Abstract
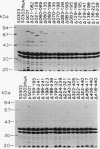
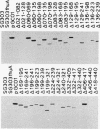
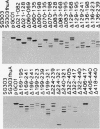
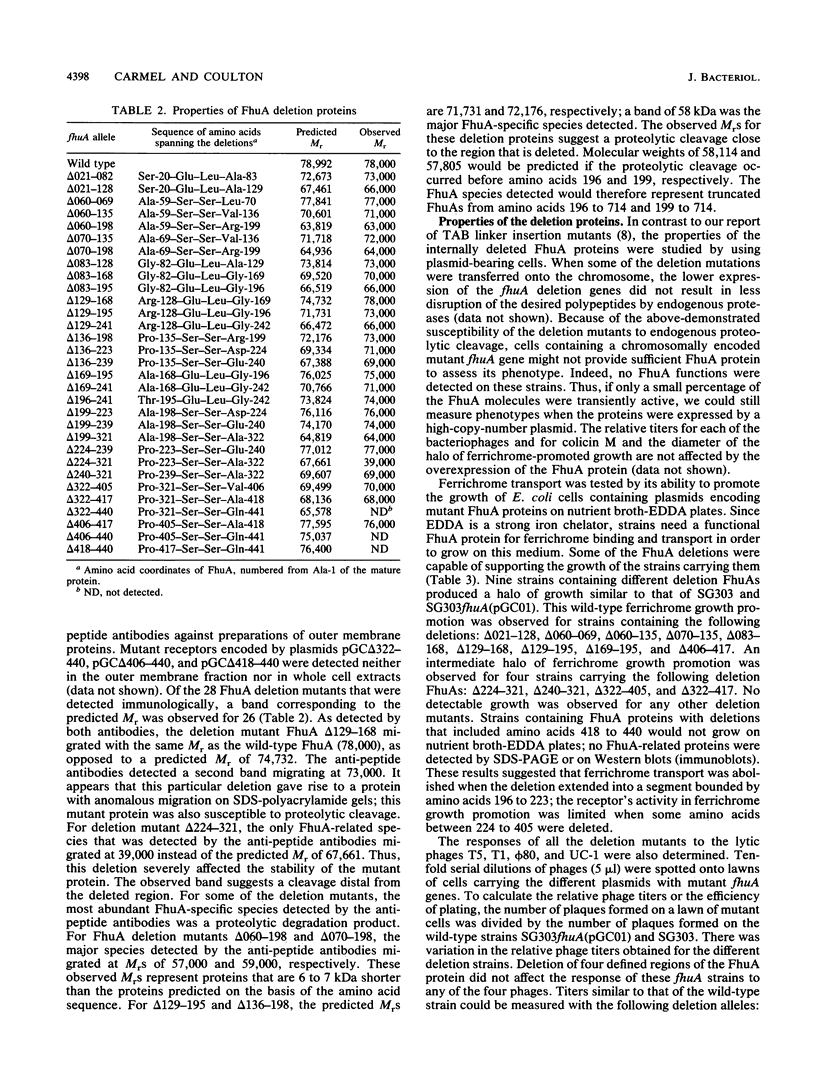
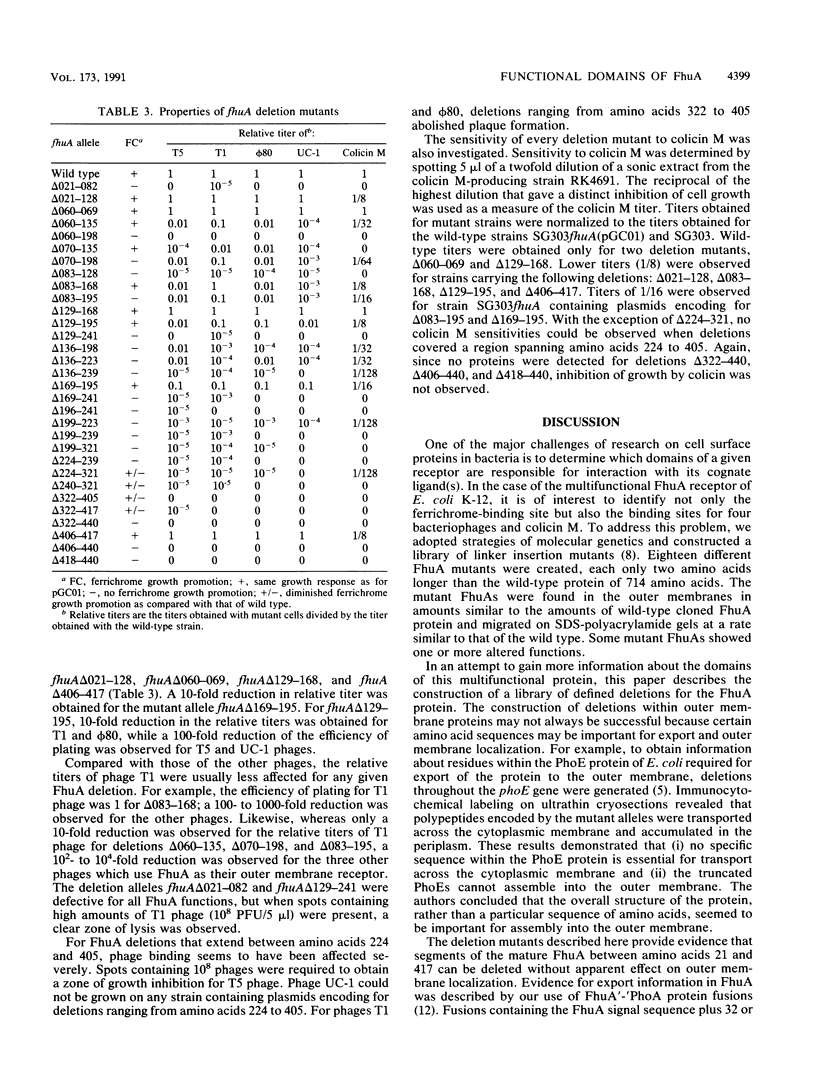
The ferrichrome-iron receptor encoded by the fhuA gene of Escherichia coli K-12 is a multifunctional outer membrane receptor required for the binding and uptake of ferrichrome and bacteriophages T5, T1, phi 80, and UC-1 as well as colicin M. To identify domains of the protein which are important for FhuA activities, a library of 31 overlapping deletion mutants in the fhuA gene was generated. Export of FhuA deletion proteins to the outer membrane and receptor functions of the deletion proteins were analyzed. All but three of the deletion mutant FhuA proteins cofractionated with the outer membrane; no FhuA proteins were detected in outer membrane preparations or in cell extracts when the deletions spanned amino acids 418 to 440. Most deletion proteins were susceptible to cleavage by endogenous proteolytic activity; some degradation products were detected on Coomassie blue-stained gels and on Western blots (immunoblots). Receptor functions were measured with the mutated genes present on multicopy plasmids. Two deletion mutants, FhuA delta 060-069 and FhuA delta 129-168, conferred wild-type phenotypes: they demonstrated growth promotion by ferrichrome and the same efficiency of plating of bacteriophages as that of wild-type FhuA; killing by colicin M was also unaffected. For FhuA delta 021-128 and FhuA delta 406-417, reduced sensitivity to colicin M was detected; wild-type phenotypes were observed for all other FhuA functions. Deletions from amino acids 169 to 195 slightly reduced sensitivities to bacteriophages and to colicin M; ferrichrome growth promotion was unaffected. When deletions extended into the region of amino acids 196 to 405, all FhuA functions were either reduced or abolished. The results indicate that selected regions of the FhuA protein have receptor activities and demonstrate the presence of both shared and unique ligand-responsive domains.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong S. K., Francis C. L., McIntosh M. A. Molecular analysis of the Escherichia coli ferric enterobactin receptor FepA. J Biol Chem. 1990 Aug 25;265(24):14536–14543. [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barany F. Single-stranded hexameric linkers: a system for in-phase insertion mutagenesis and protein engineering. Gene. 1985;37(1-3):111–123. doi: 10.1016/0378-1119(85)90263-x. [DOI] [PubMed] [Google Scholar]
- Barany F. Two-codon insertion mutagenesis of plasmid genes by using single-stranded hexameric oligonucleotides. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4202–4206. doi: 10.1073/pnas.82.12.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch D., Leunissen J., Verbakel J., de Jong M., van Erp H., Tommassen J. Periplasmic accumulation of truncated forms of outer-membrane PhoE protein of Escherichia coli K-12. J Mol Biol. 1986 Jun 5;189(3):449–455. doi: 10.1016/0022-2836(86)90316-5. [DOI] [PubMed] [Google Scholar]
