Abstract
B cell lymphoma 2 (Bcl-2) homology domain 3 (BH3)–only proteins of the Bcl-2 family are important functional adaptors that link cell death signals to the activation of Bax and/or Bak. The BH3-only protein Nbk/Bik induces cell death via an entirely Bax-dependent/Bak-independent mechanism. In contrast, cell death induced by the short splice variant of Bcl-x depends on Bak but not Bax. This indicates that Bak is functional but fails to become activated by Nbk. Here, we show that binding of myeloid cell leukemia 1 (Mcl-1) to Bak persists after Nbk expression and inhibits Nbk-induced apoptosis in Bax-deficient cells. In contrast, the BH3-only protein Puma disrupts Mcl-1–Bak interaction and triggers cell death via both Bax and Bak. Targeted knockdown of Mcl-1 overcomes inhibition of Bak and allows for Bak activation by Nbk. Thus, Nbk is held in check by Mcl-1 that interferes with activation of Bak. The finding that different BH3-only proteins rely specifically on Bax, Bak, or both has important implications for the design of anticancer drugs targeting Bcl-2.
Introduction
B cell lymphoma 2 (Bcl-2) family members are critical regulators of programmed cell death (van Delft and Huang, 2006). Proteins of this family share homology in four conserved regions termed Bcl-2 homology (BH) domains and can be divided into anti- and proapoptotic proteins. The antiapoptotic proteins Bcl-2, Bcl-xL, Bcl-w, myeloid cell leukemia (Mcl) 1, and Bfl-1/A1 are characterized by all four BH domains. Proapoptotic homologues can be further subdivided into two subfamilies. The multi–BH domain Bax homologues, including Bax, Bak, and Bok/Mtd, contain BH1–3, whereas the proteins of the BH3-only subfamily, which comprise Bad, Bid, Bim, Bmf, Puma, Noxa, Nbk/Bik, and Hrk, only share the BH3 domain. BH3-only proteins are essential initiators of apoptosis, and once activated, they regulate the ability of the multi–BH domain members Bax and Bak to undergo a conformational switch and to oligomerize in the outer mitochondrial membrane (Desagher et al., 1999; Wei et al., 2000). Activated Bax and Bak then induce mitochondrial membrane permeabilization and subsequent release of proapoptotic factors, e.g., cytochrome c, from the intermembrane space into the cytosol. Cytosolic cytochrome c induces formation of the apoptosome and ultimately triggers execution of the intrinsic apoptosis signaling cascade.
Deregulation of Bcl-2 family proteins has been implicated in the development of many malignancies (Cory et al., 2003). In addition to deregulated expression of antiapoptotic Bcl-2 (Strasser et al., 1990), disruption of multidomain proapoptotic Bcl-2 homologues is also critically involved in tumorigenesis. Loss of Bax is a frequent event in human cancer and is related to tumor progression, poor prognosis, and clinical resistance to anticancer therapy (Bargou et al., 1995; Sturm et al., 2001). Furthermore, loss of the bax gene contributes to oncogenic transformation and tumor development in mice (Yin et al., 1997). Recently, it has been shown that inactivation of BH3-only proteins is implicated in human cancer (Tagawa et al., 2005). In line with a tissue-specific expression of the BH3-only protein Nbk/Bik (Boyd et al., 1995; Han et al., 1996) with strong expression in the kidney (Daniel et al., 1999), loss of Nbk and the more broadly expressed Bim is a common feature of clear cell renal cell carcinoma (Sturm et al., 2006; Zantl et al., 2007).
Bcl-2 family members can form homo- and heterodimers with other members of the protein family. It is considered that the BH3 domain constitutes an amphipathic α helix, which binds to a hydrophobic groove formed by the BH1, BH2, and BH3 domain of antiapoptotic Bcl-2 family members. Binding to and inactivation of antiapoptotic Bcl-2 family members is crucial for BH3-only proteins to initiate apoptosis (Willis et al., 2005). The ability of proapoptotic Bcl-2 family proteins to interact with their antiapoptotic siblings led to the so-called rheostat model: the ratio of pro- to antiapoptotic Bcl-2 members determines the apoptotic fate of the cell (Korsmeyer et al., 1993). A recent study revealed, however, that BH3-only proteins selectively bind to specific sets of prosurvival proteins and that only certain pairs associate with each other under physiological conditions (Chen et al., 2005). These complementary binding profiles implicate two classes of antiapoptotic Bcl-2 proteins, one comprising Mcl-1 and Bfl-1/A1, the other Bcl-2, Bcl-xL, and Bcl-w. It has been suggested that efficient induction of apoptosis depends on neutralization of both classes of prosurvival proteins.
Moreover, numerous studies show a central role of Bax rather than Bak in Bcl-2–regulated cell death. This is especially true for Puma and Nbk, which both have been shown to act via a Bax-dependent/Bak-independent pathway (Theodorakis et al., 2002; Gillissen et al., 2003; Yu et al., 2003). There is also accumulating evidence that distinct BH3-only proteins act specifically through the activation of Bak (Cartron et al., 2003; Lindenboim et al., 2005). We have shown that disruption of Bax is sufficient to confer resistance to Nbk under conditions of abiding Bak expression. This indicates that induction of cell death by Nbk is mediated specifically via a Bax-dependent and apparently Bak-independent pathway (Gillissen et al., 2003). In this light, the role of Bak and putative Bak inhibitors in Nbk-induced apoptosis remains enigmatic. Thus, we cannot rule out the possibility that loss of Bax protects cells just by decreasing the amount of Bax/Bak-like molecules under a critical threshold, which would be necessary for Nbk to induce apoptosis.
Here, we show that Bak is fully functional and sufficient to mediate apoptosis by specific activators, e.g., Bcl-xS. Moreover, evidence is provided for the mechanism underlying Nbk resistance in Bax-deficient cells. Although Nbk triggers Bax activation, it nevertheless fails to relieve Mcl-1–mediated inhibition of Bak. Moreover, Mcl-1 protein levels are increased upon Nbk expression. By this mechanism, Nbk enforces inhibition of Bak by Mcl-1. Collectively, these results provide a molecular rationale for the Bax dependency of the BH3-only protein Nbk.
Results
We have shown previously that loss of Bax protects cancer cells from apoptosis induced by the BH3-only protein Nbk (Gillissen et al., 2003). To further investigate the mechanism of Nbk- induced cell death and to study the regulation of the Bax homologous protein Bak in detail, we induced expression of a myc-tagged Nbk protein in the parental wild-type (wt) HCT116 cell line and in isogeneic cell lines devoid of Bax (Bax−) or Bak (Bak−) expression alone or of both (Bax−/Bak−; Fig. 1 A). Loss of protein expression was achieved by knockout of the Bax gene (Zhang et al., 2000) and Bak knockdown by short hairpin RNA (Theodorakis et al., 2002), respectively.
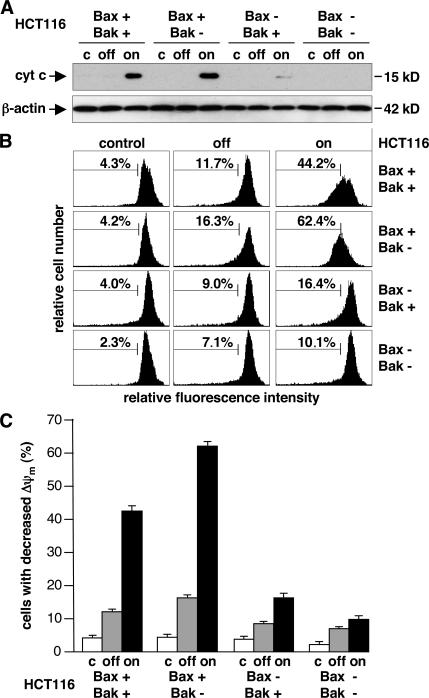
Figure 1.
Nbk expression induces caspase processing and apoptotic DNA fragmentation in HCT116 wt and HT116 Bak-deficient cells but not in Bax knockout cells. (A) Bax and Bak expression levels in different HCT116 cell lines. (B) Western blot analysis for Nbk expression and caspase 9 and 3 cleavage. HCT116 cells were infected with the recombinant adenovirus Ad-mycNbk-tTA and cultured for 24 h in the presence (off) or absence (on) of doxycycline (Dox). Control cells were mock treated and grown in the absence of doxycycline. *, unspecific band. (C) Flow cytometric measurement of DNA content. Cells were treated as described in B. Cells displaying a sub-G1 hypodiploid DNA content are considered apoptotic. Data are means ± SD from three independent experiments.
