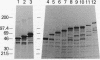
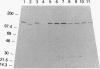
Abstract
The DNA sequence of the central regulatory locus vir of Bordetella pertussis predicts that three gene products, BvgA, BvgB, and BvgC, are encoded. Features of the predicted primary structures of these proteins and their homology to other two-component systems suggest that BvgA is located in the cytoplasm, BvgB is located in the periplasm, and BvgC spans the inner membrane. We have used gene fusions to the phoA and lacZ genes of Escherichia coli to investigate the subcellular localization and membrane topology of these proteins. PhoA fusion proteins were also purified and used to raise antibodies that allowed visualization of the vir-encoded polypeptides by Western immunoblotting. Our results have largely confirmed the predictions of the DNA sequence, with the exception that BvgB and BvgC were found to constitute one larger protein that was homologous to the sensor class of two-component systems. We propose that this protein be named BvgS (for sensor) and that its gene be named bvgS.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aricó B., Miller J. F., Roy C., Stibitz S., Monack D., Falkow S., Gross R., Rappuoli R. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6671–6675. doi: 10.1073/pnas.86.17.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong S. K., Parker C. D. Surface proteins of Bordetella pertussis: comparison of virulent and avirulent strains and effects of phenotypic modulation. Infect Immun. 1986 Nov;54(2):308–314. doi: 10.1128/iai.54.2.308-314.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri J. T., Moloney B. K., Mende-Mueller L. M. Expression and secretion of the S-1 subunit and C180 peptide of pertussis toxin in Escherichia coli. J Bacteriol. 1989 Aug;171(8):4362–4369. doi: 10.1128/jb.171.8.4362-4369.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black W. J., Falkow S. Construction and characterization of Bordetella pertussis toxin mutants. Infect Immun. 1987 Oct;55(10):2465–2470. doi: 10.1128/iai.55.10.2465-2470.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bourret R. B., Hess J. F., Borkovich K. A., Pakula A. A., Simon M. I. Protein phosphorylation in chemotaxis and two-component regulatory systems of bacteria. J Biol Chem. 1989 May 5;264(13):7085–7088. [PubMed] [Google Scholar]
- Brennan M. J., Li Z. M., Cowell J. L., Bisher M. E., Steven A. C., Novotny P., Manclark C. R. Identification of a 69-kilodalton nonfimbrial protein as an agglutinogen of Bordetella pertussis. Infect Immun. 1988 Dec;56(12):3189–3195. doi: 10.1128/iai.56.12.3189-3195.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlie R. M., Coote J. G., Parton R. Adenylate cyclase activity during phenotypic variation of Bordetella pertussis. J Gen Microbiol. 1985 Jan;131(1):27–38. doi: 10.1099/00221287-131-1-27. [DOI] [PubMed] [Google Scholar]
- Charles I. G., Dougan G., Pickard D., Chatfield S., Smith M., Novotny P., Morrissey P., Fairweather N. F. Molecular cloning and characterization of protective outer membrane protein P.69 from Bordetella pertussis. Proc Natl Acad Sci U S A. 1989 May;86(10):3554–3558. doi: 10.1073/pnas.86.10.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson B. T., Berg D. E., Goldman W. E. Mutagenesis of Bordetella pertussis with transposon Tn5tac1: conditional expression of virulence-associated genes. J Bacteriol. 1990 Apr;172(4):1681–1687. doi: 10.1128/jb.172.4.1681-1687.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRita V. J., Mekalanos J. J. Periplasmic interaction between two membrane regulatory proteins, ToxR and ToxS, results in signal transduction and transcriptional activation. Cell. 1991 Jan 11;64(1):29–37. doi: 10.1016/0092-8674(91)90206-e. [DOI] [PubMed] [Google Scholar]
- Ditta G., Schmidhauser T., Yakobson E., Lu P., Liang X. W., Finlay D. R., Guiney D., Helinski D. R. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid. 1985 Mar;13(2):149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Gennity J., Goldstein J., Inouye M. Signal peptide mutants of Escherichia coli. J Bioenerg Biomembr. 1990 Jun;22(3):233–269. doi: 10.1007/BF00763167. [DOI] [PubMed] [Google Scholar]
