Abstract
Lens development is an excellent model for genetic and biochemical studies of embryonic induction, cell cycle regulation, cellular differentiation and signal transduction. Differentiation of lens is characterized by lens-preferred expression and accumulation of water-soluble proteins, crystallins. Crystallins are required for light transparency, refraction and maintenance of lens integrity. Here, we review mechanisms of lens-preferred expression of crystallin genes by employing synergism between developmentally regulated DNA-binding transcription factors: Pax6, c-Maf, MafA/L-Maf, MafB, NRL, Sox2, Sox1, RARβ/RXRβ, RORα, Prox1, Six3, γFBP-B and HSF2. These factors are differentially expressed in lens precursor cells, lens epithelium and primary and secondary lens fibers. They exert their function in combination with ubiquitously expressed factors (e.g. AP-1, CREB, pRb, TFIID and USF) and co-activators/chromatin remodeling proteins (e.g. ASC-2 and CBP/p300). A special function belongs to Pax6, a paired domain and homeodomain-containing protein, which is essential for lens formation. Pax6 is expressed in lens progenitor cells before the onset of crystallin expression and it serves as an important regulatory factor required for expression of c-Maf, MafA/L-Maf, Six3, Prox1 and retinoic acid signaling both in lens precursor cells and the developing lens. The roles of these factors are illustrated by promoter studies of mouse αA-, αB-, γF- and guinea pig ζ-crystallins. Pax6 forms functional complexes with a number of transcription factors including the retinoblastoma protein, pRb, MafA, Mitf and Sox2. We present novel data showing that pRb antagonizes Pax6-mediated activation of the αA-crystallin promoter likely by inhibiting binding of Pax6 to DNA.
Keywords: eye, lens, crystallin, gene regulation, Pax6, pRb
Introduction
The developing eye is an excellent model to understand many important biological problems including embryonic induction, signal transduction, cellular differentiation, cellular migration and apoptosis. The eye contains tissues with various levels of complexity. The lens is comprised of one cell type, epithelial (precursor) cells and terminally differentiated lens fiber cells. In contrast, neuroretina contains seven major cell types (rod and cone photoreceptors, amacrine, bipolar, ganglion, horizontal and Muller cells), with amacrine, bipolar and ganglion cells present in many varieties. Both tissues, lens and retina, have to form in precise temporal/spatial regiments, otherwise the optical function of the eye would be compromised. Both natural visual systems and cameras follow the laws of optics. Cameras are manufactured from isolated components and assembled together at once. In contrast, eyes are formed through a series of interactions between cells of different embryonic origins resulting in the establishment of various lineages of ocular precursor cells. Ectodermal cells from the head surface will give rise to the lens and corneal epithelium. Neuroectodermal cells form the retina and part of the iris. Neural crest cells form a portion of cornea and iris and trabecular meshwork. Upon receiving signals in the form of secreted molecules from either surrounding and/or more distal cells, the individual precursor cells undergo terminal differentiation forming specific ocular tissues. At the genetic level, eye development is governed by selective implementation of specific transcriptional programs establishing and/or accompanying individual physiological states of the cells including cell growth and division, cell lineage commitment, terminal differentiation, changes of mutual cell position and long-range migration and apoptosis. Gene-specific transcriptional activation combined with gene-specific repression play major roles in establishing distinct cellular phenotypes by the virtue of selecting such genes that are needed for specialized function of individual ocular cells and tissues. Lens would be marked by the expression of proteins required for its transparency and light refraction and unique shape of lens fiber cells, while retinal photoreceptors would require expression of light sensitive rhodopsins and a number of enzymes associated with light transduction.
Numerous studies in the area of eye development and gene regulation provide evidence that a single gene, Pax6, plays essential roles in the developing eye, though it is also expressed outside of the eye, i.e. in the developing brain, olfactory, pituitary, pancreas and spinal cord (for a review, see Simpson and Price, 2002). Pax6 plays cell-autonomous roles in the lens (Quinn et al., 1996; Collinson et al., 2001) and both cell-autonomous and noncell autonomous functions in other ocular cells (Collinson et al., 2003; 2004). The current data on specific functions of Pax6 during ocular development are consistent with the hypothesis that tissue-restricted roles of Pax6 originate from its interactions with tissue-restricted (e.g. c-Maf, AP-2α and Sox2) and ubiquitously expressed (e.g. pRb and TFIID) transcription factors (see below) and by the ability of Pax6 to regulate expression of a number of other transcription factors including c-Maf, MafA/L-Maf, Six3 and Prox1 (Ashery-Padan et al., 2000; Sakai et al., 2001; Goudreau et al., 2002; Reza et al., 2002)
Human PAX6 locus is located on chromosome 11p13 and mouse Pax6 locus is located on a syntenic region of chromosome 2 (see van Heyningen and Williamson, 2002). A single wild type PAX6/Pax6 allele is not sufficient for normal eye development in human, mouse and rat implicating haploinsufficiency. Rodents harboring mutated Pax6 alleles are comprised of: spontaneously deleted alleles, including mouse SeyDey and SeyH (for a review, see Glaser et al., 1995) and rat rSey (Matsuo et al., 1993), chemically-induced mutations, including mouse Pax61 to 10 Neu (Ehling et al., 1982; Favor et al., 2001), engineered disruption of Pax6 locus in mouse embryonic stem cells combined with insertion of a lacZ (St-Onge et al., 1996) and floxed Pax6 allele used for conditional inactivation of Pax6 (Ashery-Padan et al., 2000). These animal models provide excellent opportunities for understanding Pax6 function in vivo in the eye and elsewhere.
In humans, heterozygous mutations in PAX6 cause a diverse spectrum of ocular abnormalities (see Prosser and van Heyningen, 1998; and van Heyningen and Williamson, 2002) and subtle changes in the olfactory system and brain (Sisodiya et al., 2001; Ellison-Wright et al., 2004). Nearly 80% of human PAX6 mutations result in typical aniridia (for a review, see Prosser and van Heyningen, 1998). The hallmark of aniridia is iris hypoplasia, often combined with cataracts, corneal abnormalities, glaucoma, nystagmus and foveal and optic nerve hypoplasia (see Nelson et al., 1984, Hittner, 1989). About 10% of mutants involve regulatory mutations (Lauderdale et al., 2000; Kleinjan et al., 2001). In contrast, in about one half of the remaining 10% cases, missense mutations generating single amino acid substitutions cause less severe phenotypes, e.g. foveal hypoplasia, Peters’ anomaly (i.e. presence of the corneal-lenticular stalk), congenital cataracts and autosomal dominant keratitis (see Prosser and van Heyningen, 1998; van Heyningen and Williamson, 2002). However, identical mutations may or may not behave similarly, even among family members (Sale et al., 2002). A rare case of a human PAX6 compound homozygote resulted in anophthalmia and severe brain defects lethal after birth (Glaser et al., 1994).
Pax6 is a member of an evolutionary conserved Pax family of genes (for reviews, see Gruss and Walther, 1992; Mansouri et al., 1999; Chi and Epstein, 2002; Simpson and Price, 2002) that control pattern formation, cellular proliferation and differentiation and in specific instances also cellular migration and apoptosis during mammalian development. They are likely required for tissue maintenance in adults (Zhang et al., 2001), for formation of adult corneal stem cells (Collinson et al., 2004) and for response to tissue injury (Sivak et al., 2004; Zaniolo et al., 2004). The Pax genes (Pax1 to Pax9) encode a 128 amino acid DNA-binding domain, the paired domain (PD) (see Gruss and Walther, 1992). Pax3, 4, 6 and 7 also contain a paired-type homeodomain (HD). Alternative splicing of mRNAs encoding Pax proteins was observed with Pax2, Pax3, Pax5, Pax6, Pax7 and Pax8 (Dressler and Douglas, 1992; Kozmik et al., 1993; Epstein et al., 1994b; Vogan et al., 1996; Kozmik et al., 1997; Jaworski et al., 1998; Lowen et al., 2001). Alternatively spliced Pax proteins possess different functional properties (Kozmik et al., 1993; Epstein et al., 1994b; Kozmik et al., 1997; Singh et al., 2001; Chauhan et al., 2004a).
Here, we review the molecular structure and the expression pattern of Pax6 in the eye. Major focus is placed on models of Pax6-mediated regulation of crystallin gene expression in lens. Functional interactions of Pax6 with other transcription factors, including large Maf proteins, Sox proteins and retinoic acid activated nuclear receptors appear to be critical for tissue-preferred expression of crystallin genes in lens. Finally, we present novel data supporting functional significance of Pax6 interaction with the retinoblastoma protein, pRb.
Biochemistry of Pax6: binding to DNA and transcriptional regulation
The molecular structure of Pax6 proteins is critical to understand their biological activity. The paired domain (PD) can be structurally and functionally separated into two independent DNA-binding subdomains, PAI and RED (Czerny et al., 1993; Epstein et al., 1994b; Jun and Desplan, 1996). Mammalian Pax6 genes encode predominantly two forms of the Pax6 protein (see Fig. 1A), Pax6 (p46) and Pax6(5a) (p48) (Carriere et al., 1993; Richardson et al., 1995; Zaniolo et al., 2004). Additional shorter forms, p43, p33 and p32, are less abundant, mostly localized in the cytoplasm and have not been functionally characterized (Carriere et al., 1993; Jaworski et al., 1998). Pax6 contains a canonical 128 amino acid PD (Epstein et al., 1994a). In contrast, Pax6(5a) contains a 14 amino acid insertion within the PAI domain forming a distinct paired domain, PD5a. The insertion destructs the DNA-binding capacity of the PAI subdomain, leaving the RED subdomain and HD for DNA recognition (Epstein et al., 1994b; Kozmik et al., 1997). Consequently, these two Pax6 variants recognize different DNA sequences with three prototypes illustrated in Fig. 1B. Additional permutations of Pax6- and Pax6(5a)-binding sites are possible as each PAI, RED and HD can independently bind to DNA (Czerny et al., 1993; Wilson et al., 1993; Czerny and Busslinger, 1995; Jun and Desplan, 1996). The “founding” Pax6-binding sequence, known as P6CON (Epstein et al., 1994a; Fig. 1B), is a bipartite region of 20 base pairs with its 5′-half recognized by the PAI subdomain and its 3′-half recognized by the RED subdomain (Czerny et al., 1993; Epstein et al., 1994a). P6CON was derived as an “optimal” binding site deduced from a series of enrichments for high affinity binding oligonucleotides from an initial pool of random oligonucleotides (Epstein et al., 1994a). Similarly, “optimal” binding sites were identified for PD(5a) and HD, called 5aCON and HDCON (Fig. 1B), respectively (Epstein et al., 1994b; Czerny and Busslinger, 1995). Other Pax proteins interact with similar but non-identical sequences (Fig. 1B) (Czerny et al., 1993; Epstein et al., 1994a,b; Epstein et al., 1996; Vogan et al., 1996; Kalousova et al.1999; Fujitani et al., 1999). P6CON is recognized by monomeric PD (Epstein et al., 1994a). In contrast, 5aCON can bind up to four PD5a units (Epstein et al., 1994b; Kozmik et al., 1997). Comparison of sequences selected for PD and PD(5a) revealed partial sequence homology between 3′-half of P6CON and 5aCON, as only 5aCON but not P6CON is strongly recognized by the RED subdomain. Further characterization of binding of Pax6 PD and PD(5a) to P6CON revealed a dominant role of the PAI over the RED subdomain in selection of the “optimal” P6CON (Epstein et al., 1994b; Duncan et al., 2000b). The crystal structure of Pax6 PD complex with a fragment of P6CON established direct contacts between both PAI and RED subdomains, the linker region between PAI and RED subdomain and the N-terminal β-turn subdomain with individual bases and phosphate residues of cognate DNA (Xu et al., 1999) as depicted in Fig. 1C. Studies of Pax6 HD binding to DNA revealed cooperative binding of two HDs to a symmetric binding site containing two symmetric binding motifs, ATTA, each found in a number of binding sites recognized by other HD-containing proteins (Wilson et al., 1993). Crystallographic analysis of HD dimers pointed to a serine residue in the third helix of HD promoting this cooperativity (Wilson et al., 1995). Biochemical and structural studies of Pax6 and other Pax proteins serve as essential tools to understand molecular mechanism of various missense and nonsense human PAX6 mutations (Singh et al., 1998; Singh et al., 2001; Mishra et al., 2002; Chauhan et al., 2004a,b)
Fig. 1. Schematic representation of functional domains in Pax6 and Pax6(5a) and their “optimal” DNA-binding sites.
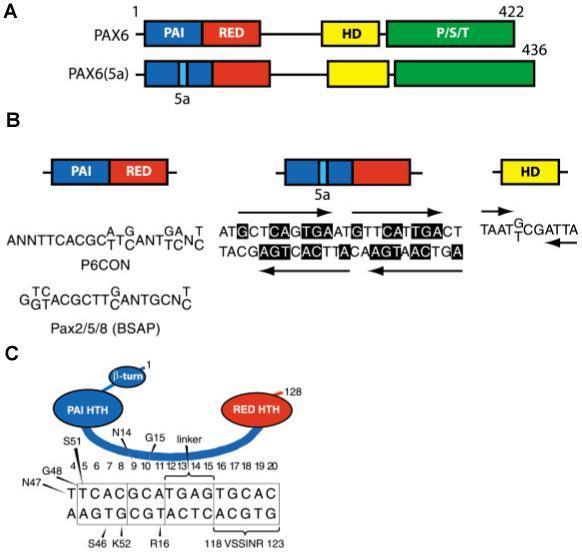
(A) Pax6 and Pax6(5a) differ in a 14 amino acid insertion in the PAI subdomain. (B) “Optimal” DNA-binding sites for Pax6 PD, PD5a and HD. P6CON sequence generated by selection procedure (Epstein et al., 1994a) is compared to a consensus binding site for Pax2/5/8 (BSAP) derived from natural binding sites (Czerny et al., 1993). Note that 5aCON site contains 4 binding sites for PD5a. The “optimal” binding site of Pax6 HD is a dimer with typical HD binding sequences (ATTA/TAAT) separated by three nucleotides, (G/T)CG (Czerny and Busslinger, 1995). (C) Contacts between individual amino acid residues and P6CON deduced from crystallographic studies (Xu et al., 1999). HTH, Helix-turn-helix.
Although isolated Pax6 PD, PD5a and HD can independently interact with DNA, the DNA-binding properties of full length Pax6 and Pax6(5a) proteins show increased complexity as functions of the PAI subdomain are influenced by the RED subdomain and functions of the HD are affected by the PD and vice versa. Early evidence for a cooperativity between Pax6 PD and HD has been shown in the promoter of neural cell adhesion molecule L1 (Chalepakis et al., 1994). Cooperative interactions between PAI and RED subdomains of the PD and HD were established for a number of Pax proteins (Jun and Desplan, 1996). Functional analysis of two Pax6 missense mutants, R26G and R128C, in transient reporter assays revealed that both PAI and RED subdomains of the PD could negatively regulate transactivation of both Pax6 and Pax6(5a) depending on the DNA-binding site used, P6CON or 5aCON (Yamaguchi et al., 1997). More recent studies of human PAX6 and PAX6(5a) missense mutants, including G18W, R26G, A33P, S43P, G64V, I87R, V126D and R128C, confirmed that mutations located in PAI subdomain influenced functional properties of Pax6 proteins bound to 5aCON sequences, although this site is only recognized by the RED subdomain (Chauhan et al., 2004a). Similarly, mutations in RED subdomain influenced transcriptional activation from P6CON-driven promoters (Chauhan et al., 2004a), although RED subdomain could not bind alone to P6CON (Epstein et al., 1994b). Studies of missense mutants in the PAI subdomain also negatively affected the HD function (Mishra et al., 2002). In addition, both deletions and missense mutations within the Pax6 activation domain influence Pax6 HD function (Singh et al., 1998; 2001). In summary, the conformation of Pax6 as well as other Pax proteins bound to DNA cannot be completely derived from the structure of their individual subdomains. In addition, presence of transcription factors bound next to Pax proteins, including members of Sox and Ets families, may significantly influence their DNA recognition (Fitzsimmons et al., 1996; Bondurand et al., 2000; Kamachi et al., 2001; Garvie et al., 2001; Lang and Epstein, 2003).
