Abstract
β-Amyloid (Aβ) peptide has been proposed to be a causal factor in Alzheimer’s disease (AD). Currently being investigated, active and passive Aβ-immunotherapy significantly reduce Aβ plaque deposition, neuritic dystrophy, and astrogliosis in the brains of APP transgenic (APP/Tg) mice. Immunization with Aβ42 formulated in the Th1-type adjuvant QS21 was beneficial for AD patients with significant titers of anti-Aβ antibodies, however, 6% of participants developed meningoencephalitis, likely due to anti-Aβ-specific autoimmune Th1 cells. Thus, successful Aβ vaccination requires the development of strong antibody responses without Th1-type cellular immunity. In this study, we compared the induction of humoral immune responses with Th1-type (Quil A) and Th2-type (Alum) adjuvants singly and in combination, using our novel epitope vaccine composed of self B cell epitope Aβ1–15 and foreign T cell epitope PADRE (PADRE-Aβ1–15-MAP). Formulated in Quil A, this vaccine resulted in significantly higher anti-Aβ antibody responses in both BALB/c (H-2d) and C57BL/6 (H-2b) mice, compared with Alum. Anti-Aβ antibodies induced by Alum were predominantly IgG1 type accompanied by lower levels of IgG2a and IgG2b. Quil A induced robust and almost equal titers of anti-Aβ antibodies of IgG1 and IgG2a isotypes and slightly lower levels of IgG2b. Switching adjuvants from Alum to Quil A induced higher concentrations of antibodies than injections with Alum only, however slightly lower than Quil A only. Switching both adjuvants did not change the profile of antibody responses generated by the initial adjuvant injected. These results suggest that switching from Alum to Quil A would be beneficial for AD patients because anti-Aβ antibody production was enhanced without changing the initially generated and likely beneficial Th2-type humoral response.
Keywords: Alzheimer’s disease (AD), Epitope vaccine immunization, Th1 and Th2 adjuvants
1. Introduction
The cause of Alzheimer’s disease (AD) remains unclear, greatly hindering the development of successful treatments for this devastating disease. The neuropathological features of AD include neurofibrillary tangles (NFT), deposition of β-amyloid (Aβ) in senile plaques, and neuronal loss in affected brain regions [1]. The Aβ peptide is thought to have a central role in the onset and progression of AD [2,3]. This is supported by results of studies using different transgenic mouse models of AD that express mutated forms of human amyloid precursor protein (APP), from which Aβ is cleaved [4–6]. Such expression of APP induces cerebral deposition of Aβ in the form of monomers, oligomers, protofibrills, and plaques, and mimics different aspects of AD in mice [7–9]. It is believed that accumulation of toxic forms of Aβ deposits in the brain have a significant, possibly central role in the onset and progression of AD.
Various strategies currently proposed as therapies for AD are aimed at reducing the level of Aβ in the brain or blocking the assembly of the peptide into pathological forms. One potentially powerful strategy for reducing the level of Aβ in the brain is immunotherapy, in which antibodies specific to Aβ facilitate the clearance of amyloid deposits [10–18]. The first human AD vaccine consisted of fibrillar Aβ42 formulated in QS21 adjuvant (AN-1792 trial) that induced strong Th1-type anti-Aβ immune responses [19], even in Th2-prone BALB/c mice [20]. This AN-1792 clinical trial was halted due to the development of meningoencephalitis in a small proportion of the subjects [21–26], but follow up studies have demonstrated that strong anti-Aβ antibody responses specific to the linear Aβ1–8 peptide [27] in some patients reduced AD pathology and diminished the cognitive decline associated with the disease [24,27–33]. These data along with preclinical studies demonstrated that anti-Aβ antibodies directed to N-terminal region of Aβ42 are effective in clearance of amyloid plaques [16,34,35]. On the contrary, the strong autoreactive Th1-type response specific to Aβ is thought to underlie the development of meningoencephalitis observed in the AN-1792 trial [30,31,33]. It is known that Th1-type pro-inflammatory immune responses that are important for the generation of protection against viral infections and cancer are also implicated in autoimmune disorders, whereas Th2-type anti-inflammatory responses have generally been shown to inhibit autoimmune disease. It is important, therefore, that future AD immunotherapy results in the clearing of Aβ peptides by specific anti-Aβ antibodies while limiting the Th1-type immune response [28–33]. To reduce the risk of an adverse T cell-mediated immune response to Aβ-immunotherapy, we developed an epitope vaccine, PADRE-Aβ1–15-MAP, and demonstrated that immunizations with this vaccine induced high titers of anti-Aβ antibodies, but not anti-PADRE antibodies. Formulation of our immunogen in the Th2-type adjuvant, Alum, was performed to direct the antibody response toward a Th2 phenotype in an attempt to avoid a strong Th1 humoral response, while maintaining potentially beneficial anti-Aβ antibody response [36]. Alum, however, is much less potent than the majority of Th1-type adjuvants including saponin (QS21) [19,37–40], sometimes as much as 200-fold [41]. Therefore, we suggested that the combined use of both Th1- and Th2-type adjuvants may be more effective and safe immunotherapy for AD.
