Abstract
Accurate chromosome segregation is controlled by the spindle checkpoint, which senses kinetochore– microtubule attachments and tension across sister kinetochores. An important step in the tension-signaling pathway involves the phosphorylation of an unknown protein by polo-like kinase 1/Xenopus laevis polo-like kinase 1 (Plx1) on kinetochores lacking tension to generate the 3F3/2 phosphoepitope. We report here that the checkpoint protein BubR1 interacts with Plx1 and that phosphorylation of BubR1 by Plx1 generates the 3F3/2 epitope. Formation of the BubR1 3F3/2 epitope by Plx1 requires a prior phosphorylation of BubR1 on Thr 605 by cyclin-dependant kinase 1 (Cdk1). This priming phosphorylation of BubR1 by Cdk1 is required for checkpoint-mediated mitotic arrest and for recruitment of Plx1 and the checkpoint protein Mad2 to unattached kinetochores. Biochemically, formation of the 3F3/2 phosphoepitope by Cdk1 and Plx1 greatly enhances the kinase activity of BubR1. Thus, Cdk1-mediated phosphorylation of BubR1 controls checkpoint arrest and promotes the formation of the kinetochore 3F3/2 epitope.
Introduction
Mitosis is controlled by precise temporal and spatial regulation of protein kinases and ubiquitin ligases (Murray, 2004). The activity of the Cdk1/cyclin B kinase is activated at mitotic entry and remains high until anaphase onset, during which destruction of cyclin B, mediated by the anaphase-promoting complex/cyclosome (APC/C), inactivates Cdk1. The timing of APC/C activation and subsequent chromosome segregation is controlled by the spindle checkpoint, which delays the onset of anaphase until all chromosomes are properly aligned at the metaphase plate. The absence of microtubule attachment or tension at kinetochores activates the checkpoint and arrests cells in mitosis. Protein phosphorylation mediated by checkpoint kinases, such as monopolar spindle 1 (Mps1), Aurora B, Bub1, BubR1, and polo-like kinase 1 (Plk1), plays an essential role in checkpoint signaling on kinetochores. In fact, unattached or untense kinetochores are hyperphosphorylated (Gorbsky and Ricketts, 1993; Nicklas et al., 1998), and the lack of tension promotes the formation of a kinetochore phosphoepitope recognized by the 3F3/2 monoclonal antibody (Cyert et al., 1988). We and others have recently identified Plk1/Xenopus laevis polo-like kinase 1 (Plx1) as the 3F3/2 kinase (Ahonen et al., 2005; Wong and Fang, 2005). Despite the extensive efforts of over a decade, the molecular identity of the kinetochore protein harboring the physiological 3F3/2 epitope remains unknown. We report here that the checkpoint protein BubR1 is the kinetochore 3F3/2 antigen phosphorylated by Plx1. Furthermore, we show that formation of the BubR1 3F3/2 epitope requires a priming phosphorylation by Cdk1/cyclin B on Thr 605 and that this phosphorylation is required for checkpoint arrest.
Results and discussion
BubR1 is a biochemical 3F3/2 antigen that interacts with Plx1
Our previous work on kinetochore assembly suggests BubR1 as a potential 3F3/2 antigen (Wong and Fang, 2006). To test this possibility, we immunoprecipitated BubR1 from X. laevis meiotic metaphase (cytostatic factor [CSF] arrested) and spindle checkpoint extracts (CSF extracts with addition of sperm chromosomes and nocodazole), followed by Western blotting with the 3F3/2 antibody. BubR1 purified from both extracts reacted with the 3F3/2 antibody to a similar extent, whereas treatment with λ-phosphatase removed the 3F3/2 signals (Fig. 1 A). Thus, BubR1 is a 3F3/2 antigen whose phosphorylation occurs before checkpoint activation. As Plk1/Plx1, which usually interacts with its substrates (Barr et al., 2004), is the 3F3/2 kinase (Ahonen et al., 2005; Wong and Fang, 2005), we tested whether BubR1 associates with Plx1. Indeed, endogenous Plx1 coimmunoprecipitated with BubR1 (Fig. 1 B), and recombinant polo box domain (PBD) from Plx1 also binds to endogenous BubR1 in extracts (unpublished data).
Figure 1.