Transduction of these cell lines with an adenoviral vector, Ad-mycNbk-tTA, for the regulated expression of Nbk leads to high levels of Nbk protein in the absence of doxycycline, i.e., under on conditions, whereas expression of the transgene was almost completely repressed by the addition of doxycycline to the culture medium, i.e., under off conditions (Fig. 1 B). Western blot analyses showed that forced expression of Nbk is accompanied by cleavage of the initiator caspase-9 and the effector caspase-3 in HCT116 wt and in HCT116 Bak− (knockdown) cells. In contrast, neither caspase-9 nor caspase-3 cleavage could be detected in HCT116 cells devoid of Bax expression, regardless of whether or not they expressed Bak. This is not caused by lower Nbk expression because Western blot analyses affirmed equal expression levels of Nbk under on conditions in all four cell lines.
To confirm that loss of Bak does not affect regulation of apoptosis upon Nbk expression, we performed flow cytometric analyses to quantify fragmentation of genomic DNA. Apoptotic cells were identified as cells with hypodiploid, i.e., sub-G1, DNA content. After 24 h of transduction with Ad-mycNbk-tTA, a mean of ∼40% of the HCT116 wt and 45% of the HCT116 knockdown cells became apoptotic under on conditions (Fig. 1 C). In sharp contrast, identical transduction with Ad-mycNbk-tTA failed to significantly induce apoptosis in Bax knockout and Bax/Bak double deficient HCT116 cells. These results establish that, at least in Nbk-induced apoptosis, Bax and Bak do not exert redundant functions. Whereas Bak knockdown does not affect Nbk-induced apoptosis, loss of Bax efficiently protects HCT116 cells from Nbk-induced apoptosis. Furthermore, the presence of Bak does not influence the proportion of cells undergoing Nbk-induced apoptosis, regardless of whether or not they express Bax.
To corroborate that all death occurs by apoptosis and to control viability, we analyzed cells induced to express Nbk by propidium iodide (PI)/Annexin V–FITC staining. As in the case of the DNA fragmentation analysis, Nbk induced apoptosis in the HCT116 wt cells and in Bak-deficient HCT116 cells, as analyzed by detection of phosphatidylserine exposure and PI negativity (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200703040/DC1). Under on conditions, 41% of wt cells were detected as Annexin V positive/PI negative (early apoptotic) and an additional 13% showed Annexin V/PI positivity (late apoptotic) at 24 h after transduction with Ad-mycNbk-tTA. In contrast to Bak knockdown cells, Bax-deficient cell lines displayed <6% of Annexin V–positive/PI-negative cells and only 6% of double positive cells.
To further address the role of Bax and Bak and of mitochondria in Nbk-induced apoptosis, we determined the release of cytochrome c and dissipation of the mitochondrial membrane potential upon exposure to Ad-mycNbk-tTA. Western blot analysis of cytosolic extracts obtained at 24 h after infection with Ad-mycNbk-tTA showed that Nbk expression induces the release of cytochrome c in both HCT116 wt and HCT116 Bak− cells but not in Bax-deficient cell lines (Fig. 2 A).
Figure 2.
Nbk-induced cytochrome c release and mitochondrial transmembrane permeability transition are impaired in Bax- but not in Bak-deficient cells. HCT116 wt, Bax−, Bak−, and Bax−/Bak− cells were transduced with Ad-mycNbk-tTA and cultured for 24 h under off or on conditions. Control cells were mock treated and grown in the absence of doxycycline. (A) Cytosolic extracts were prepared from HCT116 cell lines and subjected to Western blot analysis for cytochrome c. (B) HCT116 cells were labeled with JC-1. Decreased red fluorescence intensity was measured by flow cytometry (representative experiment). The percentage of cells with Δψm loss is indicated between markers. (C) Data are means ± SD from three independent experiments of cells with Δψm loss.
Using the potential-sensitive dye JC-1, we used flow cytometry to analyze whether release of cytochrome c is accompanied by mitochondrial permeability transition and loss of mitochondrial membrane potential (ΔΨm). This revealed loss of ΔΨm in Bax-proficient cells upon mycNbk expression, regardless of the presence or absence of cellular Bak. This loss of ΔΨm occurred in a mean of 42 and 62% of the HCT116 wt and HCT116 Bak− cells, respectively. Conversely, only 16% of the Bax knockout and 10% of Bak/Bax double deficient cells exhibited decreased mitochondrial membrane potential upon Ad-mycNbk-tTA transduction under on conditions (Fig. 2, B and C). Compared with HCT116 wt cells, induction of cell death by Nbk is slightly increased in Bax+ cells (Figs. 1, S1, and S2, available at http://www.jcb.org/cgi/content/full/jcb.200703040/DC1). This is not caused by a decrease in the level of antiapoptotic proteins such as Bcl-2 or Bcl-xL (not depicted), but it seems to reflect the increased Bax expression level in these cells (Fig. 1 A).
These data demonstrate that loss of Bax, but not of Bak, expression protects HCT116 cells from Nbk-induced apoptosis. Nevertheless, regarding the simple rheostat model of a balance between pro- and antiapoptotic proteins, we could not rule out that loss of Bax protects cells just by decreasing the amount of proapoptotic multi–BH domain proteins under a critical threshold necessary for Nbk to induce apoptosis. Thus it might be that, in Bax-deficient cells, the amount of Bak protein is not sufficient to mediate apoptotic signals by Nbk. To test if increased expression levels of proapoptotic multi–BH domain proteins can sensitize cells for Nbk-induced apoptosis, we established cell lines in which Bax or Bak is constitutively overexpressed. The parental cell line DU145 does not express Bax because of a frameshift mutation in the bax gene, whereas bak is not mutated and endogenous Bak is expressed to a moderate extent. By use of a retroviral vector, HyTK-Bax, we established DU145 cells stably reexpressing the Bax-α cDNA under the control of a cytomegalovirus (CMV) promoter (von Haefen et al., 2002) and a Bax-negative vector control cell line (Fig. 3 A). Infection of these cell lines with Ad-mycNbk-tTA resulted in comparable levels of Nbk expression in both cell lines under on conditions (Fig. 3 A).
Figure 3.
Inverse role of Bax and Bak in apoptosis induced by Nbk or Bcl-xS. DU145 wt cells and DU145 Bax- or Bak-overexpressing cell lines were transduced with Ad-mycNbk-tTA and cultured under off or on conditions. Control cells were mock treated and grown in the absence of doxycycline. (A) Western blot analyses of Bax and Bak expression levels in different DU145 cell lines (top blots) and of induced Nbk expression (bottom blots). (B) Flow cytometric measurement of hypodiploid DNA after Nbk expression. The percentage of apoptotic cells was measured 24 h after adenoviral transduction. (C) Flow cytometric measurement of hypodiploid DNA after Bcl-xS expression. Cell lines were transduced with Ad-mycBcl-xS und cultured as described in Materials and methods. The percentage of apoptotic cells was measured 24 h after adenoviral tranduction. Data are means ± SD from three independent experiments.
Flow cytometric analysis of DNA fragmentation revealed that Nbk expression failed to induce apoptosis in the Bax-negative control cells. Despite strong Nbk expression, only 7% of the cells showed hypodiploid DNA content, compared with 5 and 6% under control and off conditions, respectively. In contrast, Bax-expressing DU145 cells readily underwent apoptotic DNA fragmentation with 39% of Bax-reexpressing DU145 cells showing sub-G1 DNA content (Fig. 3 B). This confirms the Bax dependency of Nbk-induced cell death observed in the HCT116 system.
In parallel, we generated DU145 cells stably overexpressing Bak by the use of a CMV promoter–driven pcDNA3-Bak vector as previously described (Hemmati et al., 2006). DU145 control cells, transfected with an empty vector, show moderate Bak expression, whereas transfection of DU145 cells with pcDNA3-Bak resulted in high Bak protein expression (Fig. 3 A). Transduction with Ad-mycNbk-tTA resulted in high and comparable expression of Nbk under on conditions. However, in sharp contrast to Bax, Bak failed to sensitize the DU145 cells for apoptosis induction in response to Nbk expression (Fig. 3 B). We have shown previously that Bak overexpression in Bax-negative DU145 cells sensitizes for DNA damage–induced apoptosis by the topoisomerase II poison epirubicin (von Haefen et al., 2004). Thus, the resistance of Bax-deficient DU145 cells to Nbk- induced apoptosis is not caused by an insufficient function of the Bak protein. This underlines the idea that, in contrast to Bax, functional Bak is not sufficient to transduce the cell death signal triggered by Nbk. This is in contrast to other BH3-only proteins, which trigger apoptosis via Bak- or Bax/Bak-dependent pathways. Notably, Bcl-xS–induced apoptosis is entirely dependent on Bak, but not on Bax, in mouse embryonic fibroblasts (Lindenboim et al., 2005). This is in line with our results obtained in the DU145 system. Bax-overexpressing DU145 cells were resistant to Bcl-xS, whereas Bak-overexpressing DU145 cells were highly sensitized for induction of apoptosis by Bcl-xS (Fig. 3 C). These results confirm that Bak is functional. Thus, the failure of Bak to become activated by Nbk is not caused by an impaired Bak protein.