In the present study, we compared the immunogenicity of our novel prototype epitope vaccine that contains the immunodominant B cell epitope of Aβ1–15 in tandem with the synthetic universal helper T cell epitope, PADRE (PADRE-Aβ1–15-MAP) [36] formulated in Th1-type adjuvant (Quil A) or Th2-type adjuvant (Alum). To identify an acceptable adjuvant formulation for use in future pre-clinical and clinical trials, we tested the use of different combinations of Quil A and Alum in BALB/c and C57BL/6 mice of two different haplotypes H-2d and H-2b, respectively.
2. Materials and methods
2.1. Animals
Eight or 10-week-old female C57BL/6 or BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, ME). All animals were housed in a temperature- and light-cycle-controlled facility, and their care was according to the guidelines of the National Institutes of Health and an approved IACUC protocol at the University of California, Irvine.
2.2. Epitope vaccine, peptide and immunizations
A prototype epitope AD vaccine was synthesized exactly as we have described previously [36]. Briefly, the N-terminus of the immunodominant B cell epitope of Aβ1–15 was synthesized in tandem with a promiscuous foreign T cell epitope, PADRE (aK-Cha-VAAWTLKAAa, where “a” is D alanine and “Cha” is l-cyclohexylalanine) as Multiple Antigenic Peptides (MAP), which contain a core matrix of 4 branching lysines [42,43] to generate PADRE-Aβ1–15-MAP molecules (Invitrogen Inc., CA).
Mice were immunized with PADRE-Aβ1–15-MAP formulated in Th1- (Quil A) or Th2- (Alum) types of adjuvant as we have described previously [19,36,40]. Quil A (Brenntag Biosector, Denmark) is a mixture of saponins that is prepared from an aqueous extract of Q. saponaria bark and contains QS21 as the adjuvant component. Briefly, peptide was resuspended in DMSO at a concentration of 10 mM, and then diluted in PBS to 500µg/mL of peptide. Before immunization, peptide was mixed with either Alum or Quil A, and each mouse was injected subcutaneously with 50 µg of antigen and 1 mg of Alumor 20 µg of Quil A. Control groups of mice were immunized with PBS mixed with the appropriate adjuvant. After immunization, two boosts were performed at 2-week intervals. All groups of mice (total six animals per group) were bled on Day 10 after the last injection, and sera were prepared for ELISA. In switching experiments, mice were injected first with immunogen formulated in Quil A (three times biweekly) then with the same immunogen formulated in Alum (three times biweekly) or vice versa (Table 1). Again, on Day 10 after the last boost, animals were bled, and sera were prepared for ELISA.
Table 1.
Summary of immunization
| Groups | Mouse strain | Number of mice | Immunizations with antigen (Ag)a |
|---|---|---|---|
| 1 | C57BL/6 | 6 | Formulated in Alum (six injections) |
| 2 | C57BL/6 | 6 | Formulated in Alum (three injections) plus three injections in Quil A |
| 3 | C57BL/6 | 6 | Formulated in Quil A (six injections) |
| 4 | C57BL/6 | 6 | Formulated in Quil A (three injections) plus three injections in Alum |
| 5 | BALB/c | 6 | Formulated in Alum (six injections) |
| 6 | BALB/c | 6 | Formulated in Alum (three injections) plus three injections in Quil A |
| 7 | BALB/c | 6 | Formulated in Quil A (six injections) |
PADRE-Aβ1–15-MAP was used as the Ag.
