Abstract
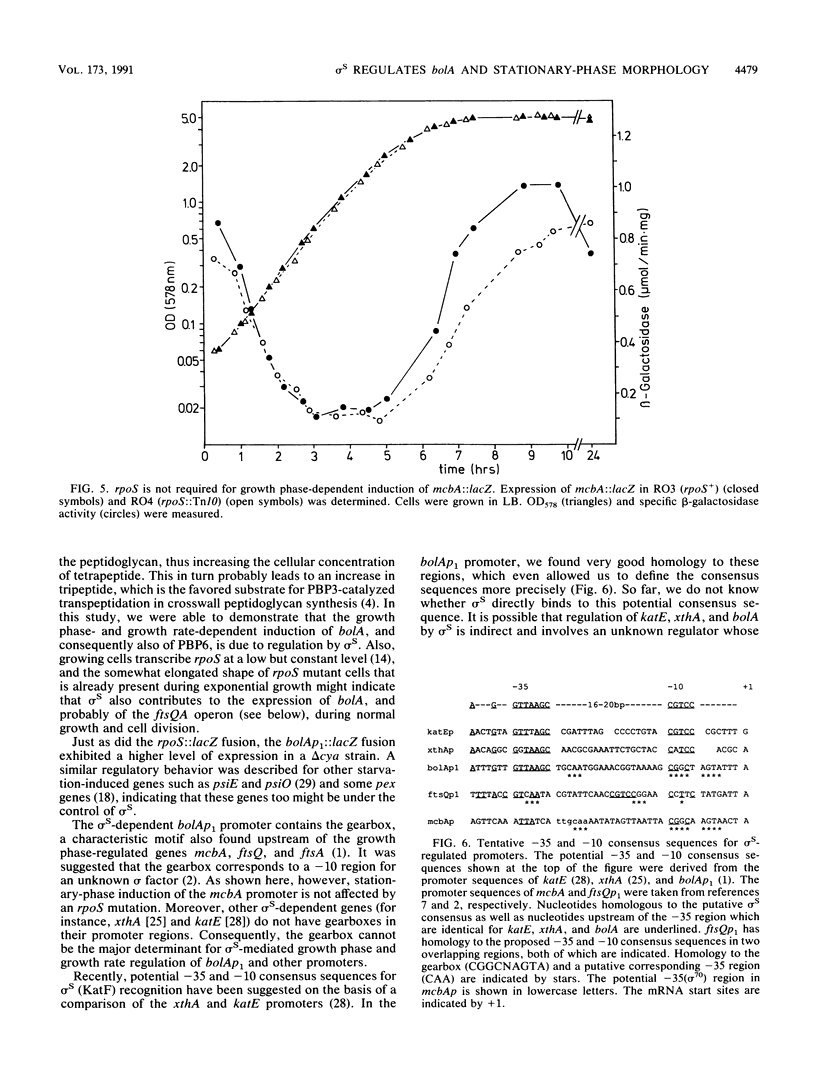
The novel sigma factor (sigma S) encoded by rpoS (katF) is required for induction of many growth phase-regulated genes and expression of a variety of stationary-phase phenotypes in Escherichia coli. Here we demonstrate that wild-type cells exhibit spherical morphology in stationary phase, whereas rpoS mutant cells remain rod shaped and are generally larger. Size reduction of E. coli cells along the growth curve is a continuous and at least biphasic process, the second phase of which is absent in rpoS-deficient cells and correlates with induction of the morphogene bolA in wild-type cells. Stationary-phase induction of bolA is dependent on sigma S. The "gearbox" a characteristic sequence motif present in the sigma S-dependent growth phase- and growth rate-regulated bolAp1 promoter, is not recognized by sigma S, since stationary-phase induction of the mcbA promoter, which also contains a gearbox, does not require sigma S, and other sigma S-controlled promoters do not contain gearboxes. However, good homology to the potential -35 and -10 consensus sequences for sigma S regulation is found in the bolAp1 promoter.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldea M., Garrido T., Hernández-Chico C., Vicente M., Kushner S. R. Induction of a growth-phase-dependent promoter triggers transcription of bolA, an Escherichia coli morphogene. EMBO J. 1989 Dec 1;8(12):3923–3931. doi: 10.1002/j.1460-2075.1989.tb08573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldea M., Garrido T., Pla J., Vicente M. Division genes in Escherichia coli are expressed coordinately to cell septum requirements by gearbox promoters. EMBO J. 1990 Nov;9(11):3787–3794. doi: 10.1002/j.1460-2075.1990.tb07592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldea M., Hernández-Chico C., de la Campa A. G., Kushner S. R., Vicente M. Identification, cloning, and expression of bolA, an ftsZ-dependent morphogene of Escherichia coli. J Bacteriol. 1988 Nov;170(11):5169–5176. doi: 10.1128/jb.170.11.5169-5176.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg K. J., Takasuga A., Edwards D. H., Dewar S. J., Spratt B. G., Adachi H., Ohta T., Matsuzawa H., Donachie W. D. The balance between different peptidoglycan precursors determines whether Escherichia coli cells will elongate or divide. J Bacteriol. 1990 Dec;172(12):6697–6703. doi: 10.1128/jb.172.12.6697-6703.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohannon D. E., Connell N., Keener J., Tormo A., Espinosa-Urgel M., Zambrano M. M., Kolter R. Stationary-phase-inducible "gearbox" promoters: differential effects of katF mutations and role of sigma 70. J Bacteriol. 1991 Jul;173(14):4482–4492. doi: 10.1128/jb.173.14.4482-4492.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan C. E., Sowell M. O. Synthesis of penicillin-binding protein 6 by stationary-phase Escherichia coli. J Bacteriol. 1982 Jul;151(1):491–494. doi: 10.1128/jb.151.1.491-494.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner M. J., Spitz E., Rickenberg H. V. Cyclic adenosine 3',5'-monophosphate in Escherichia coli. J Bacteriol. 1973 Jun;114(3):1068–1073. doi: 10.1128/jb.114.3.1068-1073.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell N., Han Z., Moreno F., Kolter R. An E. coli promoter induced by the cessation of growth. Mol Microbiol. 1987 Sep;1(2):195–201. doi: 10.1111/j.1365-2958.1987.tb00512.x. [DOI] [PubMed] [Google Scholar]
- Dewar S. J., Kagan-Zur V., Begg K. J., Donachie W. D. Transcriptional regulation of cell division genes in Escherichia coli. Mol Microbiol. 1989 Oct;3(10):1371–1377. doi: 10.1111/j.1365-2958.1989.tb00118.x. [DOI] [PubMed] [Google Scholar]