Cdk1- and Plx1-mediated phosphorylation of BubR1 generated the 3F3/2 epitope. (A) BubR1 was immunoprecipitated (IP) from either checkpoint extracts (lanes 1 and 2) or CSF extracts (lanes 3 and 4) and treated with (lanes 1 and 4) or without (lanes 2 and 3) λ-phosphatase. Control immunoprecipitation was performed in CSF extracts using nonspecific rabbit IgG (lane 5). Samples were assayed by SDS-PAGE, and identical regions of the duplicated blots were analyzed by anti-3F3/2 and -BubR1 antibodies. (B) Immunoprecipitates of control or BubR1 antibodies from CSF extracts were analyzed by Western blotting with anti-Plx1 and -BubR1 antibodies. (C and D) Chromosomes purified from checkpoint extracts were dephosphorylated by λ-phosphatase, treated with NEM, and subsequently rephosphorylated (ReP) by incubation with ATP alone or ATP plus Plx1 and/or Cdk1/cyclin B. Phosphorylated chromosomes were stained for the indicated antigens (C), and mean fluorescence intensities of kinetochore 3F3/2 signals (n = 20 kinetochores) were quantified and normalized to the corresponding value derived from samples rephosphorylated by both Plx1 and Cdk1/cyclin B (D). Error bars represent SEM. Bar, 5 μm. (E) Recombinant GST-BubR1 and GST-BubR1-T605A were first incubated at 25°C for 75 min, with or without Cdk1/cyclin B, in the presence of 0.2 mM of unlabeled ATP. Samples were then split equally and incubated for 40 min in the presence of purvalanol A with γ-[32P]ATP alone (middle) or γ-[32P]ATP plus Plx1 (top). The amounts of recombinant GST-BubR1 and GST-BubR1-T605A from duplicated samples were shown by Coomassie blue (CB) staining. The amount of 32P incorporated in the top panel was quantified and plotted. Compared with BubR1, more BubR1-T605A was used in the reaction, which explains more efficient phosphorylation of BubR1-T605A by Plx1 in the absence of Cdk1. (F and G) 1 μg each of recombinant GST-BubR1, GST-BubR1-T605A, and GST-BubR1 kinase-dead mutant (KD; K788R) were incubated with the indicated kinases in the presence of 0.2 mM ATP for 2 h, and then subjected to SDS-PAGE followed by Western blotting with the 3F3/2 antibody. (H) 3 μg each of recombinant GST-BubR1, GST-BubR1-T605A, GST-BubR1-T605E, and GST control were incubated with 140 μl CSF extracts for 20 min, immunoprecipitated with an anti-GST antibody, and then subjected to Western blotting for the associated Plx1. (I and J) Chromosomes purified from checkpoint extracts that had undergone immunodepletion (ID) and addback (AB) of equal amounts of the indicated proteins were dephosphorylated (De-P) by λ-phosphatase, treated with NEM, and subsequently rephosphorylated by Plx1 and Cdk1/cyclin B. Phosphorylated chromosomes were stained for indicated antigens (I), and mean fluorescence intensities of kinetochore 3F3/2 (red) and BubR1 (green; n = 20 kinetochores) were quantified and normalized to the corresponding value derived from mock-depleted extracts (J). Error bars represent SEM. Bar, 5 μm.
Phosphorylation of BubR1 by Cdk1 and Plx1 reconstitutes the 3F3/2 epitope
Next, we determined the kinase requirement for the formation of the kinetochore 3F3/2 phosphoepitope on sperm chromosomes. Endogenous kinetochore 3F3/2 epitopes on sperm chromosomes purified from checkpoint extracts were first dephosphorylated with λ-phosphatase and endogenous kinases inactivated by N-ethylmaleimide (NEM; Wong and Fang, 2005). Chromosomes were then incubated with active recombinant kinases and formation of the 3F3/2 epitope was monitored by immunofluorescence staining. Even though Plx1 alone at high concentrations was sufficient to generate the 3F3/2 epitope in this assay (Wong and Fang, 2005), Plx1 near its physiological concentrations (10 ng/μl Plx1; Descombes and Nigg, 1998) failed to reconstitute the 3F3/2 phosphoepitope (Fig. 1, C and D). This is not surprising, as recognition and phosphorylation of substrates by Plx1 frequently require a priming phosphorylation by another kinase, such as Cdk1/cyclin B, which generates a binding site for PBD (Barr et al., 2004). Thus, we investigated the priming requirement for the 3F3/2 epitope. Although active Plx1 or Cdk1/cyclin B alone was not sufficient to generate the kinetochore 3F3/2 signals, a combination of the two kinases generated robust 3F3/2 signals (Fig. 1, C and D), suggesting that Cdk1 functions as a priming kinase to promote the 3F3/2 phosphoepitope.
We then reconstituted the 3F3/2 epitope in BubR1 in a purified system. First, we showed that both Cdk1 and Plx1 phosphorylated recombinant BubR1 (Fig. S1 A, available at http://www.jcb.org/cgi/content/full/jcb.200708044/DC1). To investigate the effect of priming phosphorylation on BubR1, we incubated recombinant BubR1 with unlabeled ATP in the presence or absence of Cdk1/cyclin B, followed by incubation with or without Plx1 in the presence of γ-[32P]ATP and purvalanol A, a Cdk1 inhibitor (Gray et al., 1998). Although Plx1 alone phosphorylated BubR1, BubR1 that had been incubated with Cdk1 was phosphorylated fourfold more efficiently by Plx1 (Fig. 1 E). Thus, priming phosphorylation of BubR1 by Cdk1 enhances its phosphorylation by Plx1.