2.3. Detection of anti-Aβ antibodies using ELISA
Plasma from the collected sera was isolated and frozen at −20 °C. Anti-Aβ antibodies were measured in plasma as we described previously [19,40]. The monoclonal antibody A20.1 was used as the standard for determining antiAβ antibody concentrations in the sera. Briefly, wells of 96-well plates (Immulon II, Dynatech) were coated with 2.5µM soluble Aβ42 (pH 9.7, 2 h, 37 °C) followed by three washes. Primary sera from experimental and control mice and A20.1 Aβ MAb were diluted in TBS and added to the wells. HRP-conjugated anti-mouse IgG (Jackson Labs, ME) and o-phenylendiamine substrate (Sigma, St. Louis, MO) in 0.05 M phosphate–citrate buffer, pH 5.0, were used for detection of bound anti-Aβ antibody. All plates were analyzed spectrophotometrically at 405 nm. To determine the specific isotypes present, sera were pooled, diluted 1:2500, and tested in duplicate. For detection of mouse IgG1, IgG2a, IgG2b, or IgM isotypes, we used anti-mouse Ig-subclass-specific HRP-conjugated secondary antibodies (Zymed, San Francisco, CA). In C57BL/6 mice, however, instead of IgG2a, we tested for IgG2ab isotype (Igh-1b), also known as IgG2c [40]. To measure the concentration of IgG1, IgG2a/IgG2ab, IgG2b, or IgM anti-Aβ antibodies, separate standard curves generated with the appropriate monoclonal antibodies of the same isotype would be required. Since it is technically difficult to perform, we just measured binding of antibodies of different isotypes to wells coated with Aβ peptide (OD405) using appropriate secondary antibodies [19,36,40].
2.4. Immunohistochemistry
Mice were sacrificed, and brains were removed, fixed in 4% paraformaldehyde for 24 h at 4 °C, and subsequently cut in serial 50 µm-thick sections. Sections were pre-treated with 0.3% H2O2 for 30 min to eliminate endogenous peroxidase activity. The following primary antibodies were used after blocking of sections: CD45 (30-F11), CD11b (M1/70), CD4 (GK1.5), CD8a (53–6.7) (all from BD Pharmingen, San Diego, CA). All antibodies were optimally diluted in 0.1 M Tris, 0.1% Triton X-100 and 2% BSA as recommended by manufacturer. Bound antibodies were detected using biotinylated anti-hamster (Vector Laboratories, CA) or pre-absorbed biotinylated anti-rat (Jackson Immunoresearch, PA) antibodies followed by incubation with avidin:biotin peroxidase complex (ABC) using the Vectastain Elite kit (Vector Laboratories, CA). Staining reactions were performed with 3,3 diaminobenzidine (DAB, Sigma,MO)according to manufacturers’ protocols. For negative control, the primary antibody was omitted from the diluents.
2.5. Statistical analysis
All statistical data (mean, standard deviation, significant differences) were calculated using GraphPad Prism software. Significant differences in antibody production between groups were analyzed by one-way analysis of variance (ANOVA) and Tukey’s multiple comparison post-test.
3. Results
3.1. Anti-Aβ antibody responses in BALB/c and C57BL/6 mice immunized with PADRE-Aβ1–15-MAP formulated in Th1- (Quil A) or Th2- (Alum) type adjuvants
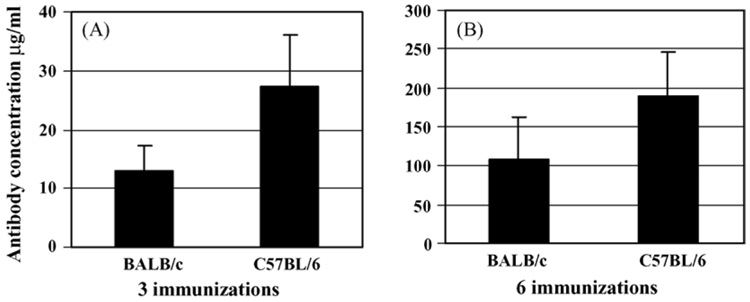
After three injections with immunogen formulated in Alum, the average concentration of anti-Aβ antibody in C57BL/6 mice was higher than that in BALB/c mice (Fig. 1A). An additional three injections dramatically enhanced the level of anti-Aβ antibodies in both strains of mice. Boosting was more effective in BALB/c mice, however, total concentrations of anti-Aβ antibodies were higher in C57BL/6 mice (Fig. 1B). Similar results were observed after immunization of these mice with PADRE-Aβ1–15-MAP formulated in Quil A (Fig. 2). Three injections with this vaccine induced higher concentrations of anti-Aβ antibodies in C57BL/6 mice than BALB/c mice, and three additional injections considerably increased the levels in both strains of mice, although this enhancement was more profound in BALB/c mice (Fig. 2). Thus, consistent with previous results with fibrillar Aβ42 immunogen [19,40], Quil A was significantly more potent and induced 269 and 425 µg (average concentrations) of anti-Aβ antibodies in BALB/c and C57BL/6 mice, respectively (Fig. 1 and Fig. 2, P < .01 for BALB/c and P < .001 for C57BL/6 mice).