To further investigate the specific role of Bax and Bak in Nbk-induced apoptosis, we generated DU145 cells stably expressing EGFP fusion proteins of Bax or Bak. To this end, the cDNA for Bax or Bak was inserted into the pEGFP-C1 vector, and resulting plasmids were transfected into DU145 cells. In agreement with an expected cytosolic localization of Bax, EGFP-Bax showed a homogenous, mostly cytoplasmic staining pattern (Fig. 4 A), with some Bax being localized constitutively at the mitochondria (Fig. 4 B). EGFP-Bak showed a different reticular localization, which corresponds to constitutive association of Bak with, e.g., mitochondrial membranes and the ER (Fig. 4 A; and see Fig. 8 C). Nbk expression induced a strong clustering of EGFP-Bax after 24 h, which indicates quantitative redistribution of Bax from the cytosol to the mitochondria and oligomerization of activated Bax. In contrast to EGFP-Bax, and despite the fact that translocation is not necessary in the case of Bak, negligible redistribution of EGFP-Bak was detected under on conditions (Fig. 4 A). This effect was far less than the effect observed for EGFP-Bax. Exposure to the anticancer drug 5-FU or Bcl-xS did, however, trigger massive clustering of Bak (von Haefen et al., 2004; unpublished data). Upon Nbk expression, EGFP-Bax coalesces into clusters that remain closely associated with mitochondria (Fig. 4 B). There, Bax localizes to mitochondrial tips and constriction sites, associates with Drp1 and Mfn2, and participates in apoptotic fragmentation of mitochondria (Nechushtan et al., 2001; Karbowski et al., 2002). Eventually, EGFP-Bax is located in small punctate structures. During this process, mitochondria cluster around the nucleus and cells undergoing apoptosis shrink and show rounded shape (Fig. S2).
Figure 4.
Nbk expression induces oligomerization and clustering of EGFP-Bax but not -Bak. (A) DU145 cells stably expressing EGFP-Bax or -Bak were transduced with Ad-mycNbk-tTA and cultured under off or on conditions. 24 h after infection of the cells, images were acquired by the use of a fluorescent microscope. Bar, 50 μm. (B) Subcellular localization pattern of EGFP-Bax. Mitochondria of DU145 EGFP-Bax cells were stained red by the use of an antibody against the Tom 20 protein and counterstained for DNA with DAPI. Bax shows a diffuse pattern of localization in nonapoptotic cells, concordant with its preferentially cytoplasmic localization (top). After Nbk expression Bax coalesces into large clusters adjacent to mitochondria (bottom). Bar, 25 μm.
Figure 8.
Knockdown of Mcl-1 facilitates conformational switch and clustering of Bak upon Nbk expression. 24 h after Mcl-1 down-regulation, cells were transduced with Ad-mycNbk-tTA and cultured for an additional 24 h under off or on conditions. (A) Cells were stained with a conformation-specific antibody against the Bak N terminus and analyzed by flow cytometry. The percentage of immunostained cells is indicated between markers. A representative experiment is shown (top). Data are means ± SD from three independent experiments (bottom). (B) Clustering of EGFP-Bak under on conditions after siRNA repression of Mcl-1. Bar, 50 μm. (C) Bak shows a more reticular localization that is in agreement with its constitutive association with mitochondria (left). In apoptotic cells, Bak coalesces into large clusters adjacent to mitochondria (right). Bar, 25 μm. (D) Coimmunoprecipitation analyses of Bcl-xL. Cell lysates were prepared as described in Materials and methods. Bcl-xL–Bax and Bcl-xL–Bak interaction were strongly diminished after Nbk expression in the presence of Mcl-1 (top) and after down-regulation of Mcl-1 by use of siRNA (bottom).
These data establish that Nbk is unable to induce apoptosis via a Bak-dependent pathway. We therefore aimed to test whether this is caused by the presence of an endogenous Bak inhibitor. Because of its constitutive membrane association, Bak is kept tightly in check by its binding to inhibitory proteins, such as VDAC2, and prosurvival members of the Bcl-2 family. As activation of Bak is reported to be specifically inhibited by two prosurvival members of the Bcl-2 family, Bcl-xL and Mcl-1 (Willis et al., 2005), we investigated interaction of Nbk with these proteins by coimmunoprecipitation studies. To this end, we used Bax-proficient DU145 cells expressing myc-tagged Nbk. MycNbk was precipitated and the resulting samples were examined for the presence of Mcl-1 and Bcl-xL by Western blot analysis. Mcl-1 could not be detected in anti-Nbk immunoprecipitates (Fig. 5 A, left, on), indicating that Nbk does not interact with Mcl-1. However, the opposite was true for Bcl-xL, which was readily detected in Nbk immunoprecipitates, thereby confirming that Nbk specifically binds to Bcl-xL. Additionally, as neither Bax nor Bak interact with Nbk, Nbk appears to act as a derepressor, which inhibits Bcl-xL function, rather than a direct activator of proapoptotic Bax-like molecules.
Figure 5.
Coimmunoprecipitation analyses of Bcl-2 family member interaction. (A) In contrast to Puma, which targets Mcl-1 and Bcl-xL, Nbk does not bind to Mcl-1. DU145 Bax cells were transduced with Ad-mycNbk-tTA or Ad-mycPuma-tTA and cultured under off or on conditions for 24 h. Lysates of cells solubilized in Triton X-100 buffer were immunoprecipitated with anti-Nbk (left) or anti-Puma (right) antibody. Immune complexes were resolved by SDS-PAGE, and proteins were detected by immunoblotting as indicated by arrows. (B) Mcl-1–Bak interaction persists despite Nbk expression, whereas Puma expression disrupts the Mcl-1–Bak complex. Cell lysates were prepared as described in A and immunoprecipitated with anti–Mcl-1 antibody. S, supernatant; P, immunoprecipitate.
On the basis of a recent publication by Chen et al. (2005), which demonstrated high affinity of the Puma BH3 peptide to all antiapoptotic Bcl-2 proteins, we expressed Puma in DU145 Bax cells as a control. Both Mcl-1 and Bcl-xL were detected in anti-Puma immunoprecipitates (Fig. 5 A, right, on). This indicates that, in contrast to Nbk, Puma targets both antiapoptotic proteins, Bcl-xL and Mcl-1, in vivo. In line with the assumption that Puma, like Nbk, functions as a derepressor, we did not observe previously reported interactions of Bax with a Puma BH3 domain in a cell-free system (Kuwana et al., 2005) or of Bax with Puma in vivo (Cartron et al., 2004). Neither did we observe an interaction of Puma with Bak. A reciprocal immunoprecipitation was also performed to confirm the aforementioned data. This time, Mcl-1 was precipitated from DU145 Bax cells overexpressing Nbk or Puma. In accordance with the immunoprecipitations described in Fig. 5 A, Nbk was not detectable in anti–Mcl-1 immunoprecipitates (Fig. 5 B, left, on), whereas Puma was readily detected in Mcl-1 immunoprecipitates (Fig. 5 B, right, on). These data demonstrate that different BH3-only proteins target different antiapoptotic Bcl-2 family members in a cellular context. This is well supported by Biacore data showing specific interactions of BH3 domain peptides with antiapoptotic Bcl-2 family proteins (Chen et al., 2005).
We next asked if Nbk or Puma might differentially disrupt the Mcl-1–Bak interaction. Under off conditions, Bak can be detected in anti–Mcl-1 immunoprecipitates (Fig. 5 B). Despite high levels of Nbk expression under on condition, this Mcl-1–Bak interaction is not disturbed by Nbk (Fig. 5 B, left). In contrast to Nbk, Puma binds to Mcl-1 and displaces Bak, as Bak is no longer detectable in anti–Mcl-1 immunoprecipitates after Puma expression (Fig. 5 B, right). Mcl-1 interacts with Bak (but not with Bax), as Bak (but not Bax) was detectable in anti–Mcl-1 immunoprecipitates (Fig. 5 B). This was observed under conditions that promote association of prosurvival proteins with Bax, i.e., the use of nonionic detergent Triton X-100. In contrast, no such findings have been reported for Bak, and Bak has also been shown to associate with Mcl-1 in the presence of CHAPS. Collectively, these interaction studies indicate that Nbk, in contrast to Puma, is insufficient to displace Bak from Mcl-1, as Nbk specifically interacts with Bcl-xL but not with Mcl-1.