- Braun V., Frenz J., Hantke K., Schaller K. Penetration of colicin M into cells of Escherichia coli. J Bacteriol. 1980 Apr;142(1):162–168. doi: 10.1128/jb.142.1.162-168.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel G., Hellstern D., Henning D., Coulton J. W. Insertion mutagenesis of the gene encoding the ferrichrome-iron receptor of Escherichia coli K-12. J Bacteriol. 1990 Apr;172(4):1861–1869. doi: 10.1128/jb.172.4.1861-1869.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton J. W., Mason P., Allatt D. D. fhuC and fhuD genes for iron (III)-ferrichrome transport into Escherichia coli K-12. J Bacteriol. 1987 Aug;169(8):3844–3849. doi: 10.1128/jb.169.8.3844-3849.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton J. W., Mason P., Cameron D. R., Carmel G., Jean R., Rode H. N. Protein fusions of beta-galactosidase to the ferrichrome-iron receptor of Escherichia coli K-12. J Bacteriol. 1986 Jan;165(1):181–192. doi: 10.1128/jb.165.1.181-192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton J. W., Mason P., DuBow M. S. Molecular cloning of the ferrichrome-iron receptor of Escherichia coli K-12. J Bacteriol. 1983 Dec;156(3):1315–1321. doi: 10.1128/jb.156.3.1315-1321.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton J. W., Reid G. K., Campana A. Export of hybrid proteins FhuA'-'LacZ and FhuA'-'PhoA to the cell envelope of Escherichia coli K-12. J Bacteriol. 1988 May;170(5):2267–2275. doi: 10.1128/jb.170.5.2267-2275.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourel D., Hikita C., Bolla J. M., Mizushima S., Pagès J. M. Characterization of ompF domains involved in Escherichia coli K-12 sensitivity to colicins A and N. J Bacteriol. 1990 Jul;172(7):3675–3680. doi: 10.1128/jb.172.7.3675-3680.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudl R., Schwarz H., Klose M., Movva N. R., Henning U. The nature of information, required for export and sorting, present within the outer membrane protein OmpA of Escherichia coli K-12. EMBO J. 1985 Dec 16;4(13A):3593–3598. doi: 10.1002/j.1460-2075.1985.tb04122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentz R., Kuys Y., Zwieb C., Taatjes D., Taatjes H., Bannwarth W., Stueber D., Ibrahimi I. Association of degradation and secretion of three chimeric polypeptides in Escherichia coli. J Bacteriol. 1988 May;170(5):2212–2220. doi: 10.1128/jb.170.5.2212-2220.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hancock R. W., Braun V. Nature of the energy requirement for the irreversible adsorption of bacteriophages T1 and phi80 to Escherichia coli. J Bacteriol. 1976 Feb;125(2):409–415. doi: 10.1128/jb.125.2.409-415.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Functional interaction of the tonA/tonB receptor system in Escherichia coli. J Bacteriol. 1978 Jul;135(1):190–197. doi: 10.1128/jb.135.1.190-197.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182(2):288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- Harkness R. E., Braun V. Colicin M inhibits peptidoglycan biosynthesis by interfering with lipid carrier recycling. J Biol Chem. 1989 Apr 15;264(11):6177–6182. [PubMed] [Google Scholar]