Fig. 1.
Epitope vaccine PADRE-Aβ1–15-MAP formulated in Alum induced a more robust humoral response in C57BL/6 mice than in BALB/c mice after three (A) or six (B) injections, however, these differences were not statistically significant (P > .05).
Fig. 2.
Epitope vaccine PADRE-Aβ1–15-MAP formulated in the Quil A induced a more robust humoral response in C57BL/6 mice than in BALB/c mice after three (A) (P > .05) or six (B) (P < .001) injections. After six injections, the anti-Aβ antibody response in both strains of mice vaccinated with PADRE-Aβ1–15-MAP formulated in Quil A was significantly higher than that in mice vaccinated with the same epitope vaccine mixed with Alum (compare Fig. 1 and Fig. 2, P < .01 for BALB/c mice, P < .001 for C57BL/6 mice).
3.2. Anti-Aβ antibody responses in BALB/c and C57BL/6 mice after switching from Alum to Quil A
We measured concentrations of anti-Aβ antibodies of all isotypes in BALB/c and C57BL/6 mice injected first three times with epitope vaccine formulated in Alum and then three more times with the same immunogen mixed with Quil A. Switching from Alum to Quil A increased the anti-Aβ immune responses generated in BALB/c and C57BL/6 mice (Fig. 3). Both strains of mice previously injected with epitope vaccine plus Alum responded better to three additional injections with PADRE-Aβ1–15-MAP formulated in Quil A, than in Alum. In terms of total immune response, however, in both groups of mice, six injections with epitope vaccine formulated in Quil A were more potent (Fig. 2B versus Fig. 3).
Fig. 3.
Switching from epitope vaccine formulated in Alum to that mixed with Quil A after three injections enhanced the immune response in both BALB/c (A) and C57BL/6 (B) mice although the concentrations of anti-Aβ antibodies were lower than that in mice injected six times with epitope vaccine formulated in Quil A (see Fig. 2B). Differences were significant (P < .05) in C57BL6 mice, but not in BALB/c mice (P > .05).
In addition to the magnitude of humoral immune responses we also analyzed the isotype of anti-Aβ42 antibodies, since this characteristic has previously been used as an indirect measure of the contribution of Th1 (IgG2a) and Th2 (IgG1) cytokines to the immune response [44–46]. Three to six injections with PADRE-Aβ1–15-MAP epitope vaccine formulated in Alum generated predominantly IgG1 anti-Aβ antibodies in BALB/c mice (Fig. 4A), consistent with previous results [36]. Switching from Alum to Quil A slightly decreased IgG1 production and induced significant increase of IgG2b isotypes. Importantly, expression of IgG2a isotype was negligible. Despite the fact that the IgG1/IgG2a ratio was still above 3 in these mice, after switching, this ratio declined for approximately two times, primarily due to suppression of the IgG1 response (Fig. 4B). In other words, switching from Alum to Quil A decreased the IgG1/IgG2a ratio in Th2-prone BALB/c mice [20].
Fig. 4.
Switching from Alum to Quil A did not change the pattern of anti-Aβ antibody responses initially generated by the same epitope vaccine formulated in Alum. Isotyping of antibody responses in BALB/c (A) and C57BL/6 (C) mice. IgG1/IgG2a ratios in BALB/c (B) and C57BL/6 (D) mice were calculated based on data presented in A and C.