Interestingly, we found that Mcl-1 expression is increased in cells expressing Nbk. Inhibition of Bak by Mcl-1 might be enforced by this stabilization of Mcl-1. In this regard, a recent study showed that the BH3-only protein Puma also stabilizes Mcl-1 levels (Mei et al., 2005). To investigate Mcl-1 stabilization, we blocked proteasomal degradation of Mcl-1 by the use of the ubiquitin proteasome inhibitor MG132 or induced expression of Puma and compared Mcl-1 levels to those upon Nbk expression in DU145 cells. Western blot analysis revealed that inhibition of the proteasome by MG132 and expression of Puma lead to increased levels of Mcl-1, and expression of Nbk gave comparable results (Fig. 6). However, in contrast to Puma, which may stabilize Mcl-1 through direct interactions (Mei et al., 2005), Nbk does not bind to Mcl-1. Thus, other and/or indirect mechanisms must be involved in the up-regulation of Mcl-1 in response to Nbk expression. As shown by coimmunoprecipitation experiments, Puma binds to Bcl-xL, and additional overexpression of Nbk strongly reduces this interaction (Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200703040/DC1). It might be that Puma is displaced from Bcl-xL after Nbk expression and then is available to bind and stabilize Mcl-1. However, expression levels of Puma are moderate in DU145 cells, and expression of Nbk leads to Mcl-1 stabilization, even after down-regulation of Puma by siRNA (Fig. 6). Thus, stabilization of Mcl-1 by Nbk is independent of Puma. Furthermore, stabilization of Mcl-1 by Nbk or Puma is specific, as expression levels of Bcl-xL and Bcl-2 (which is barely detectable in DU145) do not change after Nbk or Puma expression (Fig. 6).
Figure 6.
Nbk expression stabilizes Mcl-1 levels independently of Puma. DU145 were treated with control siRNA or, to down-regulate Puma, with siRNA against Puma mRNA. 24 h later, cells were transduced with either Ad-mycNbk-tTA or Ad-mycPuma-tTA and cultured for an additional 24 h under off or on conditions. As control, cells were cultured with or without 20 μM ubiquitin proteasome inhibitor MG132 (MG). Protein levels were analyzed by immunoblotting. Mcl-1 levels were increased in the presence of Nbk or Puma expression. Stabilization of Mcl-1 by Nbk is independent of Puma, as Nbk stabilizes Mcl-1 even after down regulation of Puma by siRNA. For Bcl-2, a positive control was added (far right).
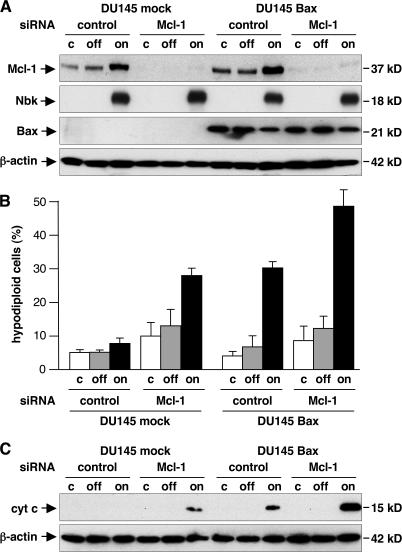
To functionally address the contribution of Mcl-1 in Bak regulation during Nbk-induced cell death, we down-regulated Mcl-1 by RNA interference in Bax-deficient and Bax-reexpressing DU145 cells (Fig. 7 A). Mcl-1 expression was specifically decreased in both cell lines by Mcl-1 siRNA but not by control siRNA. As expected, Bax-deficient DU145 cells were resistant to Nbk-induced apoptosis, and reconstitution of Bax sensitized these cells for Nbk-induced apoptosis. Lipofection of Mcl-1 siRNA sensitized both Bax-deficient and -proficient DU145 cells for Nbk-induced apoptosis, whereas an irrelevant control siRNA had no effect (Fig. 7 B). Consistent with these data, Nbk-triggered release of cytochrome c is increased after down-regulation of Mcl-1. DU145 cells treated with control siRNA did not show cytochrome c release, whereas Mcl-1 siRNA causes release of cytochrome c after Nbk expression in these Bax-deficient cells (Fig. 7 C). In Bax-proficient DU145 cells, cytochrome c release was increased by Mcl-1 siRNA but not by control siRNA. Measurement of phosphatidylserine exposure and PI uptake confirmed the increased numbers of apoptotic cells upon Mcl-1 down-regulation. Although Bax-deficient DU145 cells were resistant to Nbk-induced phosphatidylserine exposure, reconstitution of Bax sensitized these cells for Nbk-induced apoptosis. Furthermore, down-regulation of Mcl-1 sensitized both cell lines for Nbk-induced phosphatidylserine exposure. About 26% of DU145 mock cells were detected as Annexin V positive (13.5% late apoptotic [PI positive] and 12.5% early apoptotic [PI negative]). Overall, 40% of Bax-reexpressing DU145 cells were stained with Annexin V–FITC (16.5% late and 24% early apoptotic; Fig. S4, available at http://www.jcb.org/cgi/content/full/jcb.200703040/DC1). Thus, knockdown of Mcl-1 renders Bax- deficient/Bak-expressing DU145 cells susceptible to Nbk-induced cytochrome c release and apoptosis.
Figure 7.
siRNA silencing of Mcl-1 sensitizes Bak-proficient DU145 cells for Nbk-induced apoptosis and cytochrome c release. Mcl-1 was down-regulated in DU145 mock and Bak cells by use of siRNA. 24 h later, cells were transduced with Ad-mycNbk-tTA to induce Nbk expression and cultured for additional 24 h under off or on conditions. (A) SiRNA silencing of Mcl-1, overexpression of Nbk, and the Bax expression status were confirmed by Western blot analysis. (B) The percentage of apoptotic cells was determined by flow cytometric measurement of hypodiploid DNA. Data are means ± SD from three independent experiments. (C) Cytosolic extracts were prepared and subjected to immunoblotting to analyze Nbk-induced cytochrome c release after down-regulation of Mcl-1.
During apoptosis, Bak undergoes a conformational change leading to the exposure of an N-terminal epitope. To study if Nbk-induced apoptosis results in Bak activation after siRNA repression of Mcl-1 in Bax-deficient DU145 cells, we performed immunofluorescent stainings with a conformation-specific antibody directed against the Bak N terminus. siRNA-mediated down-regulation of Mcl-1 enabled Nbk to induce Bak activation in DU145 cells, whereas an irrelevant control siRNA had no effect (Fig. 8 A). In agreement with the conformational change of Bak, Nbk expression induced a strong clustering of EGFP-Bak after Mcl-1 knockdown (Fig. 8 B). In contrast, cells treated with control siRNA showed no EGFP-Bak clustering after induction of Nbk expression.
In Bax-deficient DU145 cells treated with Mcl-1 siRNA, EGFP-Bak, unlike EGFP-Bax, constitutively colocalized with mitochondria in healthy cells and was not detected in the cytosol (Fig. 8 C, left). Upon activation of Bak by induced expression of Nbk, EGFP-Bak, like EGFP-Bax, localized to mitochondrial tips and coalesced into large clusters that remained associated with mitochondria (Fig. 8 C, right). Eventually the phenotype is comparable to EGFP-Bax, with small punctate EGFP-Bak structures, clustering of mitochondria, and a round-up apoptotic shape off the cells (Fig. S5, available at http://www.jcb.org/cgi/content/full/jcb.200703040/DC1).
According to a recent paper, Bak is not only sequestered by Mcl-1 but also by Bcl-xL (Willis et al., 2005). To study if Nbk can inactivate Bcl-xL to release and activate Bak, we performed coimmunoprecipitation experiments using a Bcl-xL–specific antibody (Fig. 8 D). Both Bax and Bak can be detected in anti–Bcl-xL immunoprecipitates under off conditions, indicating binding and inactivation of these proteins by Bcl-xL. Upon induction of Nbk expression in the absence of doxycycline, Nbk was coprecipitated with Bcl-xL, whereas coprecipitation of Bak and, even more pronounced, of Bax with Bcl-xL was reduced. This indicates that Nbk binds to Bcl-xL, thereby partially displacing Bak from Bcl-xL and almost completely freeing Bax from its binding to Bcl-xL. Identical results were obtained after down-regulation of Mcl-1. However, as shown in Fig. 8, only after down-regulation of Mcl-1 did Nbk expression induce activation of Bak.