Second, we identified the amino acid residue important for priming phosphorylation. PBD recognizes the S-S/T-P sequence in mitotic Plx1 substrates (Barr et al., 2004; van Vugt and Medema, 2005), in which the Ser/Thr residue preceding Pro is phosphorylated. Sequence analysis indicated that there is only one S-T-P (aa 604–606) conserved between human and X. laevis BubR1 (Fig. S1 B). We found that priming phosphorylation of BubR1-T605A by Cdk1/cyclin B had no effect on the level of its subsequent phosphorylation by Plx1, in contrast to the wild-type BubR1 (Fig. 1 E). Thus, Thr 605 is a critical site for priming phosphorylation of BubR1 by Cdk1.
Third, we reconstituted the 3F3/2 epitope in BubR1 in vitro. Recombinant BubR1 and BubR1-T605A were phosphorylated with Cdk1/cyclin B and/or Plx1, followed by Western blotting with the 3F3/2 antibody (Fig. 1 F). Either kinase alone was not sufficient to form the 3F3/2 epitope. The presence of both kinases robustly generated the 3F3/2 epitope on wild-type BubR1, but not on BubR1-T605A. Furthermore, generation of the 3F3/2 epitope does not require the kinase activity of BubR1 (Fig. 1 G; Wong and Fang, 2006). Thus, Cdk1 and Plx1 act synergistically to generate the 3F3/2 epitope in a Thr 605–dependent manner. Consistent with this, recombinant GST-BubR1, but not GST-BubR1-T605A or GST-BubR1-T605E, coprecipitated with the endogenous Plx1 in CSF extracts (Fig. 1 H). Thus, Thr 605, likely through its phosphorylation, facilitates the BubR1–Plx1 interaction.
Lastly, we determined whether BubR1 is the 3F3/2 antigen in X. laevis checkpoint extracts. CSF extracts were first immunodepleted of endogenous BubR1 and then incubated with recombinant BubR1, BubR1-T605A, or BubR1-T605E together with sperm chromosomes and nocodazole. Chromosomes were purified onto coverslips and underwent the assay of rephosphorylation by Cdk1/cyclin B and Plx1, as described in Fig. 1 C. Depletion of BubR1 reduced the kinetochore 3F3/2 signals 30-fold, whereas addback of recombinant BubR1 rescued the signals (Fig. 1, I and J). However, the 3F3/2 signals failed to recover to a substantial extent in the BubR1-T605A and BubR1-T605E addback samples, even though both proteins were properly targeted to kinetochores. Thus, BubR1 is a physiological kinetochore 3F3/2 antigen, and Thr 605 is required for the generation of the 3F3/2 epitope. In contrast, the presence of residual 3F3/2 signals on BubR1-T605A/E– rescued kinetochores suggests that another kinetochore 3F3/2 antigen may exist whose phosphorylation or kinetochore localization is under the control of BubR1.
Thr 605 in BubR1 is required for spindle checkpoint arrest
We next determined whether the Thr 605 phosphorylation is required for checkpoint arrest. BubR1 was depleted from CSF extracts to >95% (Fig. 2 A, lane 1), and recombinant BubR1, BubR1-T605A, or BubR1-T605E was then added back to endogenous levels (Fig. 2 A, lanes 2–4). Subsequently, sperm chromosomes and nocodazole were added to activate spindle checkpoint, and samples were then split and incubated with or without calcium. Aliquots were then taken at various times and assayed for Cdk1 kinase activity. In the absence of calcium, Cdk1 kinase activity in all extracts remained high, indicating a stable meiotic metaphase arrest (Fig. 2 B, bottom). Upon addition of calcium, which triggers the transition from meiotic metaphase into interphase, BubR1-depleted extracts entered interphase with a low Cdk1 activity, whereas addback of BubR1 maintained the high Cdk1 activity caused by the activation of the spindle checkpoint (Fig. 2, B and C; Chan et al., 1999; Chen, 2002; Mao et al., 2003). However, addition of BubR1-T605A or BubR1-T605E failed to prevent meiotic exit, indicating that Thr 605 in BubR1 is required for the checkpoint arrest. Mutations on Thr 605 did not nonspecifically inactivate BubR1 because of misfolding of the mutant proteins, as BubR1-T605A was as active as BubR1 in inhibition of APC-Cdc20 (Fig. 2, D and E).
Figure 2.
