Abstract
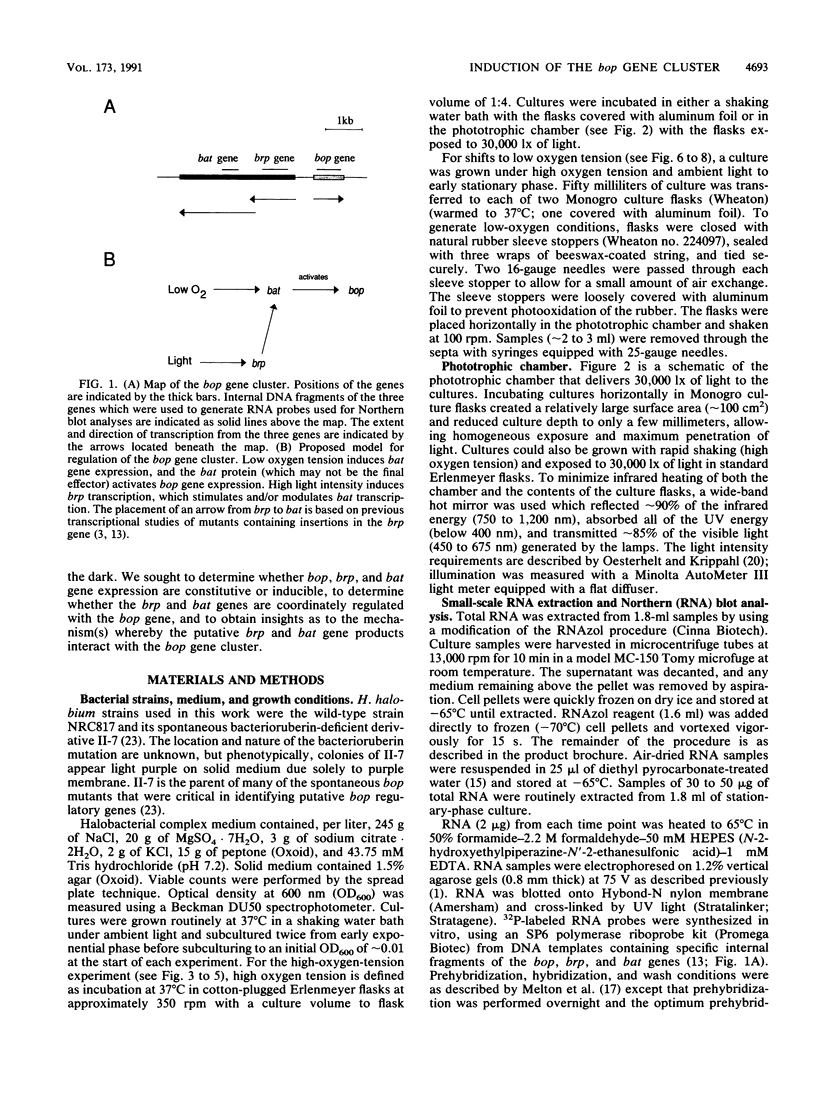