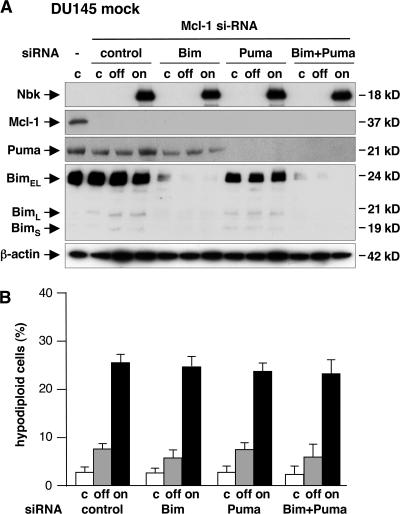
The functional studies established that Nbk-induced cell death is increased after down-regulation of Mcl-1 (Fig. 7). Regarding a displacement model for activation of Bax/Bak by BH3-only proteins, the knockdown of Mcl-1 may cause the release of BH3-only proteins bound to Mcl-1, such as Puma and Bim, which could render the system more sensitive to apoptosis. To study a putative role of these BH3-only proteins in Nbk- induced apoptosis, we analyzed the effect of siRNA-mediated down-regulation of Bim, Puma, or both in Bax-deficient DU145 mock cells after knockdown of Mcl-1. Knockdown of the prespective proteins was confirmed by Western blot analysis (Fig. 9 A). Down-regulation of Mcl-1 sensitized these Bax-deficient/Bak-expressing DU145 cells for Nbk-induced apoptosis. Nevertheless, the additional knockdown of Bim, Puma, or both failed to affect Nbk-induced apoptosis. Upon induction of Nbk, ∼26% of cells showed sub-G1 DNA content regardless of Puma or Bim expression (Fig. 9 B). Finally, the Mcl-1 inhibitor Noxa is barely detectable in DU145 cells (unpublished data). These results demonstrate that sensitization to Nbk-induced apoptosis after down-regulation of Mcl-1 is a direct effect of Nbk that is independent from these additional BH3-only proteins.
Figure 9.
Mcl-1 siRNA–induced sensitization to Nbk is independent of Bim and Puma. 24 h before transduction of Bax-deficient DU145 cells with Ad-mycNbk-tTA, Mcl-1 was down-regulated by use of siRNA to facilitate Bak activation. To assess a putative role of other BH3- only proteins, Puma and Bim expression were blocked by siRNA. DU145 cells were then cultured for 24 h under off or on conditions to repress or induce Nbk expression, respectively. Control cells were mock treated. (A) Expression levels of the indicated proteins were analyzed by immunoblotting. (B) Apoptosis was determined by flow cytometric measurement of genomic DNA fragmentation. Cells displaying sub-G1 hypodiploid DNA content are considered apoptotic. Data are means ± SD from three independent experiments.
Discussion
Nbk is a BH3-only protein that is expressed in a restricted subset of human epithelial tissues with strongest expression in the kidney (Daniel et al., 1999). In line with a potential role of Nbk in tumor suppression, loss of Nbk is a common feature of renal cell carcinoma (Sturm et al., 2006), and the chromatin locus 22p13.3, which contains nbk, is frequently deleted in human colorectal and breast cancers (Castells et al., 1999). Expression of Nbk triggers apoptosis in breast, lung, prostate, and colon carcinoma, as well as glioma- and melanoma-derived cell lines (Tong et al., 2001; Germain et al., 2002; Gillissen et al., 2003; Oppermann et al., 2005).
Because Nbk is a constitutively active protein, it is mainly regulated on the transcriptional level, and induction of Nbk in response to genotoxic stress is mediated in a p53-dependent manner (Mathai et al., 2002, 2005). Based on previous analyses that Bax deficiency protects cancer cells from Nbk-induced apoptosis, even though these cells retain expression of the Bax homologous protein Bak, it appears that the proapoptotic function of Nbk depends on the presence of Bax but not of Bak (Theodorakis et al., 2002; Gillissen et al., 2003).
Interestingly, it has been suggested most recently that BH3-only proteins can function via two mutual, yet not exclusive, modes of action (Letai et al., 2002; Kuwana et al., 2005). For the BH3-only proteins tBid, Bim, and, more controversially, Puma (Cartron et al., 2004), a physical interaction and direct activation of both Bax and Bak has been proposed. These BH3-only proteins were therefore described as direct activators.
The other mode of BH3-only proteins is derepression, which means the release of proapoptotic potential of Bax or Bak by counteracting the inhibitory effect of antiapoptotic Bcl-2 proteins. Derepressors can release Bax and/or Bak from their inhibitory antiapoptotic counterparts through competitive binding of their BH3 domain to antiapoptotic Bcl-2 proteins. Strong evidence for the derepressor model comes from the observation that Bax and Bak can mediate apoptosis without discernable association with the putative BH3-only activators (Bim, Bid, and Puma), even in cells with no Bim or Bid and reduced Puma (Willis et al., 2007).
Nbk interacts with antiapoptotic Bcl-2 family members, but not with the proapoptotic multidomain proteins Bax (Boyd et al., 1995; Han et al., 1996; Elangovan and Chinnadurai, 1997; Gillissen et al., 2003) and Bak, indicating that Nbk functions as a derepressor to activate apoptosis. However, regarding the proposed rheostat model where the relative concentration of antiapoptotic, Bcl-2–like, and proapoptotic family members determines the fate of a cell, the role of Bak in Nbk-induced apoptosis is poorly investigated.
In this study we demonstrate that specific loss of Bax, but not of Bak, confers resistance to Nbk-induced apoptosis. Furthermore, we show that, in contrast to Bax reexpression, expression of Bak does not sensitize Bax-deficient DU145 cells for Nbk-induced cell death. Nbk fails to induce clustering and oligomerization of EGFP-Bak fusion protein but induces strong EGFP-Bax translocation. This demonstrates that Nbk-mediated apoptosis ultimately depends on Bax, whereas Bak is dispensable.
Interestingly, Bak is sequestered by the antiapoptotic proteins Bcl-xL and Mcl-1 (but not by Bcl-2), and activation of Bak requires neutralization of both proteins Bcl-xL and Mcl-1 by BH3-only family members (Willis et al., 2005). In turn, we showed that Nbk interacts with Bcl-xL, but interaction with Mcl-1 could not be detected. Moreover, Nbk fails to disrupt Mcl-1–Bak interaction. Notably, Bak is sequestered and inactivated by Mcl-1, even in the presence of strong Nbk expression. This is in contrast to Puma, which binds to Bcl-xL and Mcl-1 and releases Bak from Mcl-1 binding. In this context, it is interesting that peptides derived from BH3-only domains exhibit differences in their binding selectivity to antiapoptotic Bcl-2 proteins. Determination of the binding constants by Biacore experiments revealed that Puma potently engaged all the prosurvival proteins, including Mcl-1, whereas Nbk showed very low affinity to Mcl-1 (Chen et al., 2005). Thus, it appears to be reasonable that Nbk blocks the inhibitory function of Bcl-xL on Bak, but the antiapoptotic Mcl-1 is capable of backing up and still keeping Bak in check as a second restraint. Inhibition of Bak by Mcl-1, which in turn cannot be inactivated by Nbk, is therefore responsible for the failure of Nbk to induce Bak activation. Indeed, down-regulation of Mcl-1 by siRNA sensitizes Bax- deficient DU145 cells for Nbk-induced apoptosis and enables Nbk to activate Bak. This hints at a staggered barrier formed by antiapoptotic proteins, which is counteracted by the likewise graduated proapoptotic potential of BH3-only proteins.
Mcl-1 is readily down-regulated in response to certain death stimuli. The short half-life of Mcl-1 is attributed to constitutive polyubiquitination and subsequent degradation of Mcl-1 by the proteasome, which is a prerequisite for induction of apoptosis after UV irradiation (Nijhawan et al., 2003). Polyubiquitination of Mcl-1 is catalyzed, e.g., by Mule/ARF-BP1, a novel E3 ubiquitin ligase that binds to Mcl-1 via a BH3 domain and marks Mcl-1 for proteasomal degradation (Zhong et al., 2005). The BH3-only protein Noxa also plays a crucial role in Mcl-1 degradation. By displacing Bak from Mcl-1, Noxa enhances polyubiquitination and subsequent proteasomal degradation of Mcl-1 (Willis et al., 2005). In contrast to Noxa-induced degradation of Mcl-1, we found that the cellular Mcl-1 protein level was even increased after Nbk expression. This effect of Nbk expression was specific for Mcl-1 and was not observed for Bcl-2 or Bcl-xL. A similar phenomenon has been previously reported in the context of Puma overexpression. In these experiments, Mcl-1 was stabilized by binding to Puma, and it has been suggested that the interaction between Mcl-1 and Puma and the resulting stabilization of Mcl-1 may represent a novel mechanism to regulate and prevent apoptosis (Mei et al., 2005). However, Mcl-1 is stabilized by Nbk even when Puma is knocked down. Thus it is unlikely that Puma, after being displaced from Bcl-xL by Nbk, mediates Mcl-1 stabilization in this setting. In our experimental system, persistent binding of Bak to Mcl-1 might therefore be responsible for enhanced Mcl-1 expression via stabilization, as Nbk, unlike Puma, does not bind to Mcl-1 to displace Bak. Consistent with these data, we found that adenoviral overexpression of Puma, despite stabilizing Mcl-1, can induce apoptosis in a Bak-dependent manner (in Bax-deficient DU145 Bak cells), although to a lesser extent compared with DU145 Bax cells (unpublished data). Therefore, stabilization of Mcl-1 may exert distinct regulatory functions in the fine-tuning of apoptosis and attenuating Puma-induced apoptosis. Moreover, Mcl-1 stabilization by Nbk interferes with activation of Bak because Nbk, in contrast to Puma, fails to bind and inactivate Mcl-1. Thus, in Nbk-induced apoptosis, Bak cannot compensate for Bax because of the low affinity of Nbk to Mcl-1 and the up-regulation of Mcl-1 after expression of Nbk, which may further interfere with activation of Bak. Support for this model comes from the observation that the BH3-only protein Noxa, which targets Mcl-1, cooperates with Nbk to activate mitochondrial cytochrome c release; however, the impact on Bak activation was not addressed in this study and all experiments were performed in a Bax-proficient background (Germain et al., 2005).
