Abstract
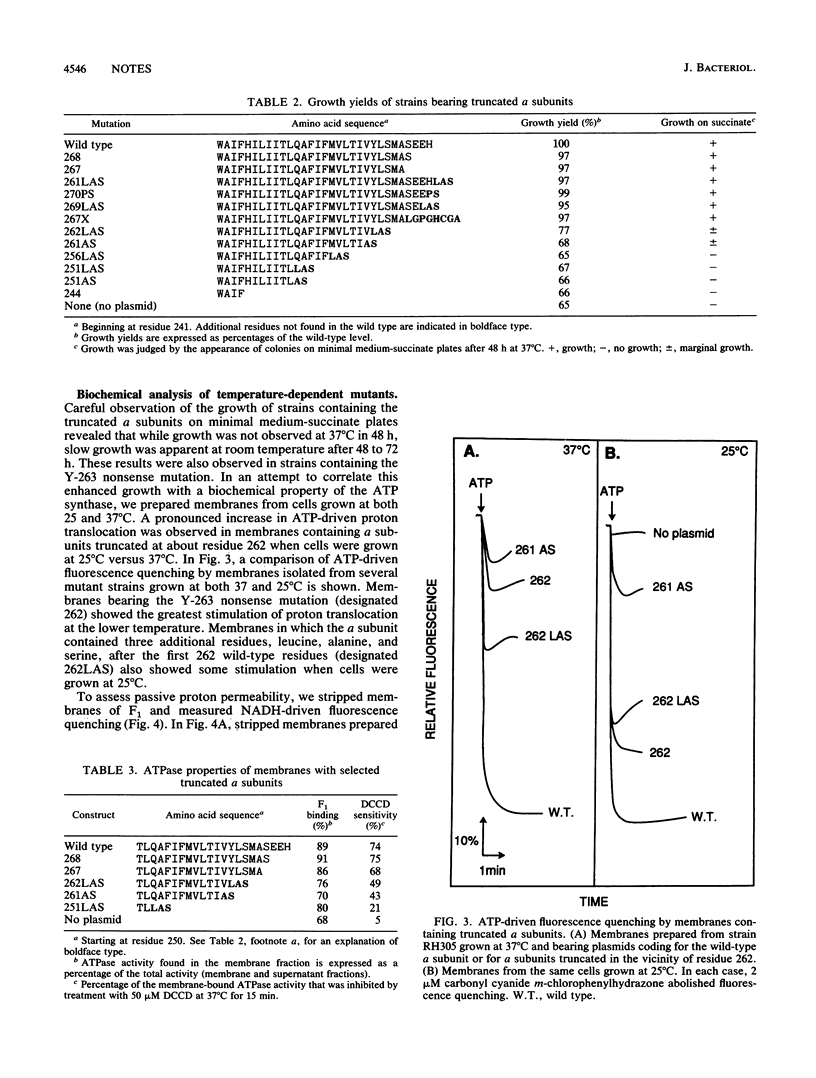
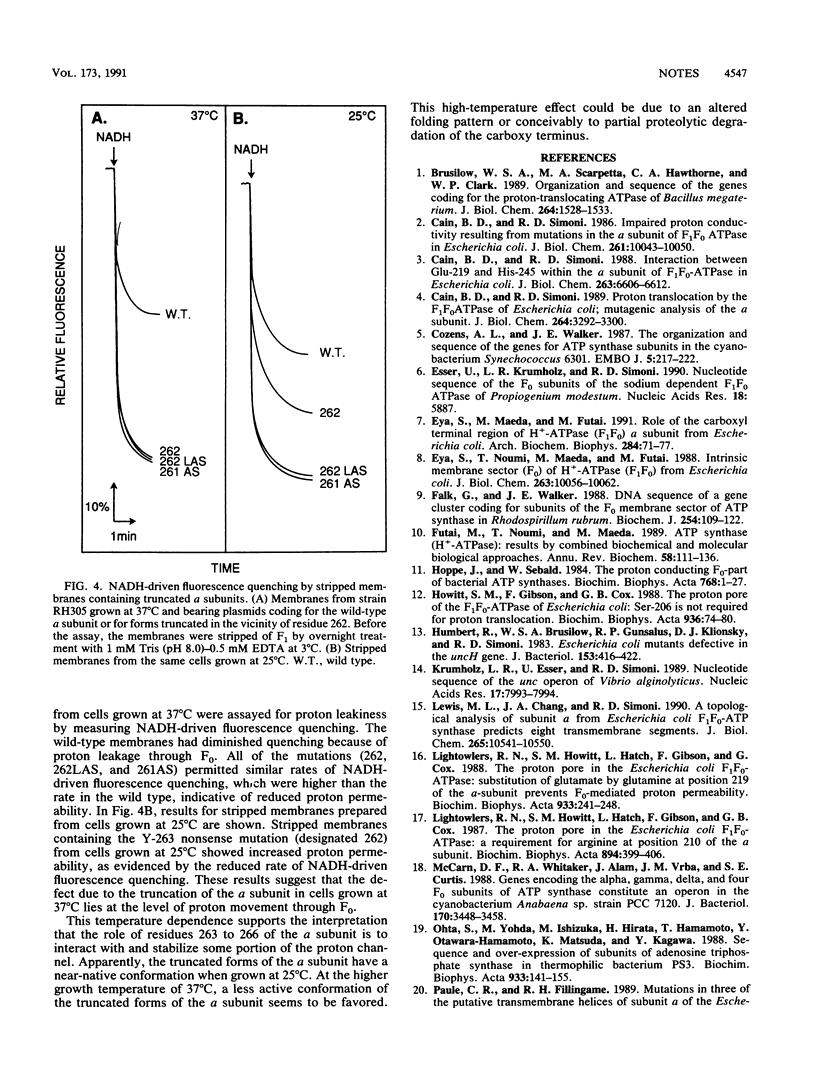
Mutations were constructed in the a subunit of the F1F0 ATP synthase from Escherichia coli. Truncated forms of this subunit showed a temperature sensitivity phenotype. We conclude that the carboxy terminus of the a subunit is not involved directly with proton translocation but that it has an important structural role.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brusilow W. S., Scarpetta M. A., Hawthorne C. A., Clark W. P. Organization and sequence of the genes coding for the proton-translocating ATPase of Bacillus megaterium. J Biol Chem. 1989 Jan 25;264(3):1528–1533. [PubMed] [Google Scholar]
- Cain B. D., Simoni R. D. Impaired proton conductivity resulting from mutations in the a subunit of F1F0 ATPase in Escherichia coli. J Biol Chem. 1986 Aug 5;261(22):10043–10050. [PubMed] [Google Scholar]
- Cain B. D., Simoni R. D. Interaction between Glu-219 and His-245 within the a subunit of F1F0-ATPase in Escherichia coli. J Biol Chem. 1988 May 15;263(14):6606–6612. [PubMed] [Google Scholar]
- Cain B. D., Simoni R. D. Proton translocation by the F1F0ATPase of Escherichia coli. Mutagenic analysis of the a subunit. J Biol Chem. 1989 Feb 25;264(6):3292–3300. [PubMed] [Google Scholar]
- Cozens A. L., Walker J. E., Phillips A. L., Huttly A. K., Gray J. C. A sixth subunit of ATP synthase, an F(0) component, is encoded in the pea chloroplast genome. EMBO J. 1986 Feb;5(2):217–222. doi: 10.1002/j.1460-2075.1986.tb04201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser U., Krumholz L. R., Simoni R. D. Nucleotide sequence of the F0 subunits of the sodium dependent F1F0 ATPase of Propionigenium modestum. Nucleic Acids Res. 1990 Oct 11;18(19):5887–5887. doi: 10.1093/nar/18.19.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eya S., Maeda M., Futai M. Role of the carboxyl terminal region of H(+)-ATPase (F0F1) a subunit from Escherichia coli. Arch Biochem Biophys. 1991 Jan;284(1):71–77. doi: 10.1016/0003-9861(91)90265-k. [DOI] [PubMed] [Google Scholar]
- Eya S., Noumi T., Maeda M., Futai M. Intrinsic membrane sector (Fo) of H+-ATPase (FoF1) from Escherichia coli. Mutations in the alpha subunit give Fo with impaired proton translocation and F1 binding. J Biol Chem. 1988 Jul 25;263(21):10056–10062. [PubMed] [Google Scholar]