- Gross R., Rappuoli R. Pertussis toxin promoter sequences involved in modulation. J Bacteriol. 1989 Jul;171(7):4026–4030. doi: 10.1128/jb.171.7.4026-4030.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp S., Mekalanos J. J. Two trans-acting regulatory genes (vir and mod) control antigenic modulation in Bordetella pertussis. J Bacteriol. 1988 Nov;170(11):5059–5066. doi: 10.1128/jb.170.11.5059-5066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laoide B. M., Ullmann A. Virulence dependent and independent regulation of the Bordetella pertussis cya operon. EMBO J. 1990 Apr;9(4):999–1005. doi: 10.1002/j.1460-2075.1990.tb08202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Li P., Inouye H., Brickman E. R., Beckwith J. Genetic studies on the inability of beta-galactosidase to be translocated across the Escherichia coli cytoplasmic membrane. J Bacteriol. 1989 Sep;171(9):4609–4616. doi: 10.1128/jb.171.9.4609-4616.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Mekalanos J. J., Beckwith J. Alkaline phosphatase fusions: sensors of subcellular location. J Bacteriol. 1990 Feb;172(2):515–518. doi: 10.1128/jb.172.2.515-518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton A. R., Weiss A. A. Environmental regulation of expression of virulence determinants in Bordetella pertussis. J Bacteriol. 1989 Nov;171(11):6206–6212. doi: 10.1128/jb.171.11.6206-6212.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monack D. M., Arico B., Rappuoli R., Falkow S. Phase variants of Bordetella bronchiseptica arise by spontaneous deletions in the vir locus. Mol Microbiol. 1989 Dec;3(12):1719–1728. doi: 10.1111/j.1365-2958.1989.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Monack D., Munoz J. J., Peacock M. G., Black W. J., Falkow S. Expression of pertussis toxin correlates with pathogenesis in Bordetella species. J Infect Dis. 1989 Feb;159(2):205–210. doi: 10.1093/infdis/159.2.205. [DOI] [PubMed] [Google Scholar]
- Ronson C. W., Nixon B. T., Ausubel F. M. Conserved domains in bacterial regulatory proteins that respond to environmental stimuli. Cell. 1987 Jun 5;49(5):579–581. doi: 10.1016/0092-8674(87)90530-7. [DOI] [PubMed] [Google Scholar]
- Roy C. R., Miller J. F., Falkow S. Autogenous regulation of the Bordetella pertussis bvgABC operon. Proc Natl Acad Sci U S A. 1990 May;87(10):3763–3767. doi: 10.1073/pnas.87.10.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy C. R., Miller J. F., Falkow S. The bvgA gene of Bordetella pertussis encodes a transcriptional activator required for coordinate regulation of several virulence genes. J Bacteriol. 1989 Nov;171(11):6338–6344. doi: 10.1128/jb.171.11.6338-6344.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Scarlato V., Prugnola A., Aricó B., Rappuoli R. Positive transcriptional feedback at the bvg locus controls expression of virulence factors in Bordetella pertussis. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6753–6757. doi: 10.1073/pnas.87.17.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira S. K., Chou J., Richaud F. V., Casadaban M. J. New versatile plasmid vectors for expression of hybrid proteins coded by a cloned gene fused to lacZ gene sequences encoding an enzymatically active carboxy-terminal portion of beta-galactosidase. Gene. 1983 Nov;25(1):71–82. doi: 10.1016/0378-1119(83)90169-5. [DOI] [PubMed] [Google Scholar]
- Stark M. J. Multicopy expression vectors carrying the lac repressor gene for regulated high-level expression of genes in Escherichia coli. Gene. 1987;51(2-3):255–267. doi: 10.1016/0378-1119(87)90314-3. [DOI] [PubMed] [Google Scholar]
- Stibitz S., Black W., Falkow S. The construction of a cloning vector designed for gene replacement in Bordetella pertussis. Gene. 1986;50(1-3):133–140. doi: 10.1016/0378-1119(86)90318-5. [DOI] [PubMed] [Google Scholar]
- Stibitz S., Weiss A. A., Falkow S. Genetic analysis of a region of the Bordetella pertussis chromosome encoding filamentous hemagglutinin and the pleiotropic regulatory locus vir. J Bacteriol. 1988 Jul;170(7):2904–2913. doi: 10.1128/jb.170.7.2904-2913.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock A. M., Mottonen J. M., Stock J. B., Schutt C. E. Three-dimensional structure of CheY, the response regulator of bacterial chemotaxis. Nature. 1989 Feb 23;337(6209):745–749. doi: 10.1038/337745a0. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Falkow S. Genetic analysis of phase change in Bordetella pertussis. Infect Immun. 1984 Jan;43(1):263–269. doi: 10.1128/iai.43.1.263-269.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983 Oct;42(1):33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems R., Paul A., van der Heide H. G., ter Avest A. R., Mooi F. R. Fimbrial phase variation in Bordetella pertussis: a novel mechanism for transcriptional regulation. EMBO J. 1990 Sep;9(9):2803–2809. doi: 10.1002/j.1460-2075.1990.tb07468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]