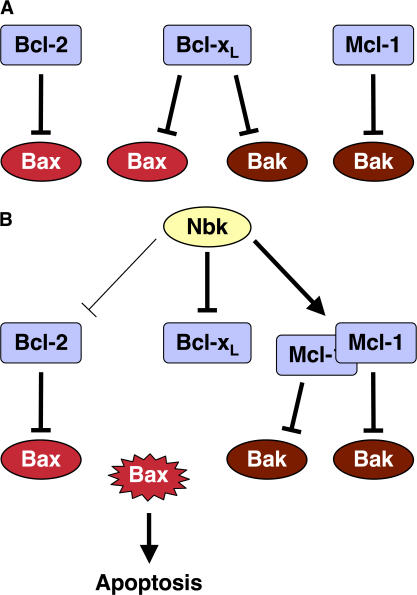
Collectively, our data support a model in which the proapoptotic activity of BH3-only proteins is governed by their propensity to interfere with an at least dual layer of protection of Bak (Willis and Adams, 2005). In our system, binding of Nbk inactivates Bcl-xL and releases Bax and Bak (Fig. 10). Although the interaction between Nbk and Bcl-xL seems to be stronger, Nbk also binds to Bcl-2 (Elangovan and Chinnadurai, 1997; Verma et al., 2001; Gillissen et al., 2003), indicating that Bcl-2 is at least partially inhibited by Nbk. Once the capacity of Bcl-2 to bind Bax is exceeded, the unbound Bax protein becomes activated. Notably, the Bax inhibitors Bcl-2 and Bcl-xL are not stabilized by Nbk. In contrast, Bak, released from Bcl-xL upon Nbk expression, is still kept in check by Mcl-1, and this is even enforced by the up-regulation of the Bak inhibitor Mcl-1 by Nbk. This Bak inhibition may be further supported by other factors that do not necessarily belong to the Bcl-2 family, such as the VDAC2 protein (Cheng et al., 2003; Chandra et al., 2005). Mcl-1–mediated repression of Bak thereby provides a molecular rationale for the strict Bax dependency of apoptosis induced by Nbk in the present setting. Thus, BH3-only proteins that, like Nbk, act as derepressors bind to distinct antiapoptotic proteins. Nevertheless, the consequently derepressed proapoptotic multidomain proteins can then be counteracted by a second row of antiapoptotic proteins. This adds a higher degree of specificity and opportunities for the fine-tuning of death signals. The outcome of triggering this tightly balanced interaction network would be dictated by both the mix of BH3-only proteins induced by a given death stimulus and the abundance of their antiapoptotic counterparts forming this second barrier to apoptosis. Support for the biological relevance of such a Bak inhibitor model comes indirectly from cancer biology. There, disruption of the bax gene impairs myc-induced islet cell depeletion upon myc expression and facilitates tumorigenesis in a myc-driven pancreatic β-cell tumor model, whereas genetic inactivation of bak has no impact (Dansen et al., 2006). In fact, loss of Bax is a frequent event in human cancer, whereas Bak expression persists in most cancers. Thus, targeting Bak inhibitors should be a rewarding strategy to overcome restraints in apoptosis signaling in cancer and resulting resistance mechanisms.
Figure 10.
Model for the regulation of Nbk-induced apoptosis by Bcl-2 family members. (A) In healthy cells, Bax is inactivated by Bcl-2 and Bcl-xL, whereas Bak is sequestered by Bcl-xL and Mcl-1. (B) Nbk activates the proapoptotic Bax through neutralization of the antiapoptotic Bcl-xL. In contrast, increased levels of the antiapoptotic Mcl-1 keep in check Bak that was released from Bcl-xL upon competition with Nbk.
Materials and methods
Cell culture and cell lines
HEK293 and DU145 cells were obtained from the American Type Culture Collection or the Deutsche Sammlung von Mikroorganismen und Zellkulturen. HCT116 wt cells and their isogeneic knockout sublines HCT116 Bax−/Bak− were provided by B. Vogelstein (Johns Hopkins Cancer Center, Baltimore, MD). The stable knockdown of Bak was performed in either HCT116 wt or HCT116 Bax knockout cells yielding Bax+/Bak− and Bax−/Bak− cells. Transfectants were generated and cells were cultured as previously described (Gillissen et al., 2003; von Haefen et al., 2004; Hemmati et al., 2006). Expression of BH3-only proteins was suppressed by addition of 1 μg/ml doxycycline to the culture medium (Tet-off condition).
Antibodies and immunoblotting
Monoclonal mouse anti-Bax antibody (clone YTH-2D2; raised against a peptide corresponding to aa 3–16) was purchased from Trevigen, Inc., and goat anti-Nbk antibody (N-19; raised against an epitope mapping to the 19-aa N terminus of human Nbk) and rabbit anti–Mcl-1 (H-260; epitope corresponding to aa 1–260 mapping at the N terminus of Mcl-1 of human origin) were purchased from Santa Cruz Biotechnology, Inc. Polyclonal rabbit anti–Bcl-xS/L antibody (raised against aa 18–233 of rat Bcl-xL) and anti-Bim (against aa 22–40 of human Bim) were purchased from BD Biosciences, monoclonal mouse anti–human cytochrome c (clone 7H8.2C12) was purchased from BD Biosciences, and goat anti–caspase-9 antibody (raised against human caspase-9 aa 139–330) was purchased from R&D Systems. The polyclonal rabbit anti-Bak antibody (raised against a peptide corresponding to aa 14–36) was purchased from Dako. Polyclonal anti–human actin antibody and anti-Puma, produced in rabbits, were obtained from Sigma-Aldrich. Polyclonal anti–human caspase-3, produced in goat, was obtained from R&D Systems. Secondary anti–rabbit, anti–goat, and anti–mouse HRP-conjugated antibodies were obtained from Promega or SouthernBiotech. After cell trypsination and washing, protein expression was detected by ECL-based Western blot analysis as described previously (Gillissen et al., 2003). For analysis of cytochrome c release, cells were lysed in a hypotonic digitonin buffer as described previously (von Haefen et al., 2003).
Construction of recombinant adenovirus
The recombinant adenovirus for Puma or Bcl-xS expression was constructed for mycNbk as described previously (Gillissen et al., 2003). In brief, the E3 region of Ad5 was replaced by tTA under the control of a CMV promoter and an SV40 poly(A) tail. A Puma or Bcl-xS cDNA was introduced into the E1 region under the control of the Tet-off system to achieve conditional expression in the absence of doxycycline. The resulting DNA construct was transfected in HEK293 packaging cells to produce vector stocks. Adenoviral stocks were propagated according to standard procedures described in Hemmati et al. (2002).
Measurement of apoptotic cell death by flow cytometry
Apoptosis was determined on a single-cell level by measuring the DNA content of individual cells with a FACScan (BD Biosciences) as described previously (Gillissen et al., 2003). Cellular DNA content was measured by flow cytometry with logarithmic amplification in the FL-3 channel. Data are given in percentage of hypoploidy (sub-G1), which reflects the percentage of apoptotic cells. Alternatively, apoptotic cell death was determined by PI staining and measuring binding of Annexin V–FITC upon exposure of phosphatidylserine to the cell surface (Gillissen et al., 2003).
Measurement of mitochondrial permeability transition
After infection with recombinant adenovirus at the MOI of 25 in the presence or absence of doxycycline, cells were collected by centrifugation at 300 g at 4°C for 5 min. Mitochondrial permeability transition was determined by staining the cells with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolylcarbocyanin iodide (JC-1; Invitrogen) as described previously (Gillissen et al., 2003). Mitochondrial permeability transition was then quantified by flow-cytometric determination of cells with decreased red fluorescence, i.e., with mitochondria displaying a lower ΔΨm.