- Genilloud O., Moreno F., Kolter R. DNA sequence, products, and transcriptional pattern of the genes involved in production of the DNA replication inhibitor microcin B17. J Bacteriol. 1989 Feb;171(2):1126–1135. doi: 10.1128/jb.171.2.1126-1135.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groat R. G., Schultz J. E., Zychlinsky E., Bockman A., Matin A. Starvation proteins in Escherichia coli: kinetics of synthesis and role in starvation survival. J Bacteriol. 1986 Nov;168(2):486–493. doi: 10.1128/jb.168.2.486-493.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins D. E., Chaisson S. A., Matin A. Starvation-induced cross protection against osmotic challenge in Escherichia coli. J Bacteriol. 1990 May;172(5):2779–2781. doi: 10.1128/jb.172.5.2779-2781.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins D. E., Schultz J. E., Matin A. Starvation-induced cross protection against heat or H2O2 challenge in Escherichia coli. J Bacteriol. 1988 Sep;170(9):3910–3914. doi: 10.1128/jb.170.9.3910-3914.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelleberg S., Hermansson M., Mårdén P., Jones G. W. The transient phase between growth and nongrowth of heterotrophic bacteria, with emphasis on the marine environment. Annu Rev Microbiol. 1987;41:25–49. doi: 10.1146/annurev.mi.41.100187.000325. [DOI] [PubMed] [Google Scholar]
- Lange R., Hengge-Aronis R. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol Microbiol. 1991 Jan;5(1):49–59. doi: 10.1111/j.1365-2958.1991.tb01825.x. [DOI] [PubMed] [Google Scholar]
- Loewen P. C., Switala J., Triggs-Raine B. L. Catalases HPI and HPII in Escherichia coli are induced independently. Arch Biochem Biophys. 1985 Nov 15;243(1):144–149. doi: 10.1016/0003-9861(85)90782-9. [DOI] [PubMed] [Google Scholar]
- Loewen P. C., Triggs B. L. Genetic mapping of katF, a locus that with katE affects the synthesis of a second catalase species in Escherichia coli. J Bacteriol. 1984 Nov;160(2):668–675. doi: 10.1128/jb.160.2.668-675.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey M. R., Loewen P. C. Nucleotide sequence of katF of Escherichia coli suggests KatF protein is a novel sigma transcription factor. Nucleic Acids Res. 1989 Dec 11;17(23):9979–9991. doi: 10.1093/nar/17.23.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey M. R., Switala J., Borys A., Loewen P. C. Regulation of transcription of katE and katF in Escherichia coli. J Bacteriol. 1990 Dec;172(12):6713–6720. doi: 10.1128/jb.172.12.6713-6720.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sak B. D., Eisenstark A., Touati D. Exonuclease III and the catalase hydroperoxidase II in Escherichia coli are both regulated by the katF gene product. Proc Natl Acad Sci U S A. 1989 May;86(9):3271–3275. doi: 10.1073/pnas.86.9.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saporito S. M., Smith-White B. J., Cunningham R. P. Nucleotide sequence of the xth gene of Escherichia coli K-12. J Bacteriol. 1988 Oct;170(10):4542–4547. doi: 10.1128/jb.170.10.4542-4547.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L. Overlapping and separate controls on the phosphate regulon in Escherichia coli K12. J Mol Biol. 1983 May 25;166(3):283–308. doi: 10.1016/s0022-2836(83)80086-2. [DOI] [PubMed] [Google Scholar]
- von Ossowski I., Mulvey M. R., Leco P. A., Borys A., Loewen P. C. Nucleotide sequence of Escherichia coli katE, which encodes catalase HPII. J Bacteriol. 1991 Jan;173(2):514–520. doi: 10.1128/jb.173.2.514-520.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]