Thr 605 in BubR1 is required for spindle checkpoint arrest. (A) CSF extracts were mock depleted (lane 5) or depleted (lanes 1–4) of BubR1. Recombinant GST-tagged BubR1 (lane 2), BubR1-T605A (lane 3), or BubR1-T605E (lane 4) was added to the depleted extracts. Different amounts of extracts were loaded to determine the depletion efficiency. (B and C) CSF extracts that had undergone immunodepletion and addback of the indicated proteins were incubated with sperm chromosomes and nocodazole to activate the spindle checkpoint, followed by incubation with (top) or without (bottom) calcium. At the indicated times, an aliquot of extracts was assayed for the Cdk1 kinase activity using histone H1 as a substrate. The kinase activity was quantified and plotted (C) upon normalization to the value at time 0 for the corresponding samples. C shows mock-depleted extracts (star), BubR1-depleted extracts (square), BubR1-depleted extracts with addback of GST-BubR1 (filled circle), GST-BubR1-T605A (open circle), and GST-BubR1-T605E (triangle). (D and E) Inhibition of APC-Cdc20–mediated ubiquitination by equal amounts of recombinant GST-BubR1 and GST-BubR1-T605A was assayed using in vitro translated 35S-securin substrate, as described previously (Fang, 2002). The amount of securin remaining was quantified and plotted (E) upon normalization to the value at time 0 for the corresponding samples. E shows interphase APC (iAPC; square), iAPC + Cdc20 (filled circle), iAPC + Cdc20 + BubR1 (triangle), and iAPC + Cdc20 + BubR1-T605A (open circle).
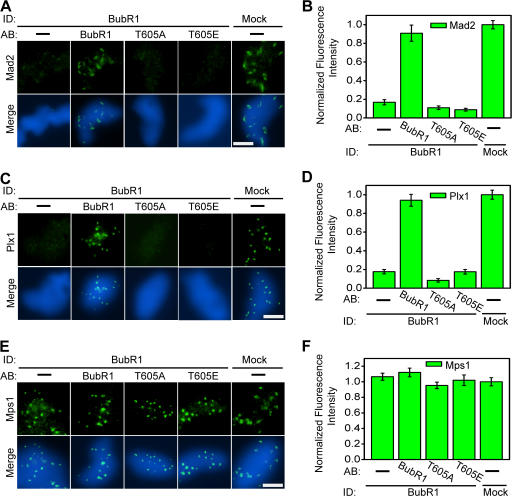
Thr 605 in BubR1 is required for the recruitment of Plx1 and Mad2 to kinetochores
We determined the cellular basis for the lack of checkpoint arrest. BubR1 controls the kinetochore localization of Plx1 and the checkpoint protein Mad2, as demonstrated in BubR1-depleted extracts (Fig. 3, A–D; Mao et al., 2003; Wong and Fang, 2006). Localization of Plx1 and Mad2 to kinetochores in BubR1-depleted extracts was recovered upon addition of recombinant BubR1, but not BubR1-T605A or BubR1-T605E, even though mutant BubR1 proteins were efficiently targeted to kinetochores (Fig. 1, I and J; and Fig. 3, A–D). This lack of recruitment of Plx1 and Mad2 was not because of a global change in the outer kinetochore structure, as the checkpoint protein Mps1 was efficiently targeted to kinetochores in all analyzed extracts (Fig. 3, E and F; Wong and Fang, 2006). Thus, Thr 605 is specifically required for the checkpoint arrest and for the recruitment of Mad2 and Plx1 to kinetochores.
Figure 3.
Thr 605 in BubR1 is required for the recruitment of Plx1 and Mad2 to kinetochores. Chromosomes were purified onto coverslips from checkpoint extracts that had undergone immunodepletion and addback of the indicated proteins, as prepared in Fig. 2 A. Purified chromosomes were stained in green for Mad2 (A), Plx1 (C), and Mps1 (E). DNA was stained in blue. Mean fluorescence intensities of Mad2 (B), Plx1 (D), and Mps1 (F) were quantified from 20 kinetochores in different fields and plotted upon normalization to the corresponding values derived from mock-depleted extracts. Error bars represent SEM. Bars, 5 μm.