- Harkness R. E., Braun V. Inhibition of lipopolysaccharide O-antigen synthesis by colicin M. J Biol Chem. 1989 Sep 5;264(25):14716–14722. [PubMed] [Google Scholar]
- Heller K., Mann B. J., Kadner R. J. Cloning and expression of the gene for the vitamin B12 receptor protein in the outer membrane of Escherichia coli. J Bacteriol. 1985 Mar;161(3):896–903. doi: 10.1128/jb.161.3.896-903.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Jackson M. E., Pratt J. M., Holland I. B. Intermediates in the assembly of the TonA polypeptide into the outer membrane of Escherichia coli K12. J Mol Biol. 1986 Jun 5;189(3):477–486. doi: 10.1016/0022-2836(86)90318-9. [DOI] [PubMed] [Google Scholar]
- Köster W., Braun V. Iron hydroxamate transport of Escherichia coli: nucleotide sequence of the fhuB gene and identification of the protein. Mol Gen Genet. 1986 Sep;204(3):435–442. doi: 10.1007/BF00331021. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Murphy C. K., Kalve V. I., Klebba P. E. Surface topology of the Escherichia coli K-12 ferric enterobactin receptor. J Bacteriol. 1990 May;172(5):2736–2746. doi: 10.1128/jb.172.5.2736-2746.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plastow G. S., Holland I. B. Identification of an Escherichia coli inner membrane polypeptide specified by a lambda-tonB transducing. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1007–1014. doi: 10.1016/0006-291x(79)91927-2. [DOI] [PubMed] [Google Scholar]
- Sauer M., Hantke K., Braun V. Sequence of the fhuE outer-membrane receptor gene of Escherichia coli K12 and properties of mutants. Mol Microbiol. 1990 Mar;4(3):427–437. doi: 10.1111/j.1365-2958.1990.tb00609.x. [DOI] [PubMed] [Google Scholar]
- Schaller K., Dreher R., Braun V. Structural and functional properties of colicin M. J Bacteriol. 1981 Apr;146(1):54–63. doi: 10.1128/jb.146.1.54-63.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz G., Ullrich F., Heller K. J., Braun V. Export and activity of hybrid FhuA'-'Iut receptor proteins and of truncated FhuA' proteins of the outer membrane of Escherichia coli. Mol Gen Genet. 1989 Apr;216(2-3):230–238. doi: 10.1007/BF00334361. [DOI] [PubMed] [Google Scholar]
- Sugimura K., Nishihara T. Purification, characterization, and primary structure of Escherichia coli protease VII with specificity for paired basic residues: identity of protease VII and OmpT. J Bacteriol. 1988 Dec;170(12):5625–5632. doi: 10.1128/jb.170.12.5625-5632.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]