Fluorescence microscopy
DU145 EGFP-Bax or EGFP-Bak cells were seeded on coverslips, infected with Ad-mycNbk-tTA, and cultured for 24 h under on or off conditions. Cells were washed with PBS and fixed for 30 min with ice-cold 1% paraformaldehyde. For immunofluorescence staining of mitochondria, cells were permeabilized with ice-cold 100% methanol for 1 min. Cells were washed again, and nonspecific binding of antibodies was blocked by incubation with 8% BSA for 30 min at room temperature. Primary antibodies (mouse anti–Tom 20 antibody [BD Biosciences]) were diluted in 1% BSA in PBS and added to the cells overnight at 4°C. Incubation with secondary antibodies (Alexa Fluor 594–conjugated chicken anti–mouse IgG [Invitrogen]) was performed for 1 h at room temperature. For staining of DNA, cells were incubated in PBS + DAPI for 5 min. Cells were washed and mounted in fluorescence mounting medium (Dako). For overview images, cells were inspected at room temperature with a fluorescent microscope (BX50; Olympus) equipped with a 40×/0.75 objective lens (UPlanFL; Olympus) and a camera (micropublisher 5.0 RTV; QImaging). For subcellular localization pattern analysis, cells were inspected at room temperature with a microscope (Axiovert 200; Carl Zeiss, Inc.) equipped with a 63×/1.4 objective lens (Plan-Apochromat; Carl Zeiss, Inc.) and a digital camera (ORCA ER; Hamamatsu Photonics). Images were acquired by Openlab software (Improvision) and vertical slices (0.2-μm separation) were deconvoluted with Openlab 5.0.2 nearest–neighbor deconvolution algorithm on Mac OSX 10.4 (Apple Inc.).
Coimmunoprecipitation
Coimmunoprecipitations were performed using the ExactaCruz IP/Western blot reagents (Santa Cruz Biotechnology, Inc.) according to the manufacturer's instructions. In brief, 1.5 × 106 cells/75-cm2 flask were infected with Ad-mycNbk-tTA or Ad-Puma-tTA and cultured for 24 h with or without doxycycline. Cells were harvested and lysed as described previously (Gillissen et al., 2003). Lysates were precleared with preclearing matrix (Santa Cruz Biotechnology, Inc.). 150 μl of the precleared cellular extract was shaken in the presence of the IP antibody–IP matrix complex at 4°C for 4 h. Immunoprecipitations were done and examined by Western blot analysis as described previously (Gillissen et al., 2003).
RNA interference
Mcl-1 siRNA and control siRNA were purchased from Santa Cruz Biotechnology, Inc. Lipofection of the cells was performed by use of transfection reagent (Santa Cruz Biotechnology, Inc.) according to the manufacturer's instructions. On-target plus siRNA against Puma, Bim, Mcl-1, and control siRNA for experiments shown in Figs. 6 and 9 was purchased from Dharmacon. Transfection of the cells was performed by use of transfection reagent (DharmaFECT; Dharmacon) according to the manufacturer's instructions. After 24 h, cells were transduced with Ad-mycNbk-tTA at an MOI of 25.
Online supplemental material
Fig. S1 shows Nbk-induced phosphatidyl serine exposure of HCT116 cell lines. Fig. S2 shows changes of the subcellular localization pattern of EGFP-Bax and mitochondria after Nbk expression. Fig. S3 shows coimmunoprecipitation studies of Puma and Bcl-xL. Fig. S4 shows Nbk-induced phosphatidyl serine exposure of DU145 cell lines after Mcl-1 knockdown. Fig. S5 shows subcellular localization pattern of EGFP-Bak and mitochondria upon Nbk expression. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200703040/DC1.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft, the Deutsche Krebshilfe e.V., and the European Union (multiple grants used).
Abbreviations used in this paper: Bcl, B cell lymphoma; BH, Bcl-2 homology; CMV, cytomegalovirus; Mcl, myeloid cell leukemia; PI, propidium iodide; wt, wild type.
References
- Bargou, R.C., P.T. Daniel, M.Y. Mapara, K. Bommert, C. Wagener, B. Kallinich, H.D. Royer, and B. Dorken. 1995. Expression of the bcl-2 gene family in normal and malignant breast tissue: low bax-alpha expression in tumor cells correlates with resistance towards apoptosis. Int. J. Cancer. 60:854–859. [DOI] [PubMed] [Google Scholar]
- Boyd, J.M., G.J. Gallo, B. Elangovan, A.B. Houghton, S. Malstrom, B.J. Avery, R.G. Ebb, T. Subramanian, T. Chittenden, R.J. Lutz, et al. 1995. Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene. 11:1921–1928. [PubMed] [Google Scholar]
- Cartron, P.F., P. Juin, L. Oliver, S. Martin, K. Meflah, and F.M. Vallette. 2003. Nonredundant role of Bax and Bak in Bid-mediated apoptosis. Mol. Cell. Biol. 23:4701–4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartron, P.F., T. Gallenne, G. Bougras, F. Gautier, F. Manero, P. Vusio, K. Meflah, F.M. Vallette, and P. Juin. 2004. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol. Cell. 16:807–818. [DOI] [PubMed] [Google Scholar]
- Castells, A., Y. Ino, D.N. Louis, V. Ramesh, J.F. Gusella, and A.K. Rustgi. 1999. Mapping of a target region of allelic loss to a 0.5-cM interval on chromosome 22q13 in human colorectal cancer. Gastroenterology. 117:831–837. [DOI] [PubMed] [Google Scholar]
- Chandra, D., G. Choy, P.T. Daniel, and D.G. Tang. 2005. Bax-dependent regulation of Bak by voltage-dependent anion channel 2. J. Biol. Chem. 280:19051–19061. [DOI] [PubMed] [Google Scholar]
- Chen, L., S.N. Willis, A. Wei, B.J. Smith, J.I. Fletcher, M.G. Hinds, P.M. Colman, C.L. Day, J.M. Adams, and D.C. Huang. 2005. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell. 17:393–403. [DOI] [PubMed] [Google Scholar]
- Cheng, E.H., T.V. Sheiko, J.K. Fisher, W.J. Craigen, and S.J. Korsmeyer. 2003. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 301:513–517. [DOI] [PubMed] [Google Scholar]
- Cory, S., D.C. Huang, and J.M. Adams. 2003. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 22:8590–8607. [DOI] [PubMed] [Google Scholar]
- Daniel, P.T., K.T. Pun, S. Ritschel, I. Sturm, J. Holler, B. Dorken, and R. Brown. 1999. Expression of the death gene Bik/Nbk promotes sensitivity to drug-induced apoptosis in corticosteroid-resistant T-cell lymphoma and prevents tumor growth in severe combined immunodeficient mice. Blood. 94:1100–1107. [PubMed] [Google Scholar]
- Dansen, T., J. Whitfield, F. Rostker, L. Brown-Swigart, and G. Evan. 2006. Specific requirement for Bax, not Bak, in Myc-induced apoptosis and tumor suppression in vivo. J. Biol. Chem. 281:10890–10895. [DOI] [PubMed] [Google Scholar]
- Desagher, S., A. Osen-Sand, A. Nichols, R. Eskes, S. Montessuit, S. Lauper, K. Maundrell, B. Antonsson, and J.C. Martinou. 1999. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 144:891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elangovan, B., and G. Chinnadurai. 1997. Functional dissection of the pro-apoptotic protein Bik. Heterodimerization with anti-apoptosis proteins is insufficient for induction of cell death. J. Biol. Chem. 272:24494–24498. [DOI] [PubMed] [Google Scholar]
- Germain, M., J.P. Mathai, and G.C. Shore. 2002. BH3-only BIK functions at the endoplasmic reticulum to stimulate cytochrome c release from mitochondria. J. Biol. Chem. 277:18053–18060. [DOI] [PubMed] [Google Scholar]
- Germain, M., J.P. Mathai, H.M. McBride, and G.C. Shore. 2005. Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis. EMBO J. 24:1546–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillissen, B., F. Essmann, V. Graupner, L. Starck, S. Radetzki, B. Dorken, K. Schulze-Osthoff, and P.T. Daniel. 2003. Induction of cell death by the BH3-only Bcl-2 homolog Nbk/Bik is mediated by an entirely Bax-dependent mitochondrial pathway. EMBO J. 22:3580–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J., P. Sabbatini, and E. White. 1996. Induction of apoptosis by human Nbk/Bik, a BH3-containing protein that interacts with E1B 19K. Mol. Cell. Biol. 16:5857–5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmati, P.G., B. Gillissen, C. von Haefen, J. Wendt, L. Starck, D. Guner, B. Dorken, and P.T. Daniel. 2002. Adenovirus-mediated overexpression of p14(ARF) induces p53 and Bax-independent apoptosis. Oncogene. 21:3149–3161. [DOI] [PubMed] [Google Scholar]
