero I, Cervantes M, Torres Y, Ferrero J, Rodriguez E, Perez J, Garcia I, Diaz G, Lopez-Saura P. Characterization of the immunoreactivity of anti-interferon alpha antibodies in myasthenia gravis patients. Epitope mapping. J Autoimmun. 2004;23:63–73. doi: 10.1016/j.jaut.2004.03.013. [DOI] [PubMed] [Google Scholar]