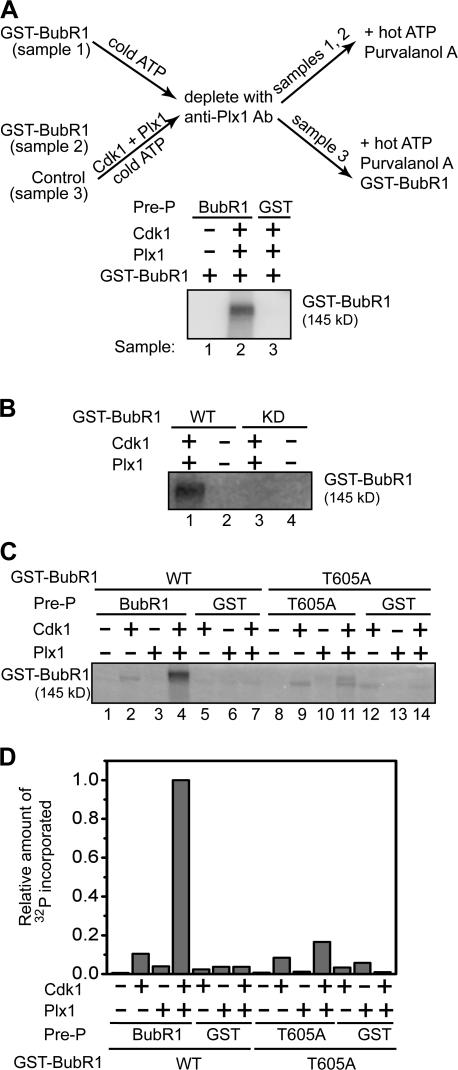
Activation of the BubR1 kinase by Plx1 and Cdk1 is dependent on Thr 605
BubR1 is a kinase whose activity is required for checkpoint arrest (Chan et al., 1999; Mao et al., 2003). Thus, we determined whether phosphorylation of BubR1 by Cdk1 and Plx1 affects its kinase activity as assayed by its autophosphorylation. Without prephosphorylation by Cdk1 and Plx1, the BubR1 kinase activity was undetectable (Fig. 4 A; Mao et al., 2003). However, prephosphorylation of BubR1, but not the kinase-dead BubR1, by Cdk1 and Plx1 increased its autophosphorylation activity at least 100-fold (Fig. 4, A and B). Thus, the Cdk1- and Plx1-mediated phosphorylation activates BubR1.
Figure 4.
Activation of the BubR1 kinase by Plx1 and Cdk1 is dependent on Thr 605. (A) BubR1 autophosphorylation was assayed as diagramed. Recombinant GST-BubR1 was prephosphorylated (Pre-P) in 0.2 mM of unlabeled ATP in the presence (sample 2) or absence (sample 1) of Plx1 and Cdk1/cyclin B. Plx1 was then removed by anti-Plx1 antibody beads, and prephosphorylated BubR1 was assayed for its autophosphorylation activity in the presence of γ-[32P]ATP and purvalanol A. In a parallel control (sample 3), recombinant GST, not BubR1, was used in the prephosphorylation reaction, and thereafter an aliquot of unphosphorylated GST-BubR1 protein was added before the autophosphorylation reaction. (B) 550 ng each of wild-type BubR1 and kinase-dead mutant was assayed for autophosphorylation after being prephosphorylated by Cdk1 and Plx1 as described in A. (C and D) 550 ng each of recombinant GST-BubR1 (lanes 1–4), GST-BubR1-T605A (lanes 8–11), and GST (lanes 5–7 and 12–14) was prephosphorylated by either Cdk1/cyclin B (lanes 2, 5, 9, and 12) or Plx1 (lanes 3, 6, 10, and 13), or by both kinases (lanes 4, 7, 11, and 14). The autophosphorylation assay was then performed as described in A. In control GST samples (lanes 5–7 and 12–14), unphosphorylated BubR1 (lanes 5–7) or BubR1-T605A (lanes 12–14) were added after the prephosphorylation step but before the autophosphorylation reaction. The extent of autophosphorylation was quantified by measuring the amount of 32P incorporated in BubR1 (D).
Next, we determined the relative contribution of Cdk1 and Plx1 and the role of Thr 605 in activation of BubR1 in a similar assay. Although prephosphorylation of BubR1 by Cdk1 or Plx1 alone enhanced its kinase activity to some extent, phosphorylation of BubR1 by both Cdk1 and Plx1 synergistically activated BubR1 by 10 or 30 times as compared with BubR1 prephosphorylated by Cdk1 or Plx1 alone, respectively (Fig. 4, C and D). This synergistic activation of BubR1 requires Thr 605 in BubR1, as the BubR1-T605A mutant only showed an additive activation by Cdk1 and Plx1 (Fig. 4, C and D). Thus, Cdk1 and Plx1 synergistically activate the kinase activity of BubR1 in a Thr 605–dependent manner.
Responses to checkpoint activation usually consist of a cell cycle arrest and repair of the cellular defects, which activates the checkpoint in the first place. In the case of the spindle checkpoint, lack of attachment or tension arrests cells in mitosis and alters the kinetochore structure to promote attachment and tension. It has been shown previously that the checkpoint protein BubR1 is not only essential for inhibition of APC/C (Sudakin et al., 2001; Tang et al., 2001; Fang, 2002) but also regulates the kinetochore–microtubule attachment and chromosome congression (Ditchfield et al., 2003; Lampson and Kapoor, 2005; Morrow et al., 2005; Draviam et al., 2007). We report here that both functions of BubR1 are coordinately regulated by Cdk1 through its phosphorylation on BubR1 Thr 605. First, this phosphorylation is required for checkpoint-mediated mitotic arrest. Mechanistically, phosphorylation on Thr 605 does not affect the in vitro inhibitory efficiency of BubR1 toward Cdc20-APC/C. Instead, this phosphorylation controls the targeting of Mad2 to kinetochores, which is essential for mitotic arrest.