As expected, immunization of C57BL/6 mice with epitope vaccine formulated in Alum also induced anti-Aβ antibodies predominantly of IgG1 isotype, although we detected low levels of IgG2b antibodies in mice that had received six injections of the vaccine (Fig. 4C). Switching from Alum to Quil A enhanced production of IgG1, IgG2b, and IgG2ab isotypes, and decreased the ratio of IgG1/IgG2ab by more than 2.5 times (Fig. 4C and D). Thus, results from BALB/c and C57BL/6 mice were similar and indicated that switching from Alum to Quil A induced strong Th2-type anti-Aβ antibody production without changing the pattern of antibody responses initiated by pre-immunizations of the same mice with epitope vaccine formulated in Alum.
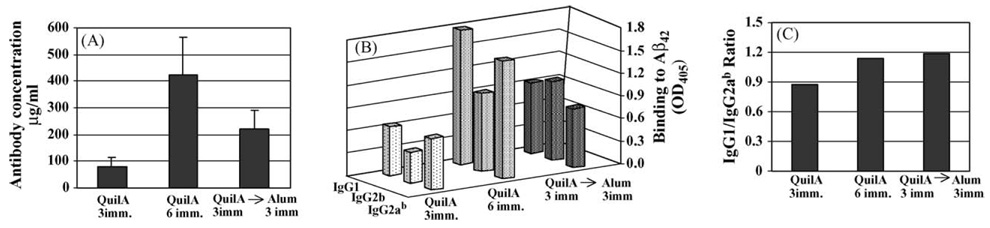
3.3. Anti-Aβ antibody responses in C57BL/6 mice after switching from Quil A to Alum
C57BL/6 mice pre-vaccinated with PADRE-Aβ1–15-MAP formulated in Quil A and then given three injections with the same immunogen mixed with Alum exhibited significantly enhanced concentrations (double) of anti-Aβ antibodies compared with mice given three injections of vaccine formulated in Quil A only (Fig. 5A). However, when we did not switch from Quil A to Alum and continue immunizations with PADRE-Aβ1–15-MAP formulated in Quil A, mice produced almost two times higher concentrations of anti-Aβ antibodies than that induced after additional injections with immunogen plus Alum (Fig. 5A). In fact PADRE-Aβ1–15-MAP formulated in Quil A was the formulation of vaccine that induced the highest concentration of antibodies specific to amyloid (Fig. 2 and Fig. 5A).
Fig. 5.
Switching from Quil A to Alum lowered the magnitude of anti-Aβ antibody response compared with using Quil A alone in C57BL/6 mice (A) (P > .05). Switching from Quil A to Alum did not change the isotype profile of these antibodies (B, C).
To detect the influence of switching adjuvants on Th1- and Th2-types of humoral immune responses, we analyzed IgG isotypes of antibodies. The epitope vaccine PADRE-Aβ1–15-MAP formulated in Quil A generated anti-Aβ antibodies of IgG1, IgG2b, and IgG2ab isotypes (Fig. 5B). After three to six injections, the IgG1/IgG2ab ratio was 0.9–1.1 (Fig. 5C), indicating predominantly Th1-type humoral responses. Switching from Quil A to Alum did not significantly change the profile of anti-Aβ antibody isotypes or the IgG1/IgG2ab ratio (Fig. 5B and C).
3.4. Epitope vaccine formulated in Th1 or Th2 adjuvants did not induce inflammation in the brains of immunized mice
To examine inflammation-related pathology in the brains of animals immunized with epitope vaccine formulated in Alum or Quil A, we conducted immunohistochemical analysis. To identify microglia activation as well as leukocyte infiltration and accumulation in the brain, we analyzed expression of CD45, CD11b, CD4, and CD8 molecules. The immunostaining in sections from the frontoparietal cortex and hippocampus revealed no observable differences between non-immunized and immunized mice. In other words, we had not observed the lymphocyte infiltration or microglia activation in the CNS of both BALB/c and C57BL/6 mice. In addition, in order to investigate toxicity and potential negative effects of vaccination, we examined Glial Fibrillary Acidic Protein (GFAP) and had not found astrocytosis.