- Falk G., Walker J. E. DNA sequence of a gene cluster coding for subunits of the F0 membrane sector of ATP synthase in Rhodospirillum rubrum. Support for modular evolution of the F1 and F0 sectors. Biochem J. 1988 Aug 15;254(1):109–122. doi: 10.1042/bj2540109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai M., Noumi T., Maeda M. ATP synthase (H+-ATPase): results by combined biochemical and molecular biological approaches. Annu Rev Biochem. 1989;58:111–136. doi: 10.1146/annurev.bi.58.070189.000551. [DOI] [PubMed] [Google Scholar]
- Hoppe J., Sebald W. The proton conducting F0-part of bacterial ATP synthases. Biochim Biophys Acta. 1984 Apr 9;768(1):1–27. doi: 10.1016/0304-4173(84)90005-3. [DOI] [PubMed] [Google Scholar]
- Howitt S. M., Gibson F., Cox G. B. The proton pore of the F0F1-ATPase of Escherichia coli: Ser-206 is not required for proton translocation. Biochim Biophys Acta. 1988 Oct 26;936(1):74–80. doi: 10.1016/0005-2728(88)90253-8. [DOI] [PubMed] [Google Scholar]
- Humbert R., Brusilow W. S., Gunsalus R. P., Klionsky D. J., Simoni R. D. Escherichia coli mutants defective in the uncH gene. J Bacteriol. 1983 Jan;153(1):416–422. doi: 10.1128/jb.153.1.416-422.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumholz L. R., Esser U., Simoni R. D. Nucleotide sequence of the unc operon of Vibrio alginolyticus. Nucleic Acids Res. 1989 Oct 11;17(19):7993–7994. doi: 10.1093/nar/17.19.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M. J., Chang J. A., Simoni R. D. A topological analysis of subunit alpha from Escherichia coli F1F0-ATP synthase predicts eight transmembrane segments. J Biol Chem. 1990 Jun 25;265(18):10541–10550. [PubMed] [Google Scholar]
- Lightowlers R. N., Howitt S. M., Hatch L., Gibson F., Cox G. B. The proton pore in the Escherichia coli F0F1-ATPase: a requirement for arginine at position 210 of the a-subunit. Biochim Biophys Acta. 1987 Dec 17;894(3):399–406. doi: 10.1016/0005-2728(87)90118-6. [DOI] [PubMed] [Google Scholar]