Molecular studies have shown that the entire C-terminal region of Pax6 (see Fig. 1A), rich in serine, threonine and proline residues (Glaser et al., 1994), is required for full activation (Tang et al., 1998; Singh et al., 1998; Duncan et al., 2000a; Mikkola et al., 2001b). The transcriptional activation domain of PAX6/PAX6(5a), when fused to the GAL4 DNA-binding domain and compared to the GAL4 activation subdomain, exhibits about 25% of the activity of a strong transcriptional activator (Glaser et al., 1994, Czerny and Busslinger, 1995). The Pax6 activation domain is phosphorylated (Carriere et al., 1993). Two types of kinases were implicated, ERK and p38 kinases, but not JNK (Mikkola et al., 2001b). The principal residue, serine 413, is evolutionary conserved; if the serine is mutated to alanine, the transcriptional activity of Pax6 is significantly reduced using an artificial promoter (Mikkola et al., 2001b). O-glycosylation of the Pax6 PD was reported (Lefebrve et al., 2002) that might modulate protein-protein interaction between Pax6 and other proteins. Additional studies are needed to identify proteins directly interacting with the Pax6 transcriptional activation domain and establish in vivo functions of its postranslational modifications.
Multiple roles of Pax6 in mammalian eye development deduced from its expression pattern, Pax6 heterozygous and homozygous phenotypes and transgenic models manipulating Pax6 and Pax6(5a) expression
Pax6 plays a crucial role in eye development in vertebrates and invertebrates, with mouse and human mutations in Pax6 impairing eye development (see below). The roles of Pax6 outside of the eye, in olfactory epithelium, pancreas, pituitary, brain and spinal cord, are reviewed elsewhere (see Mansouri et al., 1999; Chi and Epstein, 2002; Simpson and Price, 2002). Pax6 expression was found in the surface ectoderm, which will give rise to the lens and in the optic vesicle (Walther and Gruss, 1991; Grindley et al., 1995). Pax6 expression is required for further eye development since Pax6 null mice neither form a lens placode nor an optic cup and Pax6 heterozygotes exhibit delayed lens placode induction (Van Raamsdonk and Tilgham, 2000). Pax6 heterozygous lenses are smaller in size due to the absence of one round of cell division in the early lens (Van Raamsdonk and Tilgham, 2000). Pax6 is expressed highly in proliferating cells of lens epithelium, while its level in differentiating lens fiber cells is reduced (Walther and Gruss, 1991; Grindley et al., 1995; Richardson et al., 1995; Duncan et al., 1998; Zhang et al., 2001). In parallel, the surface ectoderm overlying the lens is induced to form the corneal epithelium expressing high levels of Pax6 (Walther and Gruss, 1991; Grindley et al., 1995; Koroma et al., 1997). Pax6 expression is essential for multipotency of retinal precursor cells, as the absence of Pax6 leads to the presence of only amacrine interneurons (Marquardt et al., 2001). However, only adult retinal ganglion and amacrine cells express Pax6. A large proportion of the anterior eye segment is formed from neural crest cells (see Gammill and Bronner Fraser, 2003; Cvekl and Tamm, 2004) migrating into the developing eye that transiently express Pax6 (Baulmann et al., 2002, Collinson et al., 2003). In Pax6 heterozygous eyes, the process of anterior segment formation is severely impaired as they lack the Schlemm’s canal, trabecular meshwork cells remain undifferentiated and display iris hypoplasia and vascularized corneas (Baulmann et al., 2002). Corneal epithelium in Pax6 heterozygous is thinner owing to a reduction in the number of cell layers and exhibiting abnormal adhesive properties (Davis et al., 2003). Pax6 is also expressed in conjunctival epithelium (Koroma et al., 1997), in lacrimal gland (Makarenkova et al., 2000), in corneal stem cells (Collinson et al., 2004) and in wounded corneal epithelium (Sivak et al., 2004; Zaniolo et al., 2004).
Expression studies done by in situ hybridizations (Walther and Gruss, 1991; Grindley et al., 1995) or by immunohistochemistry (Richardson et al., 1995, Koroma et al., 1997, Duncan et al., 1998) did not distinguish between Pax6 and Pax6(5a). Analysis of RNA using RNase protection (Kozmik et al., 1997) and by RT-PCR (Chauhan et al., 2002b) have shown that the ratio of Pax6 to Pax6(5a) was about 8:1 in the mouse embryo and adult mouse lens. This result was consistent with western data on lens (Richardson et al., 1995) and retinal (Carriere et al., 1993) proteins showing that Pax6 was more abundant than Pax6(5a). However, additional RT-PCR studies have shown equal levels of expression of Pax6 and Pax6(5a) transcripts in the adult human lens and cornea (Zhang et al., 2001), the monkey retina (Zhang et al., 2001) and the bovine iris (Jaworski et al., 1998).
The role of Pax6(5a) has been studied by gene targeting to eliminate the exon 5a (Singh et al., 2002). Mice expressing Pax6 but not Pax6(5a) develop a spectrum of ocular and pancreatic abnormalities. The iris is the most affected tissue and additional morphological differences were found in the lens, cornea and retina. Additional copies of the entire 450 kb Pax6 locus were inserted into the mouse genome and the resulting mice were analyzed at the morphological level (Schedl et al., 1996). An increased copy number of Pax6 loci, hence regulated as endogenous alleles, led to phenotypes similar to Pax6 haploinsufficiency, e.g. reduced size of the eye and corneal abnormalities (Schedl et al., 1996). This finding shows that the level of Pax6 expression has an optimum provided by two normal alleles and any deviation in both directions impairs normal ocular development.
In summary, expression and transgenic loss-of-function studies suggest that Pax6 expression is required to control inductive events forming the lens and cornea, lens differentiation, regulation of neural crest cell migration into the anterior eye segment, terminal differentiation of the trabecular meshwork cells and establishment of multipotency of retinal precursor cells. In addition, Pax6 is needed for the production of corneal stem cells, formation of multiple layers of corneal epithelium, development of the lacrimal gland and is involved in remodeling of the wounded corneal epithelium. Thus, all three transparent ocular tissues, namely the cornea, lens and retina and “accessory” cells (i.e. iris, lacrimal gland and trabecular meshwork) undergo a temporal or indefinite period of Pax6 expression. Utilization of a single gene, Pax6 and its genetic network, might reflect not only a successful evolutionary conserved pathway (see Gehring and Iteo, 1999), but it also provides a number of opportunities for a coordinate establishment of ocular cell lineages and for synchronization of their terminal differentiation (see Cvekl and Tamm, 2004).
Genes regulated by Pax6: from crystallins to diverse batteries of target genes
Identification of genes directly regulated by Pax6 is essential for understanding eye biology and represents a major challenge in ongoing research. To show the molecular mechanism of Pax6-dependent gene regulation, the data have to pass a series of tests (Chalepakis et al., 1993). The expression pattern of the Pax6 protein has to precede and overlap the expression pattern of the mRNA of its target gene. Regulatory regions of target genes have to contain at least one Pax6 binding site. Binding of Pax6 to this region has to be demonstrated both in vitro using footprinting and gel shift assays and in vivo using chromatin immunoprecipitations (ChIPs). Mutation of the Pax6 binding site should affect the expression level of the target gene in a cell culture experiment (using a reporter gene), in transgenic mouse and in vivo in the context of the genomic locus.
Crystallins (see below) have emerged as the first group of genes transcriptionally regulated by Pax6 a decade ago (see Cvekl and Piatigorsky, 1996). Studies also revealed that Pax6 regulates a diverse spectrum of genes including transcriptional regulators (e.g. Six 3, c-Maf and Prox1) (Ashery-Padan et al., 2000; Goudreau et al., 2002; Sakai et al., 2001) and specific cell adhesion molecules (e.g. α5β1 integrins, R-cadherin and L1CAM) (Duncan et al., 2000b; Andrews and Mastick, 2003; for a review see Simpson and Price, 2002). As the present genetic network downstream of Pax6 does not fully explain phenotypes associated with defects in Pax6 expression, novel genes directly controlled by Pax6 have to be identified. Pax6 target genes can be predicted by educated guesses (Duncan et al., 2000b) and identified by using high throughput techniques, e.g. cDNA microarrays (Chauhan et al., 2002a,c), differential display (Chauhan et al., 2002b) and chromatin immunoprecipitations (ChIPs; see Weinmann and Farnham, 2002). Paralemmin, molybdopterin synthase sulfurylase, cell adhesion molecules JAM1 and neogenin and transcription factors Brg1, Pitx3 and Etv6, were leading novel candidate direct Pax6-target genes that emerged from the above microarray studies.
Crystallins: multifunctional proteins highly expressed in lens
Crystallins are defined as water-soluble proteins highly expressed in lens. They are divided into two families: ubiquitously-expressed crystallins represented by the α- and βγ-families and taxon-specific crystallins represented by proteins found only in the lens of specific animal phyla. Most taxon-specific crystallins possess enzymatic activities and are termed enzyme crystallins. Two members of the α-family, αA and αB, are structurally and functionally related to small heat shock proteins (see Horwitz, 2002; Bhat 2003). Extralenticular expression of α-crystallins may provide protection against various forms of stress (Alge et al., 2002). Specifically, αB-crystallin has been shown to inhibit both the mitochondrial and death receptor pro-apoptotic pathways (Kamradt et al., 2001). In contrast, 14 βγ-crystallins appear to have a distant relationship to other proteins (see Wistow, 1995). In addition, the role of βγ-crystallins outside of the lens remains to be determined. The majority of data on crystallin gene regulation is based on studies of mouse and chicken genes. However, several important differences between mouse and chick lenses relevant to the molecular mechanisms of crystallin gene regulation have to be considered. First, the mouse lens crystallin gene family is comprised of crystallins αA, αB, γA-F, γS, βA1-4 and βB1-3. Chicken lens does not express the γα-F crystallin cluster, or this cluster does not exists in the chicken genome. In addition to the γS-crystallin (van Rens et al., 1991), chick lens contains two taxonspecific δ1 and δ2-crystallins. Second, δ1-crystallin appears first in the chick lens placode, followed by β- and αA-crystallin expression during the lens vesicle stage (see Piatigorsky, 1987). In mouse, αB-crystallin is found in the lens placode, followed by αA-crystallin at the transition from lens pit to lens vesicle (Robinson and Overbeek, 1996). The β- and γ-crystallins are highly expressed in differentiating fiber cells (see Wistow, 1995). Third, regulatory regions of the mouse and chicken crystallins are less evolutionary conserved than one would expect. For example, a region of 77 nucleotides upstream from the TATA-box containing multiple cis-acting elements of the mouse α-crystallin is only 63.6% conserved between mouse and chicken (Donovan et al., 1992). Nevertheless, chicken δ1- and δ2-crystallin genes, when studied in transgenic mice, exhibit remarkably high levels of expression in the lens and low expression in the retina, cornea and cerebellum, recapitulating the expression of the endogenous δ-crystallin gene in the chicken embryo and adult (Takahashi et al., 1988). Although earlier studies of transcription factors suggested the possibility that mouse and chicken genomes may contain unique genes regulating crystallins, i.e. L-Maf gene in chicken (Ogino and Yasuda, 1998), it is now evident that mouse/human MafA is a mammalian homologue of L-Maf (Benkhelifa et al., 2001, Olbrot et al., 2002, Kataoka et al., 2002). MafA/L-Maf has a much broader expression pattern in chick embryo (Lecoin et al., 2004) extending far beyond the lens and brain (Ogino and Yasuda, 1998).
Role of Pax6 in tissue-restricted and tissue-specific gene expression
Tissue-restricted and tissue-specific gene expression are governed by a combinatorial principle: a specific gene is regulated by a combination of several transcriptional activators both co-expressed and present in active forms in the given tissue. Expression of Pax6 in essentially all tissues forming the eye (i.e. lens, retina, cornea, iris, trabecular meshwork and lacrimal gland), forebrain and hindbrain/cerebellum, spinal cord, pancreas and pituitary, suggests, that the number of tissue-restricted factors needed for lens-specific expression of 15 crystallin genes is extensive. Indeed, currently known regulatory factors for crystallins with restricted patterns of expression are Pax6, c-Maf, MafA/L-Maf, MafB, NRL, Sox2, Sox1, RARβ/RXRβ, RORα, Prox1, Six3, γFBP-B and HSF2 (Fig. 2). These factors are differentially expressed in lens epithelial cells, primary and secondary lens fibers (see Fig. 2A). They differ in their onset of expression in the developing lens as shown below. In addition, some regulatory sites in crystallin promoters can interact with ubiquitously expressed AP-1, CREB, TFIID and USF transcription factors (Fig. 2B). Some of these factors (AP-1 and USF) are regulated by redox potential in the cells, with lens cells known for their highly reducing environment (Piatigorsky, 1992). Regulation of tissue-specific and tissue-preferred expression by Pax6 in other ocular and nonocular tissue follows the same combinatorial principle. Partners of Pax6 may include same or different transcription factors like AP-2α in the regulation of gelatinase B promoter in the corneal epithelium (Sivak et al., 2004).
Fig. 2. A summary of transcription factors and some growth factors known to regulate lens development and crystallin gene expression.
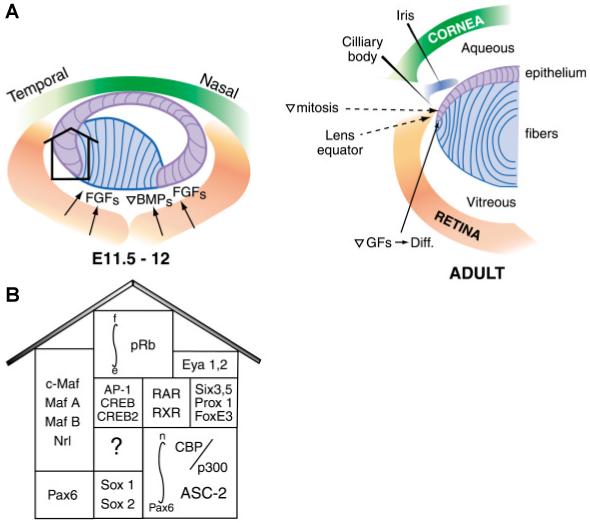
(A) A schematic representation of mouse lens development during the onset of primary lens fiber cell differentiation (left panel) and in the adult lens (right panel). (B) The diagram illustrates a diversity of transcription factors implicated in lens development and crystallin gene regulation. See text for details.