Second, Cdk1-mediated phosphorylation promotes the formation of the 3F3/2 epitope. The 3F3/2 antigen is a mysterious kinetochore protein that has been hunted by cell biologists for over a decade (Gorbsky and Ricketts, 1993). The kinetochore 3F3/2 phosphoepitope is generated by Plk1/Plx1 in response to the lack of tension across sister kinetochores (Campbell and Gorbsky, 1995; Nicklas et al., 1995, 1998; Ahonen et al., 2005; Wong and Fang, 2005). We demonstrate here that the checkpoint protein BubR1 is the kinetochore 3F3/2 antigen, whose phosphorylation requires the synergistic action of Cdk1 and Plx1, as priming phosphorylation on BubR1 Thr 605 by Cdk1 is essential for the formation of the 3F3/2 signals. Surprisingly, the 3F3/2 phosphoepitope in BubR1 is observed in both CSF extracts and checkpoint extracts (Fig. 1 A) but not in interphase extracts (unpublished data), indicating that formation of this epitope is tension independent. Thus, the mitotic/meiotic state provides a permissive environment for the formation of the 3F3/2 biochemical epitope, but its functional specificity to the lack of tension is likely determined by its kinetochore localization.
As Plk1 is not required for checkpoint arrest in human cells (Sumara et al., 2004; van Vugt et al., 2004; Lenart et al., 2007), the 3F3/2 epitope is probably not involved in mitotic arrest but is likely to act in regulating kinetochore–microtubule interactions (Gorbsky and Ricketts, 1993; Campbell and Gorbsky, 1995; Nicklas et al., 1995, 1998). Indeed, Plk1 promotes the assembly of the bipolar spindle and the generation of tension (Sumara et al., 2004; van Vugt et al., 2004; Matsumura et al., 2007). As the formation of the 3F3/2 epitope by Cdk1 and Plx1 drastically activates the kinase activity of BubR1, we speculate that the active BubR1 kinase controls the kinetochore structures and/or promotes microtubule attachment to kinetochores. Although our extract system precludes us from analyzing the exact physiological function of the 3F3/2 phosphoepitope in tension signaling, the molecular information on the 3F3/2 antigen and its kinase presented in this study nevertheless opens the door for future characterizations of the tension responses in culture cells.
Materials and methods
Antibodies and recombinant proteins
Antibodies against X. laevis Mad2, BubR1, Mps1, and Plx1, as well as 3F3/2 ascite, have been described previously (Wong and Fang, 2006). Baculoviruses for Plx1 and Cdk1/cyclin B were provided by J. Maller (University of Colorado, Denver, CO) and H. Piwnica-Worms (Washington University, St. Louis, MO), respectively. Active recombinant Plx1 and Cdk1/cyclin B were expressed in Sf9 cells for 44 h and then treated with 250 nM okadaic acid for 4 h before harvesting. BubR1-T605A and BubR1-T605E mutants were generated by site-directed mutagenesis. Recombinant BubR1, BubR1-T605A, BubR1-T605E, and BubR1 kinase dead (K788R) were expressed in Escherichia coli as GST fusion proteins and purified using glutathione agarose (GE Healthcare).
Preparation of X. laevis egg extracts, immunodepletion, and immunofluorescence
Meiotic metaphase extracts (CSF extracts) and checkpoint extracts from X. laevis eggs and demembranated sperm nuclei were prepared as described previously (Minshull et al., 1994). CSF extracts that were either mock depleted or depleted of BubR1 were incubated with demembranated sperm nuclei and nocodazole. In rescue experiments, recombinant BubR1 proteins were added to depleted extracts before the addition of sperm nuclei and nocodazole.
Immunofluorescence images were captured at 23°C on a microscope (Axiovert 200M; Carl Zeiss, Inc.) using an oil-immersion objective lens (100× 1.4 NA; Plan-Apochromat), a digital charge-coupled device camera (Orca-ER; Hamamatsu Photonics), and Openlab 5.0.1 (Improvision). For quantitative comparison of fluorescence intensities, images were acquired and processed identically. In Fig. 1 D, the intensity of each kinetochore 3F3/2 signal was determined relative to that of BubR1 obtained from the same kinetochore.
Rephosphorylation assay with sperm chromosomes
Chromosomes were purified onto coverslips from spindle checkpoint extracts (Wong and Fang, 2005). To remove the 3F3/2 phosphoepitope and inactivate endogenous kinases, coverslips were incubated with λ-phosphatase (New England Biolabs, Inc.) and subsequently treated with NEM (Wong and Fang, 2005). Coverslips were then incubated with 5 ng/μl His6-Plx1 and/or 3 ng/μl Cdk1/cyclin B in the kinase reaction buffer (KRB) (20 mM Hepes, pH 7.8, 15 mM KCl, 10 mM MgCl2, 1 mM EGTA, 0.5 μM microcystin LR, and 0.1 mg/ml BSA) supplemented with 2 mM ATP for 1.5 h at room temperature. Coverslips were next stained with the 3F3/2 ascite at a 1:8,000 dilution at 4°C overnight, with the Alexa Fluor 594 and 488 secondary antibodies (Invitrogen) for 1 h at room temperature, and with DAPI.