4. Discussion
Despite the adverse events (meningoencephalitis) in the AN-1792 clinical trial, follow up studies demonstrated that strong anti-Aβ antibody responses, rather than autoimmune T cell responses generated in AD patients, were capable of reducing AD pathology and diminishing the progressive cognitive decline associated with the disease [24,28–33]. More specifically, published data from AN-1792 clinical trials suggest that the aseptic meningoencephalitis may have been caused by a T cell-mediated autoimmune response, whereas production of high titers of anti-Aβ antibodies may have been therapeutic to the AD patients. Recently, in order to reduce the risk of an adverse T cell-mediated immune response to Aβ-immunotherapy, we developed epitope vaccine PADRE-Aβ1–15-MAP and demonstrated that immunizations with this vaccine induced high titers of anti-Aβ, but not anti-PADRE antibodies in Th2-prone BALB/c mice. On the contrary, splenocytes from immunized mice showed robust T cell stimulation in vitro in response to PADRE, but not to Aβ peptides. In these experiments in addition to replacing of the self Aβ T cell epitope with a foreign T cell epitope PADRE, we formulated immunogen in a Th2-type adjuvant, Alum in order to bias antibody response toward a Th2 phenotype [36]. Accordingly, we demonstrated that our epitope vaccine induced strong Th2-type of immune responses in Th2-prone BALB/c mice [36]. The results of the present study demonstrate that either a Th1-type adjuvant (Quil A) or a Th2-type adjuvant (Alum) incorporated in our epitope vaccine formulation was effective in generating of high titers of anti-Aβ antibodies in both BALB/c and C57BL/6 mice (Fig. 1 and Fig. 2). Quil A was the most effective adjuvant in both strains of mice tested (Fig. 2). Using a combination of the two adjuvants was also effective, switching from vaccine formulated with Quil A to Alum (or vice versa) midway through six injections (Fig. 3). Switching from Alum to Quil A produced an anti-Aβ antibody response that was almost as strong as that produced by Quil A alone. More importantly, switching adjuvants did not change the outcome of the antibody responses generated by the adjuvant used initially. The presence of IgG1 antibody is indicative of a Th2-type immune response, whereas IgG2a is indicative of a Th1-type immune response [44–48]. The results of the present study show that, in both strains of mice, switching from Alum to Quil A did not change the Th2 phenotype of antibody responses, although it reduced the IgG1/IgG2a ratio (Fig. 4). Likewise, switching from Quil A to Alum did not change the outcome of Th1 phenotype of antibodies initially generated (Fig. 5). As mentioned above, recent analysis of data from the AN-1792 clinical trial has produced encouraging results including demonstration of a reduction in plaque load and attenuation of cognitive decline in vaccinated AD patients who produced high titers of anti-Aβ antibodies [28,29,33]. On the other hand, Th1, but not Th2 type of immune response in vaccinated AD patients has been implicated in cell-mediated autoimmune response resulted in meningoencephalitis. It is important, therefore, that future AD immunotherapy results in the clearance of Aβ peptides by specific anti-Aβ antibodies while limiting the Th1-type immune response [28–33]. The results of the present study suggest that first using a Th2-type adjuvant (Alum) and then switching to the Th1-type saponin adjuvant (QS21) used in the AN-1792 clinical trial would be beneficial, because this strategy induced the levels of anti-Aβ antibodies comparable with those achieved using the Th1-type adjuvant Quil A alone, without changing the potentially beneficial Th2- type response initiated by Alum into a pro-inflammatory Th1-type response. In other words, switching from a Th2-type adjuvant to a Th1-type adjuvant enhanced the therapeutically relevant anti-Aβ antibody production without inducing the potentially harmful Th1 immune response. The data of the present study may help to develop novel immunogen-adjuvant configurations with the potential to avoid the adverse immune response observed in the first clinical trial, while maintaining the therapeutic value of the production of anti-Aβ antibodies in reducing AD pathology and improving cognitive function in AD patients.
Acknowledgements
This work was supported in part by NIH grants AG 20241 (D.H.C. and M.G.A.) NS 50895 (D.H.C. and M.G.A.), IIRG 03-6279 (M.G.A.), and AI 44809 (M.G.A.).