These specific DNA-binding transcriptional activators are assembled at the crystallin promoters and at more distal regulatory regions and play number of specific roles. Their two primary functions are to overcome the repressive influence of chromatin and to recruit general transcription factors including the RNA polymerase II. In some instances, they also counteract the action of ubiquitously or tissue-specifically expressed repressor molecules including γFBP-B (Liu et al., 1994) and δEF1(Sekido et al., 1994). Chromatin-mediated transcriptional repression is alleviated by reversible acetylations and methylations of histone tails, by chromatin remodeling, and/or by nucleosomal displacement. Experimental evidence suggests that a common co-activator CBP/p300 (Fig. 2A) harboring the histone acetylase activity (HAT) is involved in crystallin gene expression (Chen et al., 2002a,b) (see below). Expression of chromatin remodeling factor Brg1, a catalytical subunit of mammalian SWI/SNF complex, was found in the embryonic and newborn lens (Chauhan et al., 2002c). ASC-2, also termed AIB3, TRBP, RAP250, NRC and PRIP (Fig. 2A), is a co-activator interacting with histone H3-lysine 4-specific methyltransferase and its function is also required in vivo for αA-, γD- and γF-crystallins (Kim et al., 2002). Some of the DNA-binding factors, e.g. Prox1 (Duncan et al., 2002), may be regulated at the level of nuclear/cytoplasmic localization. Finally, lens fiber cell differentiation is triggered by an exit from cell cycling that is regulated by the retinoblastoma protein (pRb, Fig. 2B) in conjunction with DNA-binding factors from the E2F family (Rampalli et al., 1998; McCaffrey et al., 1999). Lack of expression of pRb in lens interferes with fiber cell differentiation and crystallin gene expression (Morgenbesser et al., 1994; Fromm et al., 1995). Finally, some of these specific DNA-binding transcription factors have to be connected with specific signal transduction pathways (FGF, TGFβ and BMP) that control lens development and lens fiber cell differentiation (Fig. 2A) and described in this issue (see Beebe et al., 2004; Chen et al., 2004). Experimental evidence points to the phosphorylation of MafA (Benkhelifa et al., 2001) and Pax6 (Mikkola et al., 2001b).
Regulation of crystallin gene expression by Pax6 in combination with a small family of specific transcription factors in ocular lens
Over ten years ago, crystallins were shown as the first known class of genes regulated by Pax6 (see Cvekl and Piatigorsky, 1996). Data accumulated over this period indicate an intricate complexity of vertebrate crystallin gene regulation (see Duncan et al., 2004). Interestingly, recent data have shown that jellyfish PaxB, an ancestral Pax gene, regulates expression of invertebrate J3-crystallin promoter (Kozmik et al., 2003; Piatigorsky and Kozmik, 2004). The experimental data were mostly collected on mouse and chicken genes tested in both homologous and heterologous transgenic and cell culture systems. Although these studies have been conducted for over 20 years, we do not have a complete understanding of all aspects of their regulation. The ultimate goals of these studies are as follows: 1. Elucidation of the genomic organization of crystallin loci that involves defining the genomic region that is sufficient to drive the expression of the endogenous gene at a level comparable to the endogenous locus in a position-independent and copy-number dependent manner in transgenic animals. 2. Identification of regulatory regions of the gene and their role in the embryonic, neonatal and adult lens. These regions control the tissue-specificity, the temporal and spatial pattern of expression and quantity of the expression. 3. Dissection of these regions into individual cis-regulatory elements and determining their roles. 4. Identification of a full complement of transcriptional activators and repressors interacting with cis-elements. 5. Characterization of chromatin remodeling processes associated with crystallin gene activation and repression. 6. Elucidation of quantitative aspects of crystallin gene transcription involving measurements of crystallin mRNA turnover and determination of the amounts of mRNA per lens epithelial and fiber cell. 7. Identification and dissection of signal transduction pathways in lens cells and their association with crystallin gene expression. Here, we will review the mechanisms of transcriptional regulation of mammalian αA-, αB-, γF- and ζ-crystallins. Studies of chicken crystallins are separately presented in this issue and were also recently reviewed elsewhere (see Duncan et al., 2004).
Mouse αA-crystallin is predominantly expressed in the lens. However, immunological studies detected the protein at low levels in rat thymus, spleen, cerebellum, skin, small intestine, liver and kidney (Kato et al., 1991; Srinivasan et al., 1992). More recent studies found considerable expression of αA-crystallin in mouse retina, with expression in ganglion cells, inner and outer nuclear layers of photoreceptors (Li et al., 2003). In situ hybridizations (Fig. 3A) detected transcription of αA-crystallin in the lens pit of the 10-10.5 day mouse embryo and equal expression in the anterior and posterior portions of the lens vesicle (Robinson and Overbeek, 1996). With the beginning of fiber cell differentiation at E11.5 of mouse development, the elongating posterior lens cells dramatically increase the expression of this gene. Coordination may exist between increased expression of αA-crystallin and signal transduction pathways triggering fiber cell differentiation (see Beebe et al., 2004; Chen et al., 2004, this issue). The posterior cells of the lens vesicle are exposed to different growth factors including members of FGF and TGFβ/BMP families of factors and Wnt/β-catenin signaling originating from the developing retina compared to lens anterior cells. Although the mechanism enhancing the αA-crystallin gene expression in primary lens fiber cells is at present not known, there are at least two, mutually not exclusive, possibilities. The first possibility is sequential; the growth factors impose cellular differentiation through cyclins and their modulatory kinases, followed by the full activation of transcriptional machinery responsible for transcription of crystallin genes. The second possibility involves parallel effects of growth factors on the regulatory components of the cell cycle and transcriptional machineries.
Fig. 3. Schematic representation of transcript levels encoding αA- and αB-crystallins in mouse embryonic, neonatal and postnatal lens. (A) Expression of αA-crystallin.
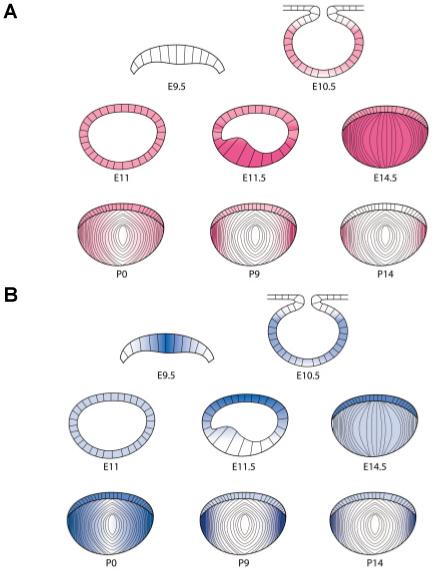
(B) Expression of αB-crystallin. Diagrams are based on in situ hybridization data from Robinson and Overbeek (1996). Note that these data are different from protein accumulation that persists in the entire lens.
When the primary lens fiber cells reach the anterior epithelial cells, the embryonic lens is formed. In parallel, the anterior chamber formation is on its way, as the iris is formed from the peripheral edge of the optic cup (see Cvekl and Tamm, 2004). The formation of iris separates the cavity between lens and cornea into the anterior and posterior chamber and may result in a redistribution of gradients of growth factors and the creation of a mitotic zone in the area of lens epithelial cells, a transitional zone, close to the lens equator. Lens epithelial cells at the equatorial region undergo terminal differentiation due to their exposure to specific differentiation signals (see Lang, 1999). Consequently, the lens continues its growth by adding new layers of “secondary” lens fibers in the equatorial regions of the lens. In secondary fibers, αA-crystallin transcripts are highly expressed throughout the remainder of lens development well into adulthood.
Mouse αA-crystallin gene regulation at the promoter level was studied in lens culture systems and in transgenic mice (see Sax and Piatigorsky, 1994; Bhat, 2003; Duncan et al., 2004). Transgenic studies demonstrated that mouse αA-crystallin promoter fragments originating from positions -366, -111 and -88 to +46 fused to the chloramphenicol acetyltransferase (CAT) reporter gene were all highly active in lens fiber cells. Additional shortening of the promoter to a fragment of -60 to +44 resulted in loss of activity (Sax et al., 1993). It appears that the fragment of -366 to +46 is active only in lens fiber cells (see Sax and Piatigorsky, 1994), although its initial study raised the possibility that it was also active in lens epithelium (Overbeek et al., 1985). These transgenes exhibited variability of expression within three orders of magnitude due to the positional and copy-number dependent effects (Sax et al., 1993). Multiple sequence alignments of mouse, human, mole rat, hamster and chicken genomic loci containing αA-crystallin gene revealed evolutionary conserved regions both 5′- and 3′-of the gene suggesting the possibility that these regions contain novel distant control regions (Cvekl et al., 2002).
Transcription factor binding sites of the mouse αA-crystallin promoter (see Fig. 4) were identified using site-directed mutagenesis and transient transfections in cultured lens cells (Sommer et al., 1988; Nakamura et al., 1989, Donovan et al., 1992, Cvekl et al., 1995a). These studies identified two regions, a 13 base pair cis-element -110 to -98 and a wider 48 base pair region -87 to -40 (Nakamura et al., 1989, Donovan et al., 1992). The region of -110 to -98 contains a binding site (MARE) for large members of the Maf family of transcription factors, e.g. c-Maf (Ring et al., 2000), overlapping a functional cAMP responsive element (CRE) (Cvekl et al., 1995a). The binding of transcription factors from the region -87 to -40 is less understood. Both in vitro and in vivo footprinting studies revealed interactions with nuclear proteins (Kantorow et al., 1993a). The first identified protein to bind in vitro to this region was a ubiquitously expressed large zinc finger transcription factor αA-CRYBP1/PRDII-BFI/MBP-1(Nakamura et al., 1989; Kantorow et al., 1993b; Brady et al., 1995). A Pax6-binding site was found upstream from the TATA-box (Cvekl et al., 1995a), followed by the identification of an AP-1 binding site downstream of the TATA-box (Ilagan et al., 1999). Data to establish specific actions of Pax6, large Maf proteins, αA-CRYBP1 and AP-1 factors in vivo are currently available only for c-Maf. Three studies of lens in c-Maf null mice revealed significant reduction of αA-crystallin transcripts (Kawauchi et al., 1999; Kim et al., 1999; Ring et al., 2000) that were linked to the presence of MARE (-110 to -98) in the promoter (see Table 1). However, deletion/mutagenesis of this site did not eliminate lens-specific expression in transgenic mice (Wawrousek et al., 1990; Sax et al., 1993) implying two possibilities. Either different large Maf proteins, e.g. MafA and NRL, may be involved in the process, or other MARE sites may exist both within the -88 to +46 fragment or in the novel distant control regions (Cvekl et al., 2002). The role of Pax6 in αA-crystallin also requires further studies. DNase I footprinting experiments using recombinant Pax6 proteins indicated the presence of two additional binding sites within the -111 to +46 fragment and at least one upstream binding site (Cvekl et al., 2002).
Fig. 4. Diagrammatic representation of four crystallin promoters/enhancers and their regulatory sites and factors.
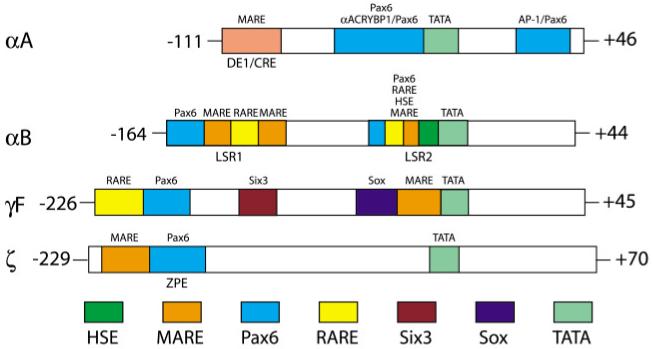
The numbering corresponds to the mouse αA-, αB- and γF-, together with guinea pig ζ-crystallin promoter fragments. The regions are not shown to scale. Note that these and many other crystallin promoters contain canonical TATA-boxes (see Cvekl and Piatigorsky, 1996).
TABLE 1.
SUMMARY OF DIFFERENTIALLY EXPRESSED CRYSTALLINS IN LENSES OF LOSS-OF-FUNCTION AND TRANSGENIC MODELS*
| Crystallin | ASC-2 (DN tg) | CREB2/ATF4 (-/-) | c-Maf (-/-) | Pax6 (+/-) | Prox1 (-/-) | Sox1(-/-) |
|---|---|---|---|---|---|---|
| αA | reduced | normal | reduced | normal | normal | normal |
| αB | normal | ND | reduced | reduced | normal | normal |
| βB1 | ND | ND | reduced | normal | normal | ND |
| βB2 | ND | ND | no | ND | ND | ND |
| βA3/A1 | normal | ND | no | reduced | normal | normal |
| βA4 | ND | ND | no | ND | ND | ND |
| γB | normal | ND | reduced | ND | no | no |
| γD | reduced | ND | no | ND | no | reduced |
| γE | ND | normal | reduced | normal | normal | reduced |
| γF | reduced | normal | reduced | normal | normal | reduced |
The data were combined from the following references (Chauhan et al., 2002b; Kawauchi et al., 1999; Kim et al., 1999; Kim et al., 2002; Nishiguchi et al., 1998; Ring et al., 2000; Tanaka et al., 1998; Wigle et al., 1999). Different developmental and adult stages were analyzed in the individual reports.
DN tg, dominant negative transgenics; +/-, heterozygous lenses; -/-, homozygous lenses; ND, not determined.
The present data suggest a following model. The relatively low expression of the αA-crystallin gene in lens pit and lens vesicle is mediated by lens epithelial transcription factors including Pax6 and MafB, with possible roles of other factors including CREB. In the differentiating lens fibers, MafB is replaced by MafA, c-Maf and NRL with concomitant downregulation of Pax6 (Walther and Gruss, 1991; Duncan et al., 1998; Lecoin et al., 2004) resulting in significant upregulation of this gene. Recruitment of co-activators CBP/p300 (see above) by a DNA-binding factor c-Maf (Chen et al., 2002a,b) and likely by other large Maf factors and Pax6 (Hussain and Habener, 1999) provides a link between activation and repression of the αAcrystallin locus at the chromatin level. Interestingly, ASC-2 null lenses exhibit reduced expression of the αA- but not αB-crystallin (see Table 1). ASC-2 is a transcriptional coactivator of nuclear receptors, AP-1 and other transcription factors that function in the lens (Kim et al., 2002). Additional studies of the mouse αA-crystallin locus including its promoter are required to fully understand regulatory mechanisms that account for its temporal and spatial regulation and links to the fiber-cell differentiation signals.
Although mouse αB-crystallin, like its cousin αA-crystallin, is predominantly expressed in the lens, its expression outside of the lens reaches significant levels in the heart, skeletal muscle and lung and appreciable expression is evident in the brain and kidney (Bhat and Nagineni, 1989; Dubin et al., 1989). In addition, αB-crystallin is also expressed in the cornea, ciliary body, optic nerve, extraocular muscle and trabecular meshwork (Tamm et al., 1996). More recent studies found specific expression of αB-crystallin in mouse retina. The expression of αB-crystallin was found in ganglion cells, inner and outer nuclear layers of photoreceptors and retinal pigment epithelium (Li et al., 2003). In situ hybridizations (Fig. 3B) detected transcription of αB-crystallin in the lens placode of the E9.5 mouse embryo and equal expression in the anterior and posterior portions of the lens vesicle at E10.5-11 (Robinson and Overbeek, 1996). During subsequent stages of lens development, αB-transcripts were more abundant in lens epithelium compared to αA-crystallin (Fig. 3). Expression of αB-crystallin is also elevated in differentiating primary lens fibers as well as in secondary fibers. Its expression in lens epithelium appears to persist longer than αA-crystallin expression.