In vitro kinase assays
Phosphorylation of BubR1 by Cdk1 or Plx1 was performed in a total volume of 10 μl KRB for 30 min at room temperature, using 1 μg BubR1 and 0.2 mM ATP, with either 250 ng Cdk1/cyclin B or 30 ng His6-Plx1. The radiolabeling assay was performed similarly, except in the presence of 2 μCi γ-[32P]ATP.
To assay BubR1 autophosphorylation in Fig. 4, 550 ng of recombinant BubR1 was first phosphorylated by Cdk1/cyclin B in KRB supplemented with 0.2 mM of unlabeled ATP for 75 min at 25°C, and then by Plx1 for 45 min in the presence of 65 nM purvalanol A, a potent inhibitor of Cdk1/cyclin B (Gray et al., 1998). The Plx1 kinase was then removed from phosphorylated BubR1 by incubating with anti-Plx1 antibody/protein A beads for 1 h at room temperature. Subsequently, phosphorylated BubR1 was assayed for autophosphorylation by incubating in KRB plus 0.2 mM ATP, 2 μCi γ-[32P]ATP, 65 nM purvalanol A, and 0.1 mg/ml ovalbumin for 40 min at room temperature. Control experiments were done in parallel using GST protein as a substrate to undergo prephosphorylation by Cdk1/cyclin B and Plx1. After subsequent depletion of Plx1, the control samples were mixed with 550 ng of recombinant BubR1, which had not been phosphorylated by either kinase, and assayed for BubR1 autophosphorylation.
Online supplemental material
Cdk1 and Plx1 phosphorylate BubR1 in vitro. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200708044/DC1.
Acknowledgments
We thank Drs. James Maller and Helen Piwnica-Worms for reagents, A. Seki and Dr. H. Du for technical assistance, and members of the Fang laboratory for discussions.
This work was supported by a Burroughs-Wellcome Career Award in Biomedical Research and a grant from National Institutes of Health (GM062852) to G. Fang.
O.K. Wong's present address is Sunesis Pharmaceuticals, South San Francisco, CA 94080.
Abbreviations used in this paper: APC/C, anaphase-promoting complex/cyclosome; CSF, cytostatic factor; KRB, kinase reaction buffer; Mps1, monopolar spindle 1; NEM, N-ethylmaleimide; PBD, polo box domain; Plk1, polo-like kinase 1; Plx1, Xenopus laevis polo-like kinase 1.
References
- Ahonen, L.J., M.J. Kallio, J.R. Daum, M. Bolton, I.A. Manke, M.B. Yaffe, P.T. Stukenberg, and G.J. Gorbsky. 2005. Polo-like kinase 1 creates the tension-sensing 3F3/2 phosphoepitope and modulates the association of spindle-checkpoint proteins at kinetochores. Curr. Biol. 15:1078–1089. [DOI] [PubMed] [Google Scholar]
- Barr, F.A., H.H. Sillje, and E.A. Nigg. 2004. Polo-like kinases and the orchestration of cell division. Nat. Rev. Mol. Cell Biol. 5:429–440. [DOI] [PubMed] [Google Scholar]
- Campbell, M.S., and G.J. Gorbsky. 1995. Microinjection of mitotic cells with the 3F3/2 anti-phosphoepitope antibody delays the onset of anaphase. J. Cell Biol. 129:1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, G.K., S.A. Jablonski, V. Sudakin, J.C. Hittle, and T.J. Yen. 1999. Human BUBR1 is a mitotic checkpoint kinase that monitors CENP-E functions at kinetochores and binds the cyclosome/APC. J. Cell Biol. 146:941–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R.H. 2002. BubR1 is essential for kinetochore localization of other spindle checkpoint proteins and its phosphorylation requires Mad1. J. Cell Biol. 158:487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert, M.S., T. Scherson, and M.W. Kirschner. 1988. Monoclonal antibodies specific for thiophosphorylated proteins recognize Xenopus MPF. Dev. Biol. 129:209–216. [DOI] [PubMed] [Google Scholar]
- Descombes, P., and E.A. Nigg. 1998. The polo-like kinase Plx1 is required for M phase exit and destruction of mitotic regulators in Xenopus egg extracts. EMBO J. 17:1328–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditchfield, C., V.L. Johnson, A. Tighe, R. Ellston, C. Haworth, T. Johnson, A. Mortlock, N. Keen, and S.S. Taylor. 2003. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J. Cell Biol. 161:267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draviam, V.M., F. Stegmeier, G. Nalepa, M.E. Sowa, J. Chen, A. Liang, G.J. Hannon, P.K. Sorger, J.W. Harper, and S.J. Elledge. 2007. A functional genomic screen identifies a role for TAO1 kinase in spindle-checkpoint signalling. Nat. Cell Biol. 9:556–564. [DOI] [PubMed] [Google Scholar]