References
- 1.Price DL, Sisodia SS. Cellular and molecular biology of Alzheimer’s disease and animal models. Annu Rev Med. 1994;45:435–446. doi: 10.1146/annurev.med.45.1.435. [DOI] [PubMed] [Google Scholar]
- 2.Hardy JA, Higgins GA. A Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 4.Selkoe D. Amyloid protein and Alzheimer’s disease. Sci Am. 1991;265(5):68–71. 74–76, 78. doi: 10.1038/scientificamerican1191-68. [DOI] [PubMed] [Google Scholar]
- 5.Selkoe DJ. Alzheimer’s disease: a central role for amyloid. J Neuropath Exp Neurol. 1994;53(5):438–447. doi: 10.1097/00005072-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Esler WP, Wolfe MS. A portrait of Alzheimer Secretases-new features and familiar faces. Science. 2001;293:1449–1454. doi: 10.1126/science.1064638. [DOI] [PubMed] [Google Scholar]
- 7.Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, et al. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20(11):4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuoka Y, Picciano M, Malester B, LaFrancois J, Zehr C, Daeschner JM, et al. Inflammatory responses to amyloidosis in a transgenic mouse model of Alzheimer’s disease. Am J Pathol. 2001;158(4):1345–1354. doi: 10.1016/S0002-9440(10)64085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dodart JC, Mathis C, Bales KR, Paul SM. Does my mouse have Alzheimer’s disease? Genes Brain Behav. 2002;1(3):142–155. doi: 10.1034/j.1601-183x.2002.10302.x. [DOI] [PubMed] [Google Scholar]
- 10.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse (see comments) Nature. 1999;400(6740):173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 11.Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408(6815):982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 12.Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, et al. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 13.Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, et al. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer’s disease. Nature. 2000;408:975–979. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- 14.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6(8):916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 15.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;98(15):8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klyubin I, Walsh DM, Lemere CA, Cullen WK, Shankar GM, Betts V, et al. Amyloid beta protein immunotherapy neutralizes Abeta oligomers that disrupt synaptic plasticity in vivo. Nat Med. 2005;11(6):556–561. doi: 10.1038/nm1234. [DOI] [PubMed] [Google Scholar]
- 17.Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43(3):321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Cribbs DH, Agadjanyan MG. Immunotherapy for Alzheimer’s disease: potential problems and possible solutions. Curr Immunol Rev. 2005;1(2):95. [Google Scholar]
- 19.Cribbs DH, Ghochikyan A, Tran M, Vasilevko V, Petrushina I, Sadzikava N, et al. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. Int Immunol. 2003;15(4):505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charles PC, Weber KS, Cipriani B, Brosnan CF. Cytokine, chemokine and chemokine receptor mRNA expression in different strains of normal mice: implications for establishment of a Th1/Th2 bias. J Neuroimmunol. 1999;100(1–2):64–73. doi: 10.1016/s0165-5728(99)00189-7. [DOI] [PubMed] [Google Scholar]
- 21.Steinberg D. Companies halt first Alzheimer’s vaccine trial. Scientist. 2002:22–23. [Google Scholar]
- 22.Birmingham K, Frantz S. Set back Alzheimer vaccine studies. Nat Med. 2002;8(3):199–200. doi: 10.1038/nm0302-199b. [DOI] [PubMed] [Google Scholar]
- 23.Schenk D. Opinion: amyloid-beta immunotherapy for Alzheimer’s disease: the end of the beginning. Nat Rev Neurosci. 2002;3(10):824–828. doi: 10.1038/nrn938. [DOI] [PubMed] [Google Scholar]
- 24.Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, et al. Antibodies against beta-amyloid slow cognitive decline in Alzheimer’s disease. Neuron. 2003;38(4):547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 25.Orgogozo JM, Gilman S, Dartigues JM, Laurent B, Puel M, Kirby LC, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61(1):46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 26.Senior K. Dosing in phase II trial of Alzheimer’s vaccine suspended. Lancet Neurol. 2002;1(1):3. doi: 10.1016/s1474-4422(02)00023-6. [DOI] [PubMed] [Google Scholar]
- 27.Lee M, Bard F, Johnson-Wood K, Lee C, Hu K, Griffith SG, et al. Abeta42 immunization in Alzheimer’s disease generates Abeta N-terminal antibodies. Ann Neurol. 2005;58(3):430–435. doi: 10.1002/ana.20592. [DOI] [PubMed] [Google Scholar]