Transgenic studies of the αB-crystallin gene appear to be more complete compared to those of αA-crystallin. A fragment of 4 kb 5′-flanking sequence of the mouse αB-crystallin gene fused to the lac Z reporter gene was sufficient to reproduce the developmental pattern of the endogenous gene (Haynes et al., 1996). Promoter fragments from -164 and -115 to +44 were sufficient to activate CAT reporter expression in transgenic lenses. In contrast, a fragment -68 +44 was inactive. Positional and copy-number effects indicated that additional regulatory regions were necessary (Gopal-Srivastava et al., 1996). Two regulatory regions, LSR1 and LSR2, were found in the 5′-flanking region (Fig. 4). Both elements contain Pax6-sites and RAREs (Gopal-Srivastava et al., 1996, 1998). LSR1 also contains two putative MAREs (Chauhan et al., 2004b, Yang et al., 2004). LSR2 also contains a Pax6 site overlapping a putative MARE and a heat shock responsive element (HSE) (Somasundaram and Bhat, 2000; Yang et al., 2004). The in vivo evidence supports roles of Pax6 and c-Maf in transcriptional regulation of the αB-crystallin locus (see Table 1). Both Pax6 heterozygous (Chauhan et al., 2002b) and c-Maf homozygous (Kawauchi et al., 1999; Kim et al., 1999; Ring et al., 2000) lenses contain reduced amounts of αB-crystallin transcripts. The regulation of αB-crystallin by RARβ/RXRβ may function between E9.5 to E12.5 as retinoic acid/retinoid signaling is lost in lens by E12.5 and is Pax6-dependent (Enwright and Grainger, 2000).
Interestingly, the mammalian αB-crystallin loci contain a gene, HspB2, encoding a protein structurally similar to the αB-crystallin (Iwaki et al., 1997). Transcription of this gene, although not detected in the lens, initiates at position -940 relative to the start siteof transcription for mouse αB-crystallin and in the opposite direction. Since the αB-crystallin contains an enhancer (-427 to -255, MLH enhancer) activating its expression in lens and non-lens tissues (e.g. skeletal muscle and heart), it was of general interest to determine why this “enhancer” operates only with the αB-crystallin promoter and not a nearby HspB2 promoter. The studies revealed directionality of the MLH enhancer and suggested the presence of a genomic boundary element, the insulator (Swamynathan and Piatigorsky, 2002).
Studies of transcriptional control of seven mammalian γ-crystallins (Dirks et al., 1996) also greatly advanced the field of crystallin gene regulation with the majority of functional studies focused on γF-crystallin. Recent studies have detected the expression of some members of βγ-crystallins in the lens epithelium (M. Kantorow, personal communication), in different retinal cells (Li et al., 2003) and in the brain and testis (Magabo et al., 2000). In mammals, γ-crystallin genes are highly expressed in differentiating primary fibers. A genomic fragment from -759 to +45 of the mouse γF-crystallin fused to the lac Z gene essentially reproduced the expression of the endogenous gene (Goring et al., 1987). Shorter fragments -171 to +45 and -67 to +45 were expressed in central lens fibers (Goring et al., 1993). Cell culture experiments identified two enhancer-like regions, -392 to -278 and -226 to -121(Yu et al., 1990) and several cis-elements in the -67 to +45 region (Liu et al., 1991). A retinoic acid responsive element (RARE) was shown in the region of -210 to -190 interacting with RARβ/RXRβ heterodimers (Tini et al., 1993) and the retinoic acid receptor-related orphan nuclear receptor RORα (Tini et al., 1995). More recently, Pax6- and Pax6(5a)-binding sites were found next to RARE; Pax6 binding was required for retinoic acid induced activation of the γF-promoter (Kralova et al., 2002; Chauhan et al., 2004b). A repressor-binding site capable of interacting with Six3 was found between the promoter and Pax6 binding site (Lengler et al., 2001). The repression of γF-crystallin promoter by Six3 is likely due to the recruitment of transcriptional co-repressors Grg4 and Grg5 (Zhu et al., 2002). It appears that early expression of Pax6 and Six3 inhibit the γF-crystallin promoter expression which is mediated by Sox1/2 (Kamachi et al., 1995) and large Maf proteins (Civil et al., 2002) interacting with a tandem of adjacent binding sites 5′-from the TATA-box (Yang et al., 2004). Indeed, in both Sox1 and c-Maf null lenses, γF-crystallin transcripts were almost eliminated (Table 1). Although sequence homologies between six γ-crystallin genes suggested that they are likely regulated by common factors (Peek et al., 1990), Prox1 null lenses (Table 1) contained only reduced amounts of γB- and γD-crystallins but not of γE- and γF-crystallins (Wigle et al., 1999).
Finally, studies of the enzyme ζ-crystallin gene expression provided invaluable data (Lee et al., 1994, Richardson et al., 1995; Sharon-Friling et al., 1998). The lens-specific 5′-flanking region of guinea pig ζ-crystallin gene appears to contain only a single Pax6-binding site. Site-directed mutagenesis of this Pax6 binding site abolished lens-specific expression in transgenic mice (Richardson et al., 1995). Another important regulatory site is MARE, just upstream from the Pax6-binding site (ZPE), functionally interacting with the large Maf protein, NRL (Sharon-Friling et al., 1998) and perhaps with other large Mafs. The guinea pig ζ-crystallin differs from its mouse counterpart in the genomic region harboring MARE and a Pax6 site that is probably inserted as a result of a transposon insertion between two nine base pair direct repeats (Lee et al., 1994). Recruitment of crystallins from preexisting genes due to modifications in their transcriptional regulatory regions was reviewed extensively elsewhere (for reviews, see Piatigorsky, 1992; Wistow, 1995; Piatigorsky, 2003).
Molecular interactions of Pax6 provides clues into tissue-specific roles of Pax6
Transcriptional studies of Pax6 in conjunction with other DNA-binding transcription factors described above have revealed that tissue-specific actions of Pax6 depend on a combination of at least two principles. Regulatory regions of crystallin promoters are organized as arrays of specific binding sites, sometimes using a repetition of motifs to enhance the stringency of regulation. In a number of crystallin promoters including the mouse αB- and chicken βB1-crystallins, Pax6-binding sites overlap with MAREs (Chauhan et al., 2004b, Cai et al., 2004; Yang et al., 2004). Each αB-crystallin LSR1 and LSR2 contains a binding site for Pax6, a MARE and a RARE (Chauhan et al., 2004b; Yang et al., 2004). Chicken θ1-crystallin “core” enhancer contains at least three Pax6-binding sites (Cvekl et al., 1995b, Kamachi et al., 2001, Muta et al., 2002), interrupted by at least one Sox-binding site (Kamachi et al., 2001). Adjacent and/or overlapping binding sites for Pax6, large Maf proteins and other proteins suggest formation of direct protein-protein interactions between these DNA-binding transcription factors. Indeed, studies of these interactions in lens and other cells expressing Pax6 is generating an increasing number of proteins interacting with Pax6. Biochemical data support direct protein-protein interactions between Pax6 and Cdx-2/3 in β-cells of pancreas mediated by p300 (Hussain and Habener, 1999), Pax6 and Sox2 in lens cells (Kamachi et al., 2001), Pax6 and c-Maf in β-cells of pancreas (Planque et al., 2001b), Pax6 and MITF in neuroretina (Planque et al., 2001a), Pax6 and pRb in lens (Cvekl et al., 1999), Pax6 and TFIID in lens (Cvekl et al., 1999) and Pax6 and AP-2α in corneal epithelium (Sivak et al., 2004). In addition, Pax6 HD is capable to functionally interact with a number of HD-containing proteins co-expressed with Pax6 in ocular and non-ocular tissues including Six3, Lhx2, Chx10, Prox1, Optx2/Six6, Msx2 and Otx2 (Mikkola et al., 2001a; Chauhan et al., 2004b).
Identification of non-DNA binding transcription factors including transcriptional co-activators and chromatin remodeling complexes interacting with Pax6, large Maf and other proteins in lens requires special attention. Co-activators CBP/p300 directly bind c-Maf (Chen et al., 2002b) raising the possibility of Pax6/p300/c-Maf ternary complex formation similar to the complex Pax6/Cdx2/p300 found in β-cells of pancreas (Hussain and Habener, 1999). Functional data support direct or indirect interactions between Pax6 and retinoic acid activated nuclear receptors RARβ and RXRβ (Kralova et al., 2002; Chauhan et al., 2004b), Pax6 and Pax6(5a) (Chauhan et al., 2004b) and Pax6 proteins with MafA/c-Maf (Chauhan et al., 2004b). A summary of selected protein-protein interactions involving Pax6 is given in Fig. 5.
Fig. 5. Pax6 regulates transcription of a variety of DNA-binding transcription factors and some of these factors biochemically interact with Pax6 proteins.
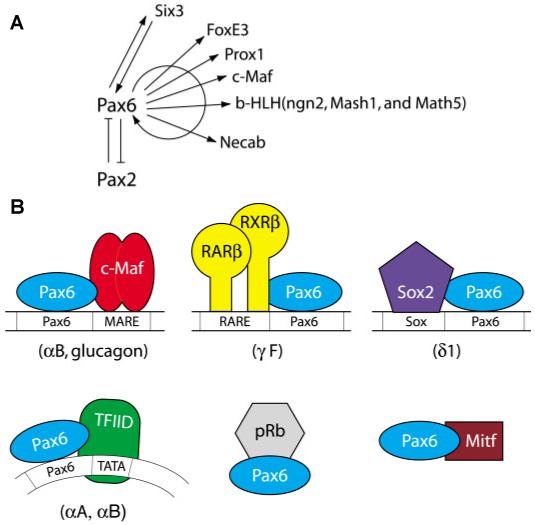
(A) A summary of currently known genes encoding specific DNA-binding transcription factors regulated by Pax6 (Plaza et al., 1993; Ashery-Padan et al., 2000; Schwarz et al., 2000; Marquardt et al., 2001; Bernier et al., 2001; Sakai et al., 2001; Goudreau et al., 2002; Reza et al., 2002). (B) Protein-protein contacts between Pax6 and other transcription factors important in gene regulation by Pax6. See text for details.
Collectively, the present data demonstrate that Pax6 regulates the expression of a diverse spectrum of specific DNA-binding transcription factors (e.g. Six3, Pax2; Prox1, c-Maf, MafA, FoxE3, ngn2, Mash1 and Math5) (Ashery-Padan et al., 2000; Blixt et al., 2000; Brownell et al., 2000; Schwarz et al., 2000; Marquardt et al., 2001; Semina et al., 2001; Goudreau et al., 2002; Reza et al., 2002), forms functional complexes with some of them (e.g. c-Maf and MafA), autoregulates its own promoter in lens lineage (Plaza et al., 1993, Grindley et al., 1995), controls retinoic acid signaling in the embryonic lens (Enwright and Grainger, 2000) and controls expression of components of signal transduction pathways including α5β1 integrin (Duncan et al., 2000b) and Necab (Bernier et al., 2001). Thus, Pax6 is interwoven into a delicate network of processes at multiple genetic (Fig. 5A) and biochemical levels (Fig. 5B). As lens development and specific crystallin gene expression including αB-crystallin is sensitive to Pax6 gene dosage effect (Chauhan et al., 2002b; Chauhan et al., 2004b), further studies of the molecular mechanism of crystallin gene expression in normal and abnormal lenses are essential for better understanding of human diseases caused by mutations in PAX6 locus.
Interactions between Pax6 and retinoblastoma protein, pRb
To gain better insight into Pax6 interactions with other nuclear factors, we tested the roles of Pax6 and pRb (Cvekl et al., 1999) in crystallin gene regulation. Using the synchronized mouse αTN4-1 cultured lens cells, we found earlier that the formation of Pax6/pRb complex is sensitive to the cell cycle with its formation observed during the G1 phase of the cell cycle. We also found that Pax6 homeodomain (HD) provided the major structural contribution for the interaction (Cvekl et al., 1999). Other Pax proteins, Pax3 and Pax5/BSAP, also bind pRb (Wiggan et al., 1998; Eberhard and Busslinger, 1999). Here, we used the mouse αA-crystallin promoter fragment -111 to +46 containing at least one Pax6 binding site (see Fig. 4). A synthetic promoter driven by four E2F binding sites upstream of the E4 TATA-box (see Fig. 6A) was used as a positive control for pRb repression (Sellers et al., 1998). Co-transfection of the mouse αA-crystallin promoter with PAX6 resulted in 3.2-fold activation (see Fig. 6A), while co-transfection with pRb did not affect the promoter activity in C-33A cells deficient in endogenous pRb (Sellers et al., 1998) and Pax6 (data not shown). Co-transfection of PAX6 together with pRb resulted in a dose-dependent transcriptional repression compared to PAX6 alone (see Fig. 6A). These results were supported by direct identification of a specific PAX6/hypophosphorylated pRb complex (Cvekl et al., 1999). Next, we performed similar studies with seven representative human mutated PAX6 proteins G18W, R26G, G64V, R128C, R242T, R317X and S353X (Chauhan et al., 2004a). Five of these mutants contained single amino acid substitution and two of them were nonsense mutations. Biochemical properties of these PAX6 mutants raised the possibility that they may be abnormally folded (Chauhan et al., 2004a) and this folding may be “sensed” by other proteins associated with Pax6, e.g. pRb. To directly test this hypothesis, we cotransfected the mouse αA-crystallin promoter together with PAX6 and/or its mutants and pRb in C33A cells. The results (Fig. 6B) indicated that specific PAX6 mutants, namely G64V, R317X, R26G and R242T, lost their abilities to be repressed by pRb. The data are consistent with our earlier hypothesis that some mutated Pax6 proteins are abnormaly folded (Chauhan et al., 2004a) and cannot functionally associate with other proteins, e.g. pRb, as indicated here.
Fig. 6. Transactivation of the mouse αA-crystallin promoter in the presence of PAX6, PAX6 mutants and pRb.
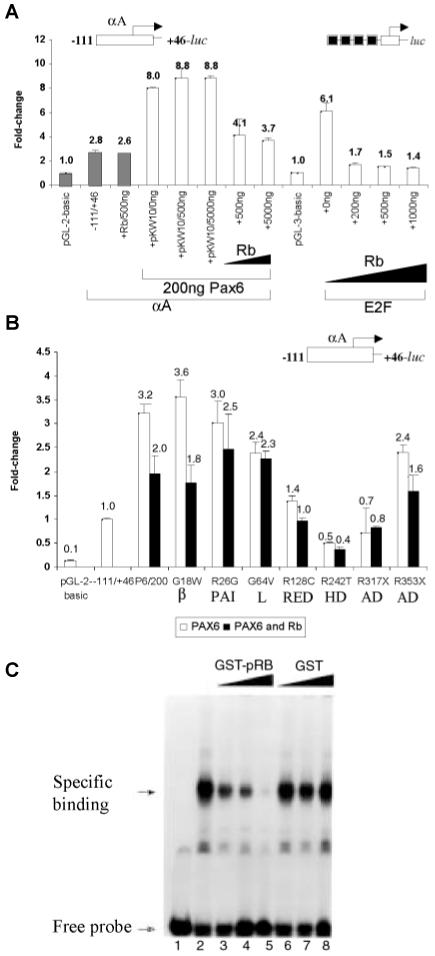
(A) PAX6-mediated activation of mouse αA-crystallin promoter is partially reduced by pRb in C-33A cells. The results with E2F-driven promoter are shown as a positive control. (B) Retinoblastoma protein, pRb, was used to test PAX6 mutants, G18W, R26G, G64V, R128C, R242T, R317X and S353X (see Chauhan et al., 2004a). Shown are the results of cotransfections with PAX6 alone (open bars) and with PAX6 and pRb (solid bars), respectively. Domain symbols, β, β-turn; PAI, PAI subdomain; L, linker between PAI and RED; RED, RED subdomain; HD, homeodomain; AD, activation domain; see also Fig. 1. (C) Electrophoretic mobility shift assays showing inhibition of Pax6 binding to DNA in the presence of pRb. Recombinant GST-Pax6 PD/HD (Duncan et al., 2000) was incubated with recombinant GST-pRb (pocket A/B) at room temperature for 10 min followed by the addition of radioactively labeled probe P6CON as described earlier (Duncan et al., 2000). Control experiments were performed with GST only.