- Fang, G. 2002. Checkpoint protein BubR1 acts synergistically with Mad2 to inhibit anaphase-promoting complex. Mol. Biol. Cell. 13:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbsky, G.J., and W.A. Ricketts. 1993. Differential expression of a phosphoepitope at the kinetochores of moving chromosomes. J. Cell Biol. 122:1311–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, N.S., L. Wodicka, A.M. Thunnissen, T.C. Norman, S. Kwon, F.H. Espinoza, D.O. Morgan, G. Barnes, S. LeClerc, L. Meijer, et al. 1998. Exploiting chemical libraries, structure, and genomics in the search for kinase inhibitors. Science. 281:533–538. [DOI] [PubMed] [Google Scholar]
- Lampson, M.A., and T.M. Kapoor. 2005. The human mitotic checkpoint protein BubR1 regulates chromosome-spindle attachments. Nat. Cell Biol. 7:93–98. [DOI] [PubMed] [Google Scholar]
- Lenart, P., M. Petronczki, M. Steegmaier, B. Di Fiore, J.J. Lipp, M. Hoffmann, W.J. Rettig, N. Kraut, and J.M. Peters. 2007. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr. Biol. 17:304–315. [DOI] [PubMed] [Google Scholar]
- Mao, Y., A. Abrieu, and D.W. Cleveland. 2003. Activating and silencing the mitotic checkpoint through CENP-E-dependent activation/inactivation of BubR1. Cell. 114:87–98. [DOI] [PubMed] [Google Scholar]
- Matsumura, S., F. Toyoshima, and E. Nishida. 2007. Polo-like kinase 1 facilitates chromosome alignment during prometaphase through BubR1. J. Biol. Chem. 282:15217–15227. [DOI] [PubMed] [Google Scholar]
- Minshull, J., H. Sun, N.K. Tonks, and A.W. Murray. 1994. A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell. 79:475–486. [DOI] [PubMed] [Google Scholar]
- Morrow, C.J., A. Tighe, V.L. Johnson, M.I. Scott, C. Ditchfield, and S.S. Taylor. 2005. Bub1 and aurora B cooperate to maintain BubR1-mediated inhibition of APC/CCdc20. J. Cell Sci. 118:3639–3652. [DOI] [PubMed] [Google Scholar]
- Murray, A.W. 2004. Recycling the cell cycle: cyclins revisited. Cell. 116:221–234. [DOI] [PubMed] [Google Scholar]
- Nicklas, R.B., S.C. Ward, and G.J. Gorbsky. 1995. Kinetochore chemistry is sensitive to tension and may link mitotic forces to a cell cycle checkpoint. J. Cell Biol. 130:929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas, R.B., M.S. Campbell, S.C. Ward, and G.J. Gorbsky. 1998. Tension-sensitive kinetochore phosphorylation in vitro. J. Cell Sci. 111:3189–3196. [DOI] [PubMed] [Google Scholar]
- Sudakin, V., G.K. Chan, and T.J. Yen. 2001. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J. Cell Biol. 154:925–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumara, I., J.F. Gimenez-Abian, D. Gerlich, T. Hirota, C. Kraft, C. de la Torre, J. Ellenberg, and J.M. Peters. 2004. Roles of polo-like kinase 1 in the assembly of functional mitotic spindles. Curr. Biol. 14:1712–1722. [DOI] [PubMed] [Google Scholar]
- Tang, Z., R. Bharadwaj, B. Li, and H. Yu. 2001. Mad2-Independent inhibition of APCCdc20 by the mitotic checkpoint protein BubR1. Dev. Cell. 1:227–237. [DOI] [PubMed] [Google Scholar]
- van Vugt, M.A., and R.H. Medema. 2005. Getting in and out of mitosis with Polo-like kinase-1. Oncogene. 24:2844–2859. [DOI] [PubMed] [Google Scholar]
- van Vugt, M.A., B.C. van de Weerdt, G. Vader, H. Janssen, J. Calafat, R. Klompmaker, R.M. Wolthuis, and R.H. Medema. 2004. Polo-like kinase-1 is required for bipolar spindle formation but is dispensable for anaphase promoting complex/Cdc20 activation and initiation of cytokinesis. J. Biol. Chem. 279:36841–36854. [DOI] [PubMed] [Google Scholar]
- Wong, O.K., and G. Fang. 2005. Plx1 is the 3F3/2 kinase responsible for targeting spindle checkpoint proteins to kinetochores. J. Cell Biol. 170:709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, O.K., and G. Fang. 2006. Loading of the 3F3/2 antigen onto kinetochores is dependent on the ordered assembly of the spindle checkpoint proteins. Mol. Biol. Cell. 17:4390–4399. [DOI] [PMC free article] [PubMed] [Google Scholar]