- 28.Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64(9):1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 29.Fox NC, Black RS, Gilman S, Rossor MN, Griffith SG, Jenkins L, et al. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64(9):1563–1572. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
- 30.Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9(4):448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 31.Ferrer I, Rovira MB, Guerra MLS, Rey MJ, Costa-Jussa F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathol. 2004;14(1):11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, et al. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005;64(1):129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- 33.Bayer AJ, Bullock R, Jones RW, Wilkinson D, Paterson KR, Jenkins L, et al. Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. Neurology. 2005;64(1):94–101. doi: 10.1212/01.WNL.0000148604.77591.67. [DOI] [PubMed] [Google Scholar]
- 34.Bard F, Barbour R, Cannon C, Carretto R, Fox M, Games D, et al. Epitope and isotype specificities of antibodies to beta -amyloid peptide for protection against Alzheimer’s disease-like neuropathology. Proc Natl Acad Sci USA. 2003;100(4):2023–2028. doi: 10.1073/pnas.0436286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bacskai BJ, Kajdasz ST, McLellan ME, Games D, Seubert P, Schenk D, et al. Non-Fc-mediated mechanisms are involved in clearance of amyloid-beta in vivo by immunotherapy. J Neurosci. 2002;22(18):7873–7878. doi: 10.1523/JNEUROSCI.22-18-07873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agadjanyan MG, Ghochikyan A, Petrushina I, Vasilevko V, Movsesyan N, Mkrtichyan M, et al. Prototype Alzheimer’s disease vaccine using the immunodominant B cell epitope from beta-amyloid and promiscuous T cell epitope pan HLA DR-binding peptide. J Immunol. 2005;174(3):1580–1586. doi: 10.4049/jimmunol.174.3.1580. [DOI] [PubMed] [Google Scholar]
- 37.Livingston PO, Adluri S, Helling F, Yao TJ, Kensil CR, Newman MJ, et al. Phase 1 trial of immunological adjuvant QS-21 with a GM2 ganglioside-keyhole limpet haemocyanin conjugate vaccine in patients with malignant melanoma. Vaccine. 1994;12(14):1275–1280. doi: 10.1016/s0264-410x(94)80052-2. [DOI] [PubMed] [Google Scholar]
- 38.Kensil CR, Kammer R. QS-21: a water-soluble triterpene glycoside adjuvant. Expert Opin Investig Drugs. 1998;7(9):1475–1482. doi: 10.1517/13543784.7.9.1475. [DOI] [PubMed] [Google Scholar]
- 39.Gupta RK, Siber GR. Adjuvants for human vaccines—current status, problems and future prospects. Vaccine. 1995;13(14):1263–1276. doi: 10.1016/0264-410x(95)00011-o. [DOI] [PubMed] [Google Scholar]
- 40.Petrushina I, Tran M, Sadzikava N, Ghochikyan A, Vasilevko V, Agadjanyan MG, et al. Importance of IgG2c isotype in the immune response to b-amyloid in APP/Tg mice. Neurosci Lett. 2003;338:5–8. doi: 10.1016/s0304-3940(02)01357-5. [DOI] [PubMed] [Google Scholar]
- 41.Kashala O, Amador R, Valero MV, Moreno A, Barbosa A, Nickel B, et al. Safety, tolerability and immunogenicity of new formulations of the Plasmodium falciparum malaria peptide vaccine SPf66 combined with the immunological adjuvant QS-21. Vaccine. 2002;20(17–18):2263–2277. doi: 10.1016/s0264-410x(02)00115-9. [DOI] [PubMed] [Google Scholar]
- 42.Panina-Bordignon P, Tan A, Termijtelen A, Demotz S, Corradin G, Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989;19(12):2237–2242. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- 43.Chai SK, Clavijo P, Tam JP, Zavala F. Immunogenic properties of multiple antigen peptide systems containing defined T and B epitopes. J Immunol. 1992;149(7):2385–2390. [PubMed] [Google Scholar]
- 44.Finkelman FD, Holmes J, Katona IM, Urban JF, Beckmann MP, Park LS, et al. Lymphokine control of in vivo immunoglobulin isotype selection. Ann Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 45.Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 46.Hasbold J, Hong JS, Kehry MR, Hodgkin PD. Integrating signals from IFN-gamma and IL-4 by B cells: positive and negative effects on CD40 ligand-induced proliferation, survival, and division-linked isotype switching to IgG1, IgE, and IgG2a. J Immunol. 1999;163(8):4175–4481. [PubMed] [Google Scholar]
- 47.Coffman RL, Savelkoul HF, Lebman DA. Cytokine regulation of immunoglobulin isotype switching and expression. Semin Immunol. 1989;1(1):55–63. [PubMed] [Google Scholar]
- 48.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]