Finally, we tested whether the Pax6/pRb complex affects DNA-binding properties of Pax6 in vitro. Herein, we incubated recombinant Pax6 with GST-human pRb (pocket A and B) and tested binding of Pax6 to a P6CON radioactively labeled probe containing an “optimal” binding site for the Pax6 paired domain (Epstein et al., 1994a). The outcome of the experiment depended on the order of the addition of Pax6 and pRb. Preincubation of both proteins in the absence of DNA resulted in the inhibition of the formation of the PAX6-DNA complex (Fig. 6C). Addition of GST alone did not affect Pax6-DNA interaction (Fig. 6C). In contrast, when Pax6 was first bound to DNA, pRb could not either form a ternary complex-DNA/Pax6/pRB-nor disrupt the binary complex of DNA-Pax6 (data not shown). In summary, the present data raise the possibility that the level of expression of the Pax6 target gene can be modulated by Pax6 interactions with the retinoblastoma protein, a key regulator of cell cycle machinery. We propose that some Pax6 target genes can be regulated in a cell cycle-dependent manner. Since pRb is essential for in vivo terminal differentiation of lens fiber cells (Morgenbesser et al., 1994; Fromm et al., 1994; Maandag et al., 1994; Williams et al., 1994), studies in the intersection of Pax6- and pRb-dependent pathways should provide new molecular insights into the process of lens formation and crystallin gene regulation.
Concluding remarks
Although molecular studies to understand lens-preferred expression of crystallin genes, including the mechanism of their temporal and spatial patterns of expression, have been performed for more then two decades, we are just beginning to reveal the details of these processes. Ultimately, studies aimed to elucidate external signals triggering fiber cell differentiation have to merge with nuclear studies addressing the structural/functional organization of individual crystallin loci and the identification of specific DNA-binding proteins associated with promoters, enhancers and other regulatory regions (i.e. silencers and insulators). Another important aspect of crystallin gene regulation is to understand which co-activators and chromatin remodeling complexes are recruited by specific DNA-binding factors to regulate crystallin promoters. Some of the factors presently known (Pax6, c-Maf, MafA/L-Maf, MafB, NRL, Sox2, Sox1, RARβ/RXRβ, RORα, Prox1, Six3, γFBP-B, HSF2, AP-1, CREB, TFIID and USF) are the ultimate downstream targets of signaling pathways active in lens cells (e.g. c-Maf), while the activities of some of these factors (e.g. AP-1 and USF) are regulated by the redox state of the cell. Co-activators CBP/p300 and ASC-2 regulate specific gene activity at the chromatin level and pRb may regulate accessibility and activity of Pax6 and other DNA-binding factors. Availability of innovative genetic and biochemical tools to further dissect lens differentiation will result in understanding the temporally and spatially regulated expression of diverse crystallin genes in the embryonic, neonatal and adult lens at the molecular level.
Acknowledgements
We thank Dr. Zbynek Kozmik for his critical reading of the manuscript. We thank Barry Shapiro for his help with illustrations and Ales Cvekl, Jr. for his editorial assistance. Supported by NIH grants EY12200 and EY14237. A.C. was a recipient of a Research to Prevent Blindness Inc. (RPB) Career Development Award.
Abbreviations used in this paper
- CRE
cAMP-responsive element
- HD
homeodomain
- MARE
Maf responsive element
- PD
paired domain
- PD(5a)
alternatively spliced paired domain
- pRb
retinoblastoma protein
- RARE
retinoic acid responsive element
- RT-PCR
reverse transcriptase-polymerase chain reaction.
References
- ALGE C, PRIGLINGER S, NEUBAUER AS, KAMPIK A, ZILLIG M, BLOEMENDAL H, WELGE-LUSSEN U. Retinal pigment epithelium is protected against apoptosis by αB-crystallin. Invest. Ophthalmol. Vis. Sci. 2002;43:3575–3582. [PubMed] [Google Scholar]
- ANDREWS GL, MASTICK GS. R-cadherin is a Pax6-regulated, growth promoting cue for pioneer axons. J. Neurosci. 2003;23:9873–9880. doi: 10.1523/JNEUROSCI.23-30-09873.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHERY-PADAN R, MARQUARDT T, ZHOU X, GRUSS P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAULMANN DC, OHLMANN A, FLUGEL-KOCH C, GOSWAMI S, CVEKL A, TAMM ER. Pax6 heterozygous eyes show defects in chamber angle differentiation that are associated with a wide spectrum of other anterior eye segment abnormalities. Mech. Develop. 2002;118:3–17. doi: 10.1016/s0925-4773(02)00260-5. [DOI] [PubMed] [Google Scholar]
- BEEBE D, GUO J, GARCIA C, WANG X, RAJAGOPAL R, FELDMEIER M, KIM JY, CHYTIL A, MOSES H, ASHERY-PADAN R, RAUCHMAN M. Contribution by members of the TGFbeta superfamily to lens development. Int. J. Dev. Biol. 2004;48:845–856. doi: 10.1387/ijdb.041869db. [DOI] [PubMed] [Google Scholar]
- BENKHELIFA S, PROVOT S, NABAIS E, EYCHENE A, CALOTHY G, FELDER-SCHMITTBUHL MP. Phosphorylation of MafA is essential for its transcriptional and biological properties. Mol. Cell. Biol. 2001;21:4441–4452. doi: 10.1128/MCB.21.14.4441-4452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNIER G, VUKOVICH W, NEIDHARDT L, HERRMANN BG, GRUSS P. Isolation and characterization of a downstream target of Pax6 in the mammalian retinal primordium. Development. 2001;128:3987–3994. doi: 10.1242/dev.128.20.3987. [DOI] [PubMed] [Google Scholar]
- BHAT SP. Crystallins, genes and cataract. Prog. Drug. Res. 2003;60:207–262. doi: 10.1007/978-3-0348-8012-1_7. [DOI] [PubMed] [Google Scholar]
- BHAT SP, NAGINENI CN. αB subunit of lens-specific protein α-crystallin is present in other ocular and non-ocular tissues. Biochem. Biophys. Res. Commun. 1989;158:319–325. doi: 10.1016/s0006-291x(89)80215-3. [DOI] [PubMed] [Google Scholar]
- BLIXT A, MAHLAPUU M, AITOLA M, PELTO-HUIKKO M, ENERBACK S, CARLSSON P. A forkhead gene, FoxE3, is essential for lens epithelial proliferation and closure of the lens vesicle. Genes Dev. 2000;14:245–254. [PMC free article] [PubMed] [Google Scholar]
- BONDURAND N, PINGAULT V, GOERICH DE, LEMORT N, SOCK E, CAIGNEC CL, WEGNER M, GOOSSENS M. Interaction among SOX10, PAX3 and MITF, three genes altered in Waardenburg syndrome. Hum. Mol. Genet. 2000;9:1907–1917. doi: 10.1093/hmg/9.13.1907. [DOI] [PubMed] [Google Scholar]
- BRADY JP, KANTOROW M, SAX CM, DONOVAN D, PIATIGORSKY J. Murine transcription factor αA-crystallin binding protein I. Complete sequence, gene structure, expression and functional inhibition via antisense RNA. J. Biol. Chem. 1995;270:1221–1229. doi: 10.1074/jbc.270.3.1221. [DOI] [PubMed] [Google Scholar]
- BROWNELL I, DIRKSEN M, JAMRICH M. Forkhead Foxe3 maps to the disgenesis lens locus and is critical in lens development and differentiation. Genesis. 2000;27:81–93. doi: 10.1002/1526-968x(200006)27:2<81::aid-gene50>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- CAI W, TOMAREV SI, PIATIGORSKY J, CHEPELINSKY AB, DUNCAN MK. Mafs, Prox1 and Pax6 can regulate chicken βB1-crystallin gene expression. J. Biol. Chem. 2004;279:11088–11095. doi: 10.1074/jbc.M312414200. [DOI] [PubMed] [Google Scholar]
- CHALEPAKIS G, STOYKOVA A G, WIJNHOLDS J, TREMBLAY P, GRUSS P. Pax: Gene regulators in the developing nervous system. J. Neurobiol. 1993;24:1367–1384. doi: 10.1002/neu.480241009. [DOI] [PubMed] [Google Scholar]
- CHALEPAKIS G, WIJNHOLDS J, GIESSE P, SCHACHNER M, GRUSS P. Characterization of Pax-6 and Hoxa-1 binding to the promoter region of the neural cell adhesion molecule L1. DNA Cell Biol. 1994;13:891–900. doi: 10.1089/dna.1994.13.891. [DOI] [PubMed] [Google Scholar]
- CARRIERE C, PLAZA S, MARTIN P, QUATANNERS B, BAILLY M, STEHELIN D, SAULE S. Characterization of quail Pax-6 (Pax-QNR) proteins expressed in the neuroretina. Mol. Cell. Biol. 1993;13:7257–7266. doi: 10.1128/mcb.13.12.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAUHAN BK, REED NA, ZHANG W, DUNCAN MK, KILIMANN MW, CVEKL A. Identification of genes downstream of Pax6 in the mouse lens using cDNA microarrays. J. Biol. Chem. 2002a;277:11539–11548. doi: 10.1074/jbc.M110531200. [DOI] [PubMed] [Google Scholar]
- CHAUHAN BK, ZHANG, CVEKLOVA K, KANTOROW M, CVEKL A. Identification of differentially expressed genes in mouse Pax6 heterozygous lenses. Invest. Ophthalmol. Vis. Sci. 2002b;43:1884–1890. [PMC free article] [PubMed] [Google Scholar]
- CHAUHAN BK, REED NA, YANG Y, CERMAK L, RENEKER L, DUNCAN MK, CVEKL A. A comparative cDNA microarray analysis reveals a spectrum of genes regulated by Pax6 in mouse lens. Genes Cells. 2002c;7:1267–1283. doi: 10.1046/j.1365-2443.2002.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAUHAN BK, YANG Y, CVEKLOVA K, CVEKL A. Functional properties of natural human PAX6 and PAX6(5a) mutants. Invest. Ophthalmol. Vis. Sci. 2004a;45:385–392. doi: 10.1167/iovs.03-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAUHAN BK, YANG Y, CVEKLOVA K, CVEKL A. Functional interactions between alternatively spliced forms of Pax6 in crystallin gene regulation and in haploinsufficiency. Nucleic Acids Res. 2004b;32:1696–1709. doi: 10.1093/nar/gkh334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN Q, DOWHAN DH, LIANG D, MOORE DD, OVERBEEK PA. CREB-binding protein/p300 co-activation of crystallin gene expression. J. Biol. Chem. 2002a;277:24081–24089. doi: 10.1074/jbc.M201821200. [DOI] [PubMed] [Google Scholar]
- CHEN Q, ASH JD, BRANTON P, FROMM L, OVERBEEK PA. Inhibition of crystallin expression and induction of apoptosis by lens-specific E1A expression in transgenic mice. Oncogene. 2002b;21:1029–1037. doi: 10.1038/sj.onc.1205050. [DOI] [PubMed] [Google Scholar]
- CHEN Y, STUMP RJW, LOVICU FJ, McAVOY JW. Expression of frizzleds and secreted frizzled-related proteins (Sfrps) during mammalian lens development. Int. J. Dev. Biol. 2004;48:867–877. doi: 10.1387/ijdb.041882yc. [DOI] [PubMed] [Google Scholar]
- CHI N, EPSTEIN JA. Getting your Pax straight: Pax proteins in development and disease. Trends Genet. 2002;18:41–47. doi: 10.1016/s0168-9525(01)02594-x. [DOI] [PubMed] [Google Scholar]
- CHOW RL, LANG RA. Early eye development in vertebrates. Annu. Rev. Cell Dev. Biol. 2001;17:255–296. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- CIVIL A, VAN GENESEN ST, LUBSEN NH. c-Maf, the γD-crystallin Maf-responsive element and growth factor regulation. Nucleic Acids Res. 2002;30:975–982. doi: 10.1093/nar/30.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLINSON JM, QUINN JC, BUCHANAN MA, KAUFMAN MH, WEDDEN SE, WEST JD, HILL RE. Primary defects in the lens underlie complex anterior segment abnormalities of the Pax6 heterozygous eye. Proc. Natl. Acad. Sci. USA. 2001;98:9688–9693. doi: 10.1073/pnas.161144098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLINSON JM, QUINN JC, HILL RE, WEST JD. The roles of Pax6 in the cornea, retina and olfactory epithelium of the developing mouse embryo. Dev. Biol. 2003;255:303–312. doi: 10.1016/s0012-1606(02)00095-7. [DOI] [PubMed] [Google Scholar]
- COLLINSON JM, CHANAS SA, HILL RE, WEST JD. Corneal development, limbal stem cell function and corneal epithelial cell migration in the Pax6 +/- mouse. Invest. Ophthalmol. Vis. Sci. 2004;45:1101–1108. doi: 10.1167/iovs.03-1118. [DOI] [PubMed] [Google Scholar]
- CVEKL A, KASHANCHI F, SAX CM, BRADY JN, PIATIGORSKY J. Transcriptional regulation of the mouse αA-crystallin gene: activation dependent on a cyclic AMP-responsive element (DE1/CRE) and a Pax-6-binding site. Mol. Cell. Biol. 1995a;15:653–660. doi: 10.1128/mcb.15.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CVEKL A, SAX CM, LI X, McDERMOTT JB, PIATIGORSKY J. Pax-6 and lens-specific transcription of the chicken θ1-crystallin gene. Proc. Natl. Acad. Sci. USA. 1995b;92:4681–4685. doi: 10.1073/pnas.92.10.4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CVEKL A, PIATIGORSKY J. Lens development and crystallin gene expression: many roles for Pax-6. BioEssays. 1996;18:621–630. doi: 10.1002/bies.950180805. [DOI] [PubMed] [Google Scholar]
- CVEKL A, KASHANCHI F, BRADY JN, PIATIGORSKY J. Pax-6 interactions with TATA-box-binding protein and retinoblastoma protein. Invest. Ophthalmol. Vis. Sci. 1999;40:1343–1350. [PubMed] [Google Scholar]
- CVEKL A, YANG Y, CHAUHAN BK, GOSWAMI S, CVEKLOVA K, MONT-GOMERY K, KUCHERLAPATI R. Transcriptional regulation of the mouse αA-crystallin locus. Invest. Ophthalmol. Vis. Sci. 2002;43S:3658. [Google Scholar]