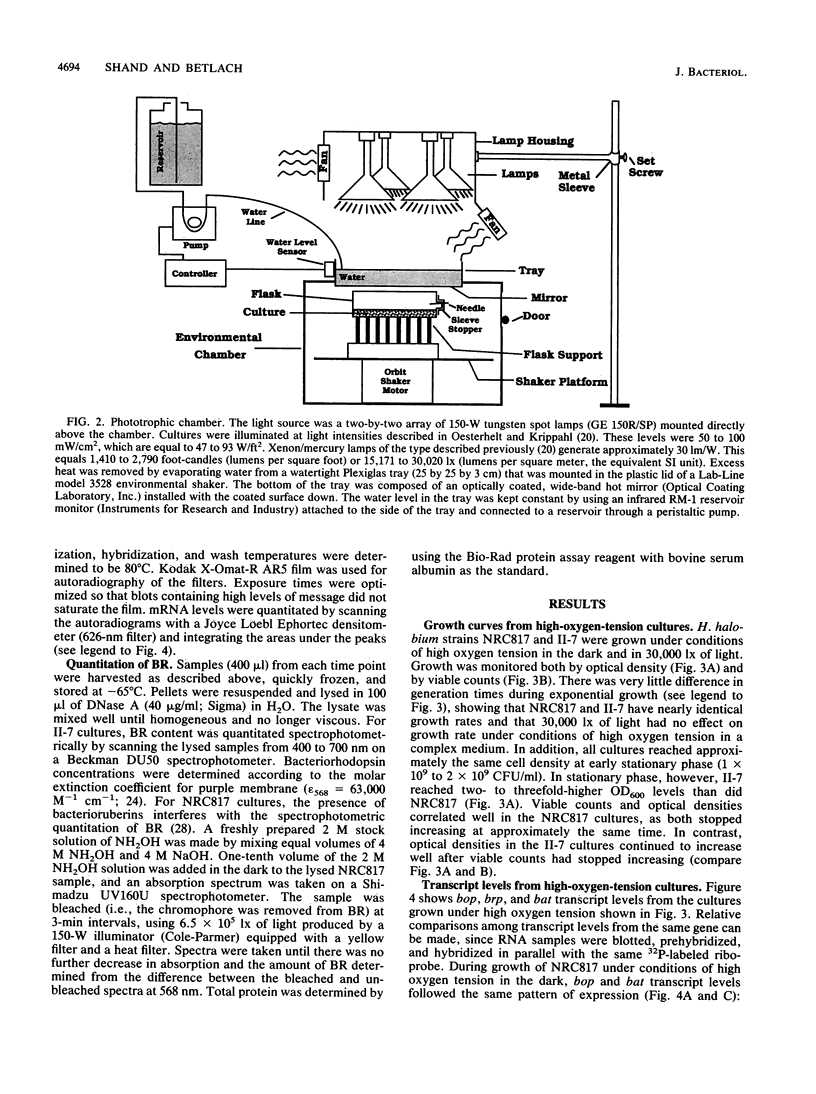
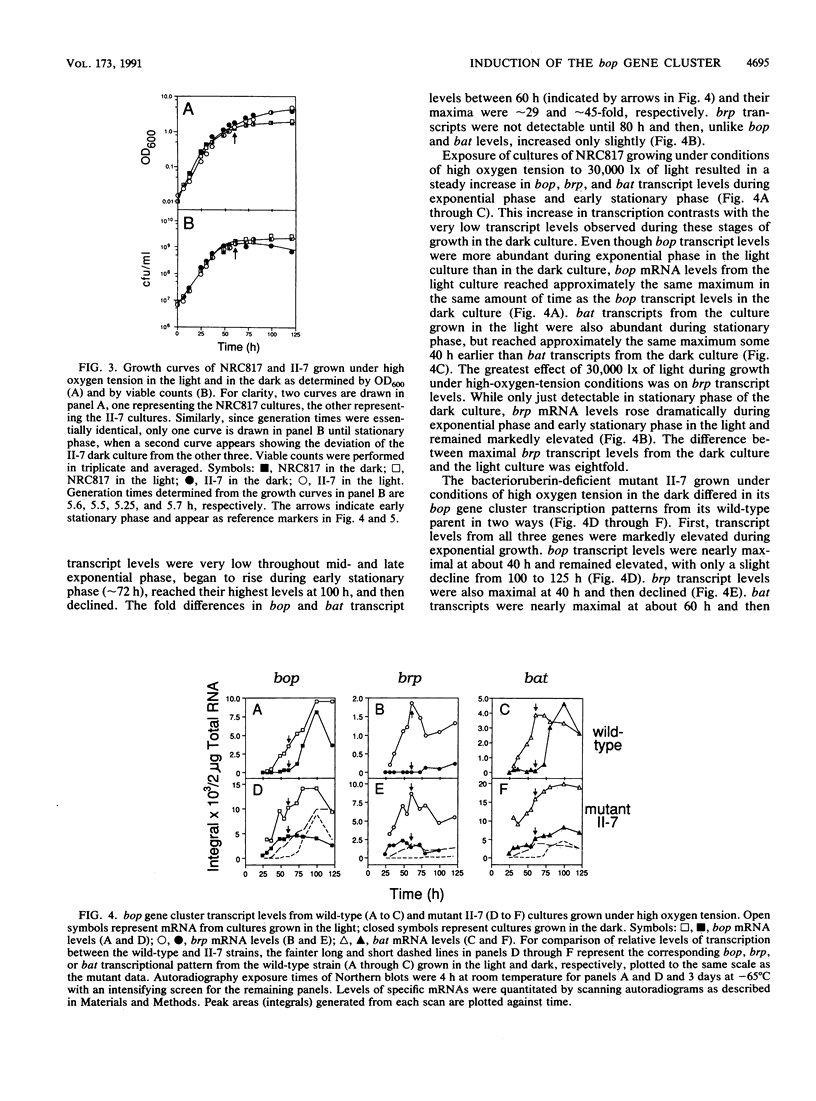