- Hemmati, P.G., D. Guner, B. Gillissen, J. Wendt, C. von Haefen, G. Chinnadurai, B. Dorken, and P.T. Daniel. 2006. Bak functionally complements for loss of Bax during p14ARF-induced mitochondrial apoptosis in human cancer cells. Oncogene. 25:6582–6594. [DOI] [PubMed] [Google Scholar]
- Karbowski, M., Y.J. Lee, B. Gaume, S.Y. Jeong, S. Frank, A. Nechushtan, A. Santel, M. Fuller, C.L. Smith, and R.J. Youle. 2002. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 159:931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer, S.J., J.R. Shutter, D.J. Veis, D.E. Merry, and Z.N. Oltvai. 1993. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin. Cancer Biol. 4:327–332. [PubMed] [Google Scholar]
- Kuwana, T., L. Bouchier-Hayes, J.E. Chipuk, C. Bonzon, B.A. Sullivan, D.R. Green, and D.D. Newmeyer. 2005. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell. 17:525–535. [DOI] [PubMed] [Google Scholar]
- Letai, A., M.C. Bassik, L.D. Walensky, M.D. Sorcinelli, S. Weiler, and S.J. Korsmeyer. 2002. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2:183–192. [DOI] [PubMed] [Google Scholar]
- Lindenboim, L., S. Kringel, T. Braun, C. Borner, and R. Stein. 2005. Bak but not Bax is essential for Bcl-xS-induced apoptosis. Cell Death Differ. 12:713–723. [DOI] [PubMed] [Google Scholar]
- Mathai, J.P., M. Germain, R.C. Marcellus, and G.C. Shore. 2002. Induction and endoplasmic reticulum location of BIK/NBK in response to apoptotic signaling by E1A and p53. Oncogene. 21:2534–2544. [DOI] [PubMed] [Google Scholar]
- Mathai, J.P., M. Germain, and G.C. Shore. 2005. BH3-only BIK regulates BAX,BAK-dependent release of Ca2+ from endoplasmic reticulum stores and mitochondrial apoptosis during stress-induced cell death. J. Biol. Chem. 280:23829–23836. [DOI] [PubMed] [Google Scholar]
- Mei, Y., W. Du, Y. Yang, and M. Wu. 2005. Puma(*)Mcl-1 interaction is not sufficient to prevent rapid degradation of Mcl-1. Oncogene. 24:7224–7237. [DOI] [PubMed] [Google Scholar]
- Nechushtan, A., C.L. Smith, I. Lamensdorf, S.H. Yoon, and R.J. Youle. 2001. Bax and Bak coalesce into novel mitochondria-associated clusters during apoptosis. J. Cell Biol. 153:1265–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhawan, D., M. Fang, E. Traer, Q. Zhong, W. Gao, F. Du, and X. Wang. 2003. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 17:1475–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppermann, M., C.C. Geilen, L.F. Fecker, B. Gillissen, P.T. Daniel, and J. Eberle. 2005. Caspase-independent induction of apoptosis in human melanoma cells by the proapoptotic Bcl-2-related protein Nbk/Bik. Oncogene. 24:7369–7380. [DOI] [PubMed] [Google Scholar]
- Strasser, A., A.W. Harris, M.L. Bath, and S. Cory. 1990. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 348:331–333. [DOI] [PubMed] [Google Scholar]
- Sturm, I., H. Petrowsky, R. Volz, M. Lorenz, S. Radetzki, T. Hillebrand, G. Wolff, S. Hauptmann, B. Dorken, and P.T. Daniel. 2001. Analysis of p53/BAX/p16(ink4a/CDKN2) in esophageal squamous cell carcinoma: high BAX and p16(ink4a/CDKN2) identifies patients with good prognosis. J. Clin. Oncol. 19:2272–2281. [DOI] [PubMed] [Google Scholar]
- Sturm, I., C. Stephan, B. Gillissen, R. Siebert, M. Janz, S. Radetzki, K. Jung, S. Loening, B. Dorken, and P.T. Daniel. 2006. Loss of the tissue-specific proapoptotic BH3-only protein Nbk/Bik is a unifying feature of renal cell carcinoma. Cell Death Differ. 13:619–627. [DOI] [PubMed] [Google Scholar]
- Tagawa, H., S. Karnan, R. Suzuki, K. Matsuo, X. Zhang, A. Ota, Y. Morishima, S. Nakamura, and M. Seto. 2005. Genome-wide array-based CGH for mantle cell lymphoma: identification of homozygous deletions of the proapoptotic gene BIM. Oncogene. 24:1348–1358. [DOI] [PubMed] [Google Scholar]
- Theodorakis, P., E. Lomonosova, and G. Chinnadurai. 2002. Critical requirement of BAX for manifestation of apoptosis induced by multiple stimuli in human epithelial cancer cells. Cancer Res. 62:3373–3376. [PubMed] [Google Scholar]
- Tong, Y., Q. Yang, C. Vater, L.K. Venkatesh, D. Custeau, T. Chittenden, G. Chinnadurai, and H. Gourdeau. 2001. The pro-apoptotic protein, Bik, exhibits potent antitumor activity that is dependent on its BH3 domain. Mol. Cancer Ther. 1:95–102. [PubMed] [Google Scholar]
- van Delft, M.F., and D.C. Huang. 2006. How the Bcl-2 family of proteins interact to regulate apoptosis. Cell Res. 16:203–213. [DOI] [PubMed] [Google Scholar]
- Verma, S., L.J. Zhao, and G. Chinnadurai. 2001. Phosphorylation of the pro-apoptotic protein BIK: mapping of phosphorylation sites and effect on apoptosis. J. Biol. Chem. 276:4671–4676. [DOI] [PubMed] [Google Scholar]
- von Haefen, C., T. Wieder, B. Gillissen, L. Starck, V. Graupner, B. Dorken, and P.T. Daniel. 2002. Ceramide induces mitochondrial activation and apoptosis via a Bax-dependent pathway in human carcinoma cells. Oncogene. 21:4009–4019. [DOI] [PubMed] [Google Scholar]
- von Haefen, C., T. Wieder, F. Essmann, K. Schulze-Osthoff, B. Dorken, and P.T. Daniel. 2003. Paclitaxel-induced apoptosis in BJAB cells proceeds via a death receptor-independent, caspases-3/-8-driven mitochondrial amplification loop. Oncogene. 22:2236–2247. [DOI] [PubMed] [Google Scholar]
- von Haefen, C., B. Gillissen, P.G. Hemmati, J. Wendt, D. Guner, A. Mrozek, C. Belka, B. Dorken, and P.T. Daniel. 2004. Multidomain Bcl-2 homolog Bax but not Bak mediates synergistic induction of apoptosis by TRAIL and 5-FU through the mitochondrial apoptosis pathway. Oncogene. 23:8320–8332. [DOI] [PubMed] [Google Scholar]
- Wei, M.C., T. Lindsten, V.K. Mootha, S. Weiler, A. Gross, M. Ashiya, C.B. Thompson, and S.J. Korsmeyer. 2000. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- Willis, S.N., and J.M. Adams. 2005. Life in the balance: how BH3-only proteins induce apoptosis. Curr. Opin. Cell Biol. 17:617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis, S.N., L. Chen, G. Dewson, A. Wei, E. Naik, J.I. Fletcher, J.M. Adams, and D.C. Huang. 2005. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 19:1294–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis, S.N., J.I. Fletcher, T. Kaufmann, M.F. van Delft, L. Chen, P.E. Czabotar, H. Ierino, E.F. Lee, W.D. Fairlie, P. Bouillet, et al. 2007. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 315:856–859. [DOI] [PubMed] [Google Scholar]
- Yin, C., C.M. Knudson, S.J. Korsmeyer, and T. Van Dyke. 1997. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature. 385:637–640. [DOI] [PubMed] [Google Scholar]
- Yu, J., Z. Wang, K.W. Kinzler, B. Vogelstein, and L. Zhang. 2003. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc. Natl. Acad. Sci. USA. 100:1931–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zantl, N., G. Weirich, H. Zall, B.M. Seiffert, S.F. Fischer, S. Kirschnek, C. Hartmann, R.M. Fritsch, B. Gillissen, P.T. Daniel, and G. Hacker. 2007. Frequent loss of expression of the pro-apoptotic protein Bim in renal cell carcinoma: evidence for contribution to apoptosis resistance. Oncogene. 26:7038–7048. [DOI] [PubMed] [Google Scholar]
- Zhang, L., J. Yu, B.H. Park, K.W. Kinzler, and B. Vogelstein. 2000. Role of BAX in the apoptotic response to anticancer agents. Science. 290:989–992. [DOI] [PubMed] [Google Scholar]
- Zhong, Q., W. Gao, F. Du, and X. Wang. 2005. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 121:1085–1095. [DOI] [PubMed] [Google Scholar]