- CVEKL A, TAMM ER. Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases. BioEssays. 2004;26:374–386. doi: 10.1002/bies.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CZERNY T, BUSSLINGER M. DNA-binding and transactivation properties of Pax-6: Three amino acids in the paired domain are responsible for the different sequence recognition of Pax-6 and BSAP (Pax-5) Mol. Cell. Biol. 1995;15:2858–2871. doi: 10.1128/mcb.15.5.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CZERNY T, SCHAFFNER G, BUSSLINGER M. DNA sequence recognition by Pax proteins: bipartite structure of the paired domain and its binding site. Genes Dev. 1993;7:2048–2061. doi: 10.1101/gad.7.10.2048. [DOI] [PubMed] [Google Scholar]
- DAVIS J, DUNCAN MK, ROBISON WG, PIATIGORSKY J. Requirement for Pax6 in corneal morphogenesis: a role in adhesion. J. Cell. Sci. 2003;116:2157–2167. doi: 10.1242/jcs.00441. [DOI] [PubMed] [Google Scholar]
- DIRKS RPH, KLOK EJ, VAN GENESEN ST, SCHOENMAKERS JGG, LUBSEN NH. The sequence of regulatory events controlling the expression of the γD-crystallin gene during fibroblast growth factor-mediated rat lens fiber cell differentiation. Dev. Biol. 1996;173:14–25. doi: 10.1006/dbio.1996.0003. [DOI] [PubMed] [Google Scholar]
- DONOVAN DM, SAX CM, KLEMENT JF, LI X, CHEPELINSKY AB, PIATIGORSKY J. Conservation of mouse αA-crystallin promoter activity in chicken lens epithelial cells. J. Mol. Evol. 1992;35:337–345. doi: 10.1007/BF00161171. [DOI] [PubMed] [Google Scholar]
- DRESSLER GR, DOUGLAS EC. Pax-2 is a DNA-binding protein expressed in embryonic kidney and Wilms’ tumor. Proc. Natl. Acad. Sci. USA. 1992;89:1179–1183. doi: 10.1073/pnas.89.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBIN RA, WAWROUSEK EF, PIATIGORSKY J. Expression of the murine αB-crystallin gene is not restricted to the lens. Mol. Cell. Biol. 1989;9:1083–1091. doi: 10.1128/mcb.9.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNCAN MK, HAYNES JI, CVEKL A, PIATIGORSKY J. Dual roles for Pax-6: a transcriptional repressor of lens fiber cell-specific β-crystallin genes. Mol. Cell. Biol. 1998;18:5579–5586. doi: 10.1128/mcb.18.9.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNCAN MK, CVEKL A, LI X, PIATIGORSKY J. Truncated forms of Pax-6 disrupt lens morphology in transgenic mice. Invest. Ophthalmol. Vis. Sci. 2000a;41:464–473. [PubMed] [Google Scholar]
- DUNCAN MK, KOZMIK Z, CVEKLOVA K, PIATIGORSKY J, CVEKL A. Overexpression of PAX6(5a) in lens fiber cells results in cataract and upregulation of α5β1 integrin expression. J. Cell. Sci. 2000b;113:3173–3185. doi: 10.1242/jcs.113.18.3173. [DOI] [PubMed] [Google Scholar]
- DUNCAN M, CAI W, OH DJ, TOMAREV S. Prox1 is differentially localized during lens development. Mech. Develop. 2002;112:195–198. doi: 10.1016/s0925-4773(01)00645-1. [DOI] [PubMed] [Google Scholar]
- DUNCAN MK, CVEKL A, KANTOROW M, PIATIGORSKY J. Lens crystallins. In: Robinson ML, Lovicu M, editors. Development of the ocular lens. Oxford University Press; Cambridge: 2004. pp. 119–150. [Google Scholar]
- EBERHARD B, BUSSLINGER M. The partial homeodomain of the transcription factor Pax-5 (BSAP) is an interaction motif for the retinoblastoma and TATA-binding proteins. Cancer Res. 1999;59S:1716s–1724s. [PubMed] [Google Scholar]
- EHLING UH, FAVOR J, KRATOCHVILOVA J, NEUHAUSER-KLAUS A. Dominant cataract mutations and specific-locus mutations in mice induced by radiation or ethylnitrosourea. Mutat. Res. 1982;92:181–192. doi: 10.1016/0027-5107(82)90222-6. [DOI] [PubMed] [Google Scholar]
- ELLISON-WRIGHT Z, HEYMAN I, FRAMPTON I, RUBIA K, CHITNIS X, ELLISON-WRIGHT I, WILLIAMS SC, SUCKLING J, SIMMONS A, BULLMORE E. Heterozygous PAX6 mutation, adult brain structure and fronto-striato-thalamic function in a human family. Eur. J. Neurosci. 2004;19:1505–1512. doi: 10.1111/j.1460-9568.2004.03236.x. [DOI] [PubMed] [Google Scholar]
- ENWRIGHT JF, III, GRAINGER RM. Altered retinoid signaling in the heads of Small eye mouse embryos. Dev. Biol. 2000;221:10–22. doi: 10.1006/dbio.2000.9652. [DOI] [PubMed] [Google Scholar]
- EPSTEIN J, CAI L, GLASER T, JEPEAL L, MAAS RL. Identification of a Pax paired domain recognition sequence and evidence for DNA-dependent conformational changes. J. Biol. Chem. 1994a;269:8355–8361. [PubMed] [Google Scholar]
- EPSTEIN JA, GLASER T, CAI L, JEPEAL L, WALTON DS, MAAS RL. Two independent and interactive DNA-binding subdomains of the Pax6 paired domain are regulated by alternative splicing. Genes Dev. 1994b;8:2022–2034. doi: 10.1101/gad.8.17.2022. [DOI] [PubMed] [Google Scholar]
- EPSTEIN JA, SHAPIRO DM, CHENG J, LAM PYP, MAAS RL. Pax3 modulates expression of the c-Met receptor during limb muscle development. Proc. Natl. Acad. Sci. USA. 1996;93:4213–4218. doi: 10.1073/pnas.93.9.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAVOR J, PETERS H, HERMANN T, SCHMAHL W, CHATTERJEE B, NEUHAUSER-KLAUS A, SANDULACHE R. Molecular characterization of Pax62Neu and Pax610Neu: An extension of the Pax6 allelic series and the identification of two possible hypomorph alleles in the mouse Mus musculus. Genetics. 2001;159:1689–1700. doi: 10.1093/genetics/159.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZSIMMONS D, HODSDON W, WHEAT W, MAIRA SM, WASYLYK B, HAGMAN J. Pax-5 (BSAP) recruits Ets proto-oncogene family proteins to form functional ternary complexes on a B-cell-specific promoter. Genes Dev. 1996;10:2198–2211. doi: 10.1101/gad.10.17.2198. [DOI] [PubMed] [Google Scholar]
- FROMM L, OVERBEEK PA. Regulation of cyclin and cyclin-dependent kinase gene expression during lens differentiation requires the retinoblastoma protein. Oncogene. 1996;12:69–75. [PubMed] [Google Scholar]
- FUJITANI Y, KAJIMOTO Y, YASUDA T, MATSUOKA TA, KANETO H, UMAYAHARA Y, FUJITA N, WATADA H, MIAZAKI J-I, YAMASAKI Y, HORI M. Identification of a portable repression domain and an E1A-responsive activation domain in Pax4: a possible role of pax4 as a transcriptional repressor in the pancreas. Mol. Cell. Biol. 1999;19:8281–8291. doi: 10.1128/mcb.19.12.8281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAMMILL LS, BRONNER-FRASE M. Neural crest specification: migrating into genomics. Nature Rev. Neurosci. 2003;4:795–805. doi: 10.1038/nrn1219. [DOI] [PubMed] [Google Scholar]
- GARVIE CW, HAGMAN J, WOLBERGER C. Structural studies of Ets-1/Pax5 complex formation on DNA. Mol. Cell. 2001;8:1267–1276. doi: 10.1016/s1097-2765(01)00410-5. [DOI] [PubMed] [Google Scholar]
- GEHRING WJ, IKEO K. Pax6: mastering eye morphogenesis and eye evolution. Trends Genet. 1999;15:371–377. doi: 10.1016/s0168-9525(99)01776-x. [DOI] [PubMed] [Google Scholar]
- GLASER T, JEPEAL L, EDWARDS JG, YOUNG RS, FAVOR J, MAAS RL. PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nature Genet. 1994;7:463–471. doi: 10.1038/ng0894-463. [DOI] [PubMed] [Google Scholar]
- GLASER T, WALTON DS, CAI J, EPSTEIN JA, JEPEAL L, MAAS RL. PAX6 gene mutations in aniridia. In: Wiggs JL, editor. Molecular Genetics of Ocular Disease. Wiley-Liss; New York: 1995. pp. 51–82. [Google Scholar]
- GOPAL-SRIVASTAVA R, CVEKL A, PIATIGORSKY J. Pax-6 and αB-crystallin/small heat shock protein gene regulation in the murine lens. Interaction with the lens-specific regions, LSR1 and LSR2. J. Biol. Chem. 1996;271:23029–23036. doi: 10.1074/jbc.271.38.23029. [DOI] [PubMed] [Google Scholar]
- GOPAL-SRIVASTAVA R, CVEKL A, PIATIGORSKY J. Involvement of retinoic acid/retinoid receptors in the regulation of murine αB-crystallin/small heat shock protein gene expression in the lens. J. Biol. Chem. 1998;273:17954–17961. doi: 10.1074/jbc.273.28.17954. [DOI] [PubMed] [Google Scholar]
- GORING DR, ROSSANT J, CLAPOFF S, BREITMAN ML, TSUI LC. In situ detection of β-galactosidase in lenses of transgenic mice with a γ-crystallin/lacZ gene. Science. 1987;235:456–458. doi: 10.1126/science.3099390. [DOI] [PubMed] [Google Scholar]
- GORING DR, BRYCE DM, TSUI LC, BREITMAN ML, LIU Q. Developmental regulation and cell type-specific expression of the murine γF-crystallin gene is mediated through a lens-specific element containing the γF-1 binding site. Dev. Dynam. 1993;196:143–152. doi: 10.1002/aja.1001960208. [DOI] [PubMed] [Google Scholar]
- GOUDREAU G, PETROU P, RENEKER LW, GRAW J, LOSTER J, GRUSS P. Mutually regulated expression of Pax6 and Six3 and its implications for the Pax6 haploinsufficient lens phenotype. Proc. Natl. Acad. Sci. USA. 2002;99:8719–8724. doi: 10.1073/pnas.132195699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRINDLEY JC, DAVIDSON DR, HILL RE. The role of Pax-6 in eye and nasal development. Development. 1995;121:1433–1442. doi: 10.1242/dev.121.5.1433. [DOI] [PubMed] [Google Scholar]
- GRUSS P, WALTHER C. Pax in development. Cell. 1992;69:719–722. doi: 10.1016/0092-8674(92)90281-g. [DOI] [PubMed] [Google Scholar]
- HAYNES JI, DUNCAN MK, PIATIGORSKY J. Spatial and temporal activity of the αB-crystallin/small heat shock protein gene promoter in transgenic mice. Dev. Dynam. 1996;207:75–88. doi: 10.1002/(SICI)1097-0177(199609)207:1<75::AID-AJA8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- HITTNER HM. Aniridia. In: Ritch R, Shields MB, Krupin T, editors. The Glaucomas. The C.V. Mosby Comp; St. Louis: 1989. pp. 869–884. [Google Scholar]
- HORWITZ J. Alpha-crystallin. Exp. Eye Res. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- HUSSAIN MA, HABENER JF. Glucagon gene transcription activation mediated by synergistic interactions of pax-6 and cdx-2 with the p300 co-activator. J. Biol. Chem. 1999;274:28950–28957. doi: 10.1074/jbc.274.41.28950. [DOI] [PubMed] [Google Scholar]
- ILAGAN JG, CVEKL A, KANTOROW M, PIATIGORSKY J, SAX CM. Regulation of αA-crystallin gene expression. J. Biol. Chem. 1999;274:19973–19978. doi: 10.1074/jbc.274.28.19973. [DOI] [PubMed] [Google Scholar]
- IWAKI A, NAGANO T, NAKAGAWA M, IWAKI T, FUKUMAKI Y. Identification and characterization of the gene encoding a new member of the α-crystallin/small hsp family, closely linked to the αB-crystallin gene in a head-to-head manner. Genomics. 1997;45:386–394. doi: 10.1006/geno.1997.4956. [DOI] [PubMed] [Google Scholar]
- JAWORSKI C, SPERBECK S, GRAHAM C, WISTOW G. Alternative splicing of Pax6 in bovine eye and evolutionary conservation of intron sequences. Biochem. Biophys. Res. Commun. 1997;240:196–202. doi: 10.1006/bbrc.1997.7623. [DOI] [PubMed] [Google Scholar]
- JUN S, DESPLAN C. Cooperative interactions between paired domain and homeodomain. Development. 1996;122:2639–2650. doi: 10.1242/dev.122.9.2639. [DOI] [PubMed] [Google Scholar]
- KALOUSOVA A, BENES V, PACES J, PACES V, KOZMIK Z. DNA binding and transactivation properties of the paired and homeobox protein Pax4. Biochem. Biophys. Res. Commun. 1999;259:510–518. doi: 10.1006/bbrc.1999.0809. [DOI] [PubMed] [Google Scholar]
- KAMACHI Y, SOCKANATHAN S, LIU Q, BREITMAN ML, LOVELL-BADGE R, KONDOH H. Involvement of SOX proteins in lens-specific activation of crystallin genes. EMBO J. 1995;14:3510–3519. doi: 10.1002/j.1460-2075.1995.tb07357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMACHI Y, UCHIKAWA M, TANOUCHI A, SEKIDO R, KONDOH H. Pax6 and SOX2 form a co-DNA-binding partner complex that regulates initiation of lens development. Genes Dev. 2001;15:1272–1286. doi: 10.1101/gad.887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMRADT MC, CHEN F, CRYNS VL. The small heat shock protein αB-crystallin negatively regulates cytochrome c- and caspase-9-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J. Biol. Chem. 2001;276:16059–16063. doi: 10.1074/jbc.C100107200. [DOI] [PubMed] [Google Scholar]
- KANTOROW M, CVEKL A, SAX CM, PIATIGORSKY J. Protein-DNA interactions of the mouse αA-crystallin control regions. J. Mol. Biol. 1993a;230:425–435. doi: 10.1006/jmbi.1993.1160. [DOI] [PubMed] [Google Scholar]
- KANTOROW M, BECKER K, SAX CM, OZATO K, PIATIGORSKY J. Binding of tissue-specific forms of αA-CRYBP1 to their regulatory sequence in the mouse aA-crystallin-encoding gene: double-label immunoblotting on UV-crosslinked complexes. Gene. 1993b;131:159–165. doi: 10.1016/0378-1119(93)90289-f. [DOI] [PubMed] [Google Scholar]
- KATAOKA K, HAN S, SHIODA S, HIRAI M, NISHIZAWA M, HANDA H. MafA is a glucose-regulated and pancreatic β-cell-specific transcriptional activator for the insulin gene. J. Biol. Chem. 2002;277:49903–49910. doi: 10.1074/jbc.M206796200. [DOI] [PubMed] [Google Scholar]