- Lightowlers R. N., Howitt S. M., Hatch L., Gibson F., Cox G. The proton pore in the Escherichia coli F0F1-ATPase: substitution of glutamate by glutamine at position 219 of the alpha-subunit prevents F0-mediated proton permeability. Biochim Biophys Acta. 1988 Apr 22;933(2):241–248. doi: 10.1016/0005-2728(88)90031-x. [DOI] [PubMed] [Google Scholar]
- McCarn D. F., Whitaker R. A., Alam J., Vrba J. M., Curtis S. E. Genes encoding the alpha, gamma, delta, and four F0 subunits of ATP synthase constitute an operon in the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1988 Aug;170(8):3448–3458. doi: 10.1128/jb.170.8.3448-3458.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta S., Yohda M., Ishizuka M., Hirata H., Hamamoto T., Otawara-Hamamoto Y., Matsuda K., Kagawa Y. Sequence and over-expression of subunits of adenosine triphosphate synthase in thermophilic bacterium PS3. Biochim Biophys Acta. 1988 Mar 30;933(1):141–155. doi: 10.1016/0005-2728(88)90064-3. [DOI] [PubMed] [Google Scholar]
- Senior A. E. The proton-translocating ATPase of Escherichia coli. Annu Rev Biophys Biophys Chem. 1990;19:7–41. doi: 10.1146/annurev.bb.19.060190.000255. [DOI] [PubMed] [Google Scholar]
- Vik S. B., Cain B. D., Chun K. T., Simoni R. D. Mutagenesis of the alpha subunit of the F1Fo-ATPase from Escherichia coli. Mutations at Glu-196, Pro-190, and Ser-199. J Biol Chem. 1988 May 15;263(14):6599–6605. [PubMed] [Google Scholar]
- Vik S. B., Lee D., Curtis C. E., Nguyen L. T. Mutagenesis of the a subunit of the F1F0-ATP synthase from Escherichia coli in the region of Asn-192. Arch Biochem Biophys. 1990 Oct;282(1):125–131. doi: 10.1016/0003-9861(90)90095-g. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Gay N. J. The unc operon. Nucleotide sequence, regulation and structure of ATP-synthase. Biochim Biophys Acta. 1984 Sep 6;768(2):164–200. doi: 10.1016/0304-4173(84)90003-x. [DOI] [PubMed] [Google Scholar]
- Yamane K., Akiyama Y., Ito K., Mizushima S. A positively charged region is a determinant of the orientation of cytoplasmic membrane proteins in Escherichia coli. J Biol Chem. 1990 Dec 5;265(34):21166–21171. [PubMed] [Google Scholar]
- von Heijne G. Control of topology and mode of assembly of a polytopic membrane protein by positively charged residues. Nature. 1989 Oct 5;341(6241):456–458. doi: 10.1038/341456a0. [DOI] [PubMed] [Google Scholar]