- KATO K, SHINOHARA H, KUROBE N, GOTO S, INAGUMA Y, OSHIMA K. Immunoreactive αA-crystallin in rat nonlenticular tissues detected with a sensitive immunoassay method. Biochem. Biophys. Acta. 1991;1080:173–180. doi: 10.1016/0167-4838(91)90146-q. [DOI] [PubMed] [Google Scholar]
- KAWAUCHI S, TAKAHASHI S, NAKAJIMA O, OGINO H, MORITA M, NISHIZAWA M, YASUDA K, YAMAMOTO M. Regulation of lens fiber cell differentiation by transcription factor c-Maf. J. Biol. Chem. 1999;274:19254–19260. doi: 10.1074/jbc.274.27.19254. [DOI] [PubMed] [Google Scholar]
- KIM JI, LI T, HO I-C, GRUSBY MJ, GLIMCHER LH. Requirement for the c-Maf transcription factor in crystallin gene regulation and lens development. Proc. Natl. Acad. Sci. USA. 1999;96:3781–3885. doi: 10.1073/pnas.96.7.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM S-W, CHEONG C, SOHN Y-C, GOO Y-H, OH WJ, PARK JH, JOE SY, KANG H-S, KIM D-K, Kee C, LEE JW, LEE H-W. Multiple developmental defects derived from impaired recruitment of ASC-2 to nuclear receptors in mice: Implication for posterior lenticonus and cataract. Mol. Cell. Biol. 2002;22:8409–8414. doi: 10.1128/MCB.22.24.8409-8414.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEINJAN DA, SEAWRIGHT A, SCHEDL A, QUINLAN RA, DANES S, VAN HEYNINGEN V. Aniridia-associated translocations, DNase hyper-sensitivity, sequence comparison and transgenic analysis redefine the functional domain of PAX6. Hum. Mol. Genet. 2001;10:2049–2059. doi: 10.1093/hmg/10.19.2049. [DOI] [PubMed] [Google Scholar]
- KOROMA B, YANG J, SUNDIN O. The Pax-6 homeobox gene is expressed throughout the corneal and conjunctival epithelia. Invest. Ophthalmol. Vis. Sci. 1997;38:108–120. [PubMed] [Google Scholar]
- KOZMIK Z, KURZBAUER R, DÖRFFLER P, BUSSLINGER M. Alternative splicing of Pax-8 gene transcripts is developmentally regulated and generates isoforms with different transactivation properties. Mol. Cell. Biol. 1993;13:6024–6035. doi: 10.1128/mcb.13.10.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOZMIK Z, CZERNY T, BUSSLINGER M. Alternatively spliced insertions in the paired domain restrict the DNA sequence specificity of Pax6 and Pax8. EMBO J. 1997;16:6793–6803. doi: 10.1093/emboj/16.22.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOZMIK Z, DAUBE M, FREI E, NORMAN B, LOS L, DISHAW LJ, NOLL M, PIATIGORSKY J. Role of Pax genes in eye evolution: A cnidarian PaxB gene uniting Pax2 and Pax6 functions. Dev. Cell. 2003;5:773–785. doi: 10.1016/s1534-5807(03)00325-3. [DOI] [PubMed] [Google Scholar]
- KRALOVA J, CZERNY T, SPANIELOVA, RATAJOVA V, KOZMIK Z. Complex regulatory element within the γE- and γF-crystallin enhancers mediates Pax6 regulation and is required for induction by retinoic acid. Gene. 2002;286:271–282. doi: 10.1016/s0378-1119(02)00425-0. [DOI] [PubMed] [Google Scholar]
- LANG RA. Which factors stimulate lens fiber cell differentiation in vivo? Invest. Ophthalmol. Vis. Sci. 1999;40:3075–3078. [PubMed] [Google Scholar]
- LANG D, EPSTEIN JA. Sox10 and Pax3 physically interact to mediate activation of a conserved c-RET enhancer. Hum. Mol. Genet. 2003;12:937–945. doi: 10.1093/hmg/ddg107. [DOI] [PubMed] [Google Scholar]
- LAUDERDALE JD, WILENSKY JS, OLIVER ER, WALTON DS, GLASER T. 3′ deletions cause aniridia by preventing PAX6 gene expression. Proc. Natl. Acad. Sci. USA. 2000;97:13755–13759. doi: 10.1073/pnas.240398797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LECOIN L, SII-FELICE K, POUPONNOT C, EYCHENE A, FELDER-SCHMITTBUHL M-P. Comparison of maf gene expression patterns during chick embryo development. Gene Express. Patterns. 2003;4:35–46. doi: 10.1016/s1567-133x(03)00152-2. [DOI] [PubMed] [Google Scholar]
- LEE DC, GONZALES P, WISTOW G. ζ-crystallin: a lens-specific promoter and the gene recruitment of an enzyme as a crystallin. J. Mol. Biol. 1994;216:669–678. doi: 10.1006/jmbi.1994.1178. [DOI] [PubMed] [Google Scholar]
- LEFEBVRE T, PLANQUE N, LELEU D, BAILLY M, CAILLET-BOUDIN M-L, SAULE S, MICHALSKI J-C. O-glycosylation of the nuclear forms of Pax-6 products in quail neuroretina cells. J. Cell. Biochem. 2002;85:208–218. [PubMed] [Google Scholar]
- LENGLER J, KRAUSZ E, TOMAREV S, PRESCOTT A, QUINLAN RA, GRAW J. Antagonistic action of Six3 and Prox1 at the γ-crystallin promoter. Nucleic Acids Res. 2001;29:515–526. doi: 10.1093/nar/29.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI J, FARJO R, YOSHIDA S, KERN TS, SWAROOP A, ANDLEY UP. A comprehensive analysis of the expression of crystallins in mouse retina. Mol. Vis. 2003;9:410–419. [PubMed] [Google Scholar]
- LIU Q, TINI M, TSUI L-C, BREITMAN ML. Interaction of a lens cell transcription factor with the proximal domain of the mouse γF-crystallin promoter. Mol. Cell. Biol. 1991;11:1531–1537. doi: 10.1128/mcb.11.3.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Q, SHALABI F, PURI MC, TANG S, BREITMAN ML. Novel zinc finger proteins that interact with the mouse γF-crystallin promoter and are expressed in the sclerotome during early somitogenesis. Dev. Biol. 1994;165:165–177. doi: 10.1006/dbio.1994.1243. [DOI] [PubMed] [Google Scholar]
- LOWEN M, SCOTT G, ZWOLLO P. Functional analyses of two alternative isoforms of transcription factor Pax5. J. Biol. Chem. 2001;276:42575–42574. doi: 10.1074/jbc.M106536200. [DOI] [PubMed] [Google Scholar]
- MAANDAG ECR, VAN DER VALK M, VLAAR M, FELTKAMP C, O’BRIEN J, VAN ROON M, VAN DER LUGT N, BERNS A, TE RIELE H. Developmental rescue of an embryonic-lethal mutation in the retinoblastoma gene in chimeric mice. EMBO J. 1994;13:4260–4268. doi: 10.1002/j.1460-2075.1994.tb06746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANSOURI A, ST-ONGE L, GRUSS P. Role of genes in endoderm-derived organs. Trends Endocrinol. Metab. 1999;10:164–167. doi: 10.1016/s1043-2760(98)00133-7. [DOI] [PubMed] [Google Scholar]
- MAGABO KS, HORWITZ J, PIATIGORSKY J, KANTOROW M. Expression of βB2-crystallin mRNA and protein in retina, brain and testis. Invest. Ophthalmol. Vis. Sci. 2000;41:3056–3060. [PMC free article] [PubMed] [Google Scholar]
- MAKARENKOVA HP, ITO M, GOVINDARAJAN V, FABER SC, SUN L, MAHON G, OVERBEEK PA, LANG RA. FGF10 is an inducer and Pax6 competence factor for lacrimal gland development. Development. 2000;127:2563–2572. doi: 10.1242/dev.127.12.2563. [DOI] [PubMed] [Google Scholar]
- MARQUARDT T, ASHERY-PADAN R, ANDREJEWSKI N, SCARDIGLI R, GUILLEMONT F, GRUSS P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105:43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- MATSUO T, OSUMI-YAMASHITA N, NOJI S, OGUCHI H, KOYAMA E, MYOKAI F, MATSUO N, TANIGUCHI S, DOI H, ISEKI S. A mutation in the Pax-6 gene in rat small eye is associated with impaired migration of midbrain neural crest cells. Nature Genet. 1993;3:299–304. doi: 10.1038/ng0493-299. [DOI] [PubMed] [Google Scholar]
- McCAFFREY J, YAMASAKI L, DYSON NJ, HARLOW E, GRIEP AE. Disruption of retinoblastoma protein family function by human papillomavirus type 16 E7 oncoprotein inhibits lens development in part through E2F-1. Mol. Cell. Biol. 1999;19:6458–6468. doi: 10.1128/mcb.19.9.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIKKOLA I, BRUUN J-A, HOLM T, JOHANSEN T. Superactivation of Pax6-mediated transactivation from paired domain-binding sites by DNA-independent recruitment of different homeodomain proteins. J. Biol. Chem. 2001a;276:4109–4118. doi: 10.1074/jbc.M008882200. [DOI] [PubMed] [Google Scholar]
- MIKKOLA I, BRUUN J-A, BJORKOY G, HOLM T, JOHANSEN T. Phosphorylation of the transactivation domain of Pax6 by extracellular signal-regulated kinase and p38 mitogen-activated protein kinase. J. Biol. Chem. 2001b;276:15115–15126. doi: 10.1074/jbc.274.21.15115. [DOI] [PubMed] [Google Scholar]
- MISHRA R, GORLOW IP, CHAO LY, SINGH S, SAUNDERS GF. PAX6, paired domain influences sequence recognition by the homeodomain. J. Biol. Chem. 2002;277:49488–49494. doi: 10.1074/jbc.M206478200. [DOI] [PubMed] [Google Scholar]
- MORGENBESSER SD, WILLIAMS BO, JACKS T, DePINHO RA. p53-dependent apoptosis produced by Rb-deficiency in the developing mouse lens. Nature. 1994;371:72–74. doi: 10.1038/371072a0. [DOI] [PubMed] [Google Scholar]
- MUTA M, KAMACHI Y, YOSHIMOTO A, HIGASHI Y, KONDOH H. Distinct roles of SOX2, Pax6 and Maf transcription factors in the regulation of lens-specific δ1-crystallin enhancer. Genes Cells. 2002;7:791–805. doi: 10.1046/j.1365-2443.2002.00560.x. [DOI] [PubMed] [Google Scholar]
- NAKAMURA T, DONOVAN DM, HAMADA K, SAX CM, NORMAN B, FLANAGAN JR, OZATO K, WESTPHAL H, PIATIGORSKY J. Regulation of the mouse αA-crystallin gene: isolation of a cDNA encoding a protein that binds to a cis-sequence motif shared with the major histocompatibility complex class I gene and other genes. Mol. Cell. Biol. 1989;10:3700–3708. doi: 10.1128/mcb.10.7.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON LB, SPAETH GL, NOWINSKI TS, MARGO CE, JACKSON L. Aniridia. A review. Surv. Ophthalmol. 1984;28:621–642. doi: 10.1016/0039-6257(84)90184-x. [DOI] [PubMed] [Google Scholar]
- NISHIGUCHI S, WOOD H, KONDOH H, LOVELL-BADGE R, EPISKOPOU V. Sox1 directly regulates the γ-crystallin genes and is essential for lens development in mice. Genes Dev. 1998;12:776–781. doi: 10.1101/gad.12.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGINO H, YASUDA K. Induction of lens differentiation by activation of a bZIP transcription factor, L-Maf. Science. 1998;280:115–118. doi: 10.1126/science.280.5360.115. [DOI] [PubMed] [Google Scholar]
- OLBROT M, RUD J, MOSS LG, SHARMA A. Identification of β-cell-specific insulin gene transcription factor RIPE3b1 as mammalian MafA. Proc. Natl. Acad. Sci. USA. 2002;99:6737–6742. doi: 10.1073/pnas.102168499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OVERBEEK PA, CHEPELINSKY AB, KHILLAN JS, PIATIGORSKY J, WESTPHAL H. Lens-specific expression and developmental regulation of the bacterial chloramphenicol acetyltransferase gene driven by the murine αA-crystallin promoter in transgenic mice. Proc. Natl. Acad. Sci. USA. 1985;82:7815–7819. doi: 10.1073/pnas.82.23.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEEK R, VAN DER LOGT P, LUBSEN NH, SCHOENMAKERS JGG. Tissue- and species-specific promoter elements of rat γ-crystallin genes. Nucleic Acids Res. 1990;18:1189–1197. doi: 10.1093/nar/18.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIATIGORSKY J. Gene expression and genetic engineering in the lens. Invest. Ophthalmol. Vis. Sci. 1987;28:9–28. [PubMed] [Google Scholar]
- PIATIGORSKY J. Lens crystallins. Innovation associated with changes in gene regulation. J. Biol. Chem. 1992;267:4277–4280. [PubMed] [Google Scholar]
- PIATIGORSKY J. Crystallin genes: specialization by changes in gene regulation may precede gene duplication. J. Struct. Funct. Genom. 2003;3:131–137. [PubMed] [Google Scholar]
- PIATIGORSKY J, KOZMIK Z. Cubozoan Jellyfish: An Evo/Devo Model for Eyes and Other Sensory Systems. Int. J. Dev. Biol. 2004;48 doi: 10.1387/ijdb.041851jp. this issue. [DOI] [PubMed] [Google Scholar]
- PLANQUE N, LECONTE L, COQUELLE FM, MARTIN P, SAULE S. Specific Pax-6/microphthalmia transcription factor involve their DNA-binding domains and inhibit transcriptional properties of both proteins. J. Biol. Chem. 2001a;276:29330–29337. doi: 10.1074/jbc.M101812200. [DOI] [PubMed] [Google Scholar]
- PLANQUE N, LECONTE L, COQUELLE FM, BENKHELIFA S, MARTIN P, FELDER-SCHMITTBUHL MP, SAULE S. Interaction of Maf transcription factors with Pax-6 results in synergistic activation of the glucagon promoter. J. Biol. Chem. 2001b;276:35751–35760. doi: 10.1074/jbc.M104523200. [DOI] [PubMed] [Google Scholar]
- PLAZA S, DOZIER C, SAULE S. Quail Pax-6 (Pax-QNR) encodes a transcription factor able to bind and trans-activate its own promoter. Cell Growth Differ. 1993;4:1041–1050. [PubMed] [Google Scholar]
- PROSSER J, VAN HEYNINGEN V. PAX6 mutations revisited. Hum. Mut. 1998;11:93–108. doi: 10.1002/(SICI)1098-1004(1998)11:2<93::AID-HUMU1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- QUINN JC, WEST JD, HILL RE. Multiple functions for Pax6 in mouse eye and nasal development. Genes Dev. 1996;10:435–446. doi: 10.1101/gad.10.4.435. [DOI] [PubMed] [Google Scholar]
- RAMPALLI AM, GAO CY, CHAUTHAIWALE, ZELENKA PS. pRb and p107 regulate E2F activity during lens fiber cell differentiation. Oncogene. 1998;16:399–408. doi: 10.1038/sj.onc.1201546. [DOI] [PubMed] [Google Scholar]
- REZA HM, OGINO H, YASUDA K. L-Maf, a downstream target of Pax6, is essential for chick lens development. Mech. Dev. 2002;116:61–73. doi: 10.1016/s0925-4773(02)00137-5. [DOI] [PubMed] [Google Scholar]
- RICHARDSON J, CVEKL A, WISTOW G. Pax-6 is essential for lens-specific expression of ζ-crystallin. Proc. Natl. Acad. Sci. USA. 1995;92:4676–4680. doi: 10.1073/pnas.92.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RING BZ, CORDES SP, OVERBEEK PA, BARSH GS. Regulation of mouse lens fiber cell development and differentiation by the Maf gene. Development. 2000;127:307–317. doi: 10.1242/dev.127.2.307. [DOI] [PubMed] [Google Scholar]
- ROBINSON ML, OVERBEEK PA. Differential expression of αA- and αB-crystallin during murine ocular development. Invest. Ophthalmol. Vis. Sci. 1996;37:2276–2284. [PubMed] [Google Scholar]
- SAKAI M, SERRIA MS, IKEDA H, YOSHIDA K, IMAKI J, NISHI S. Regulation of c-maf gene expression by Pax6 in cultured cells. Nucleic Acids Res. 2001;29:1228–1237. doi: 10.1093/nar/29.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALE MM, CRAIG JE, CHARLESWORTH JC, FITZGERALD LM, HANSON IM, DICKINSON JL, MATTHEW SJ, VAN HEYNINGEN V, FINGERT JH, MACKEY DA. Broad phenotypic variability in a single pedigree with a novel 1410delC mutation in the PST domain of the PAX6 gene. Hum. Mut. 2002;20:322. doi: 10.1002/humu.9066. [DOI] [PubMed] [Google Scholar]
- SAX CM, ILAGAN J, PIATIGORSKY J. Functional redundancy of the DE1 and αA-CRYBP1 regulatory sites of the mouse αA-crystallin promoter. Nucleic Acids Res. 1993;21:2633–2640. doi: 10.1093/nar/21.11.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAX CM, PIATIGORSKY J. Expression of the α-crystallin/small heat shock protein /molecular chaperone genes in the lens and other tissues. Adv. Enzymol. Relat. Areas Mol. Biol. 1994;69:155–201. doi: 10.1002/9780470123157.ch5. [DOI] [PubMed] [Google Scholar]
- SCHEDL A, ROSS A, LEE M, ENGELKAMP D, RASHBASS P, VAN HEYNINGEN V, HASTIE N. Influence of Pax6 gene dosage on development: overexpression causes severe eye abnormalities. Cell. 1996;86:71–82. doi: 10.1016/s0092-8674(00)80078-1. [DOI] [PubMed] [Google Scholar]
- SCHWARZ M, CECCONI F, BERNIER G, ANDREJEWSKI N, KAMMANDEL B, WAGNER M, GRUSS P. Spatial specification of mammalian eye territories by reciprocal transcriptional repression of Pax2 and Pax6. Development. 2000;127:4325–4333. doi: 10.1242/dev.127.20.4325. [DOI] [PubMed] [Google Scholar]
- SEKIDO R, MURAI K, FUNAHASHI J, MAMACHI Y, FUJISAWA-SCHARA A, NABESHIMA Y, KONDOH H. The δ-crystallin enhancer-binding protein δEF1 is a repressor of E2-box-mediated gene activation. Mol. Cell. Biol. 1994;14:5692–5700. doi: 10.1128/mcb.14.9.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELLERS WR, NOVITCH BG, MIYAKE S, HEITH A, OTTERSON GA, KAYE FJ, LASSAR AB, KAELIN WG., Jr. Stable binding to E2F is not required for the retinoblastoma protein to activate transcription, promote differentiation and suppress tumor cell growth. Genes Dev. 1998;12:95–106. doi: 10.1101/gad.12.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEMINA EV, BROWNELL I, MINTZ-HITTNER HA, MURRAY JC, JAMRICH M. Mutations in the human forkhead transcription factor FOXE3 associated with anterior segment ocular dysgenesis and cataracts. Hum. Mol. Genet. 2001;10:231–236. doi: 10.1093/hmg/10.3.231. [DOI] [PubMed] [Google Scholar]
- SHARON-FRILING R, RICHARDSON J, SPERBECK S, LEE D, RAUCHMAN M, MAAS R, SWAROOP A, WISTOW G. Lens-specific gene recruitment of ζ-crystallin through Pax6, Nrl-Maf and brain suppressor sites. Mol. Cell. Biol. 1998;18:2067–2076. doi: 10.1128/mcb.18.4.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMPSON TI, PRICE DJ. Pax6: a pleiotropic player in development. BioEssays. 2002;24:1041–1051. doi: 10.1002/bies.10174. [DOI] [PubMed] [Google Scholar]
- SINGH S, TANG HK, LEE J-Y, SAUNDERS GF. Truncation mutations in the transactivation region of PAX6 result in dominant-negative mutants. J. Biol. Chem. 1998;273:21531–21541. doi: 10.1074/jbc.273.34.21531. [DOI] [PubMed] [Google Scholar]
- SINGH S, CHAO LY, MISHRA R, DAVIES J, SAUNDERS GF. Missense mutation at the C-terminus of PAX6 negatively modulates homeodomain function. Hum. Mol. Genet. 2001;10:911–918. doi: 10.1093/hmg/10.9.911. [DOI] [PubMed] [Google Scholar]
- SINGH S, MISHRA R, ARANGO NA, DENG JM, BEHRINGER RR, SAUNDERS GF. Iris hypoplasia in mice that lack the alternatively spliced Pax6(5a) isoform. Proc. Natl. Acad. Sci. USA. 2002;99:6812–6815. doi: 10.1073/pnas.102691299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISODIYA SM, FREE SL, WILIAMSON KA, MITCHELL TN, WILIS C, STEVENS JM, KENDALL BE, SHORVON SD, HANSON IM, MOORE AT, VAN HEYNINGEN V. PAX6 haploinsufficiency causes cerebral malformation and olfactory dysfunction in humans. Nature Genet. 2001;28:214–216. doi: 10.1038/90042. [DOI] [PubMed] [Google Scholar]
- SIVAK JM, WEST-MAYS JA, YEE A, WILLIAMS T, FINI ME. Transcription factor pax6 and AP-2α interact to coordinate corneal epithelial repair by controlling expression of matrix metalloproteinase gelatinase B. Mol. Cell. Biol. 2004;24:245–257. doi: 10.1128/MCB.24.1.245-257.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMASUNDARAM T, BHAT SP. Canonical heat shock element in the αB-crystallin gene shows tissue-specific and developmentally controlled interactions with heat shock factor. J. Biol. Chem. 2000;275:17154–17159. doi: 10.1074/jbc.M000304200. [DOI] [PubMed] [Google Scholar]
- SOMMER B, CHEPELINSKY AB, PIATIGORSKY J. Binding of nuclear proteins to promoter elements of the mouse αA-crystallin gene. J. Biol. Chem. 1988;263:15666–15672. [PubMed] [Google Scholar]
- SRINIVASAN AN, NAGINENI CN, BHAT SP. αA-crystallin is expressed in non-ocular tissues. J. Biol. Chem. 1992;267:23337–23341. [PubMed] [Google Scholar]
- ST. ONGE L, SOSA-PENEDA B, CHOWDURY K, GRUSS P. Pax6 is required for differentiation of glucagon producing β-cells in mouse pancreas. Nature. 1997;387:406–409. doi: 10.1038/387406a0. [DOI] [PubMed] [Google Scholar]
- SWAMYNATHAN SK, PIATIGORSKY J. Orientation-dependent influence of an intragenic enhancer on the promoter activity of the divergently transcribed mouse Shsp/αB-crystallin and Mkbp/HspB2 genes. J. Biol. Chem. 2002;277:49700–49706. doi: 10.1074/jbc.M209700200. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI Y, HANAOKA K, HAYSAKA M, KATOH K, KATO Y, OKADA TS, KONDOH H. Embryonic stem cell-mediated transfer and correct regulation of the chicken θ-crystallin gene in developing mouse embryos. Development. 1988;102:259–269. doi: 10.1242/dev.102.2.259. [DOI] [PubMed] [Google Scholar]
- TAMM ER, RUSSELL P, JOHNSON DH, PIATIGORSKY J. Human and monkey trabecular meshwork accumulate αB-crystallin in response to heat shock and oxidative stress. Invest. Ophthalmol. Vis. Sci. 1996;37:2402–2413. [PubMed] [Google Scholar]
- TANAKA T, TSUJIMURA T, TAKEDA K, SUGIHARA A, MAEKAWA A, TERADA N, YOSHIDA N, AKIRA S. Targeted disruption of ATF4 discloses its essential role in the formation of the eye lens fibres. Genes Cells. 1998;3:801–810. doi: 10.1046/j.1365-2443.1998.00230.x. [DOI] [PubMed] [Google Scholar]
- TANG HK, SINGH S, SAUNDERS GF. Dissection of the transactivation function of the transcription factor encoded by the eye developmental gene PAX6. J. Biol. Chem. 1998;273:7210–7221. doi: 10.1074/jbc.273.13.7210. [DOI] [PubMed] [Google Scholar]
- TINI M, OTULAKOWSKI G, BREITMAN ML, TSUI L-C, GIGUERE V. An everted repeat mediates retinoic acid induction of the γF-crystallin gene: evidence of a direct role for retinoids in lens development. Genes Dev. 1993;7:295–307. doi: 10.1101/gad.7.2.295. [DOI] [PubMed] [Google Scholar]
- TINI M, FRASER RA, GIGUERE V. Functional interactions between retinoic acid receptor-related orphan nuclear receptor (RORα) and the retinoic acid receptors in the regulation of the γF-crystallin promoter. J. Biol. Chem. 1995;270:20156–20161. doi: 10.1074/jbc.270.34.20156. [DOI] [PubMed] [Google Scholar]
- VAN HEYNINGEN V, WILLIAMSON KA. PAX6 in sensory development. Hum. Mol. Genet. 2002;11:1161–1167. doi: 10.1093/hmg/11.10.1161. [DOI] [PubMed] [Google Scholar]
- VAN RAAMSDONK CD, TILGHMAN SM. Dosage requirement and allelic expression of PAX6 during lens placode formation. Development. 2000;127:5439–5448. doi: 10.1242/dev.127.24.5439. [DOI] [PubMed] [Google Scholar]
- VAN RENS GL, DE JONG WW, BLUMEDAHL H. One member of the γ-crystallin family, γS, is expressed in birds. Exp. Eye Res. 1991;53:135–138. doi: 10.1016/0014-4835(91)90156-9. [DOI] [PubMed] [Google Scholar]
- VOGAN KJ, UNDERHILL DA, Gros P. An alternative splicing event in the Pax-3 paired domain identifies the linker region as a key determinant of paired domain DNA-binding activity. Mol. Cell. Biol. 1996;16:6677–6686. doi: 10.1128/mcb.16.12.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALTHER C, GRUSS P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- WAWROUSEK EF, CHEPELINSKY AB, MCDERMOTT JB, PIATIGORSKY J. Regulation of the murine αA-crystallin promoter in transgenic mice. Dev. Biol. 1990;137:68–76. doi: 10.1016/0012-1606(90)90008-7. [DOI] [PubMed] [Google Scholar]
- WEINMANN AS, FARNHAM PJ. Identification of unknown target genes of human transcription factors using chromatin immunoprecipitations. Methods. 2002;26:37–47. doi: 10.1016/S1046-2023(02)00006-3. [DOI] [PubMed] [Google Scholar]
- WIGGAN O, TANIGUCHI-SIDLE A, HAMEL PA. Interaction of the pRB-family proteins with factors containing paired-like homeodomains. Oncogene. 1998;16:227–236. doi: 10.1038/sj.onc.1201534. [DOI] [PubMed] [Google Scholar]
- WIGLE JT, CHOWDHURY K, GRUSS P, OLIVER G. Prox1 function is critical for mouse lens-fibre elongation. Nat. Genet. 1999;21:318–322. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]
- WILLIAMS BO, SCHMITT EM, REMINGTON L, BRONSON RT, ALBERT DM, WEINBERG RA, JACKS T. Extensive contribution of Rb-deficient cells to adult chimeric mice with limited histopathological consequences. EMBO J. 1994;13:4251–4259. doi: 10.1002/j.1460-2075.1994.tb06745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON DS, SHENG G, LECUIT T, DOSTATNI N, DESPLAN C. Cooperative dimerization of paired class homeo domains on DNA. Genes Dev. 1993;7:2120–2134. doi: 10.1101/gad.7.11.2120. [DOI] [PubMed] [Google Scholar]
- WILSON DS, GUENTHER B, DESPLAN C, KURIYAN J. High resolution crystal structure of a paired (Pax) class cooperative homeodomain dimer on DNA. Cell. 1995;82:709–719. doi: 10.1016/0092-8674(95)90468-9. [DOI] [PubMed] [Google Scholar]
- WISTOW GJ. Molecular biology and evolution of crystallins: gene recruitment and multifunctional proteins in the eye lens. Springer-Verlag; Heidelberg: 1995. [Google Scholar]
- XU HE, ROULD MA, XU W, EPSTEIN JA, MAAS RL, PABO CO. Crystal structure of the human Pax6 paired domain-DNA complex reveals specific roles for the linker region and carboxy-terminal subdomain in DNA binding. Genes Dev. 1999;13:1263–1275. doi: 10.1101/gad.13.10.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAGUCHI Y, SAWADA J, YAMADA M, HANDA H, AZUMA N. Autoregulation of Pax6 transcriptional activation by two distinct DNA-binding subdomains of the paired domain. Genes Cells. 1997;2:255–261. doi: 10.1046/j.1365-2443.1997.1170315.x. [DOI] [PubMed] [Google Scholar]
- YANG Y, CAUHAN BK, CVEKLOVA K, CVEKL A. Transcriptional regulation of mouse αB- and γF-crystallin genes in lens: Dual promoter-specific functional interactions between Pax6 and large Maf transcription factors. J. Mol. Biol. 2004;344 doi: 10.1016/j.jmb.2004.07.102. in press. [DOI] [PubMed] [Google Scholar]
- YU CC-K, TSUI L-C, BREITMAN ML. Homologous and heterologous enhancers modulate spatial expression but not cell-type specificity of the murine γF-crystallin promoter. Development. 1990;110:131–139. doi: 10.1242/dev.110.1.131. [DOI] [PubMed] [Google Scholar]
- ZANIOLO K, LECLERC S, CVEKL A, VALLIERES L, BAZIN R, LAROUCHE K, GUERIN SL. Expression of the αθ4 integrin subunit gene promoter is modulated by the transcription factor Pax-6 in corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 2004;45:1692–1704. doi: 10.1167/iovs.03-0908. [DOI] [PubMed] [Google Scholar]
- ZHANG W, CVEKLOVA K, OPPERMANN B, KANTOROW M, CVEKL A. Quantitation of PAX6 and PAX6(5A) transcript levels on adult human lens, cornea and monkey retina. Mol. Vis. 2001;7:1–5. [PMC free article] [PubMed] [Google Scholar]
- ZHU CC, DYER MA, UCHIKAWA M, KONDOH H, LAGUTIN O, OLIVER G. Six3-mediated auto repression and eye development requires its interaction with members of the Groucho-related family of co-repressors. Development. 2002;129:2835–2849. doi: 10.1242/dev.129.12.2835. [DOI] [PubMed] [Google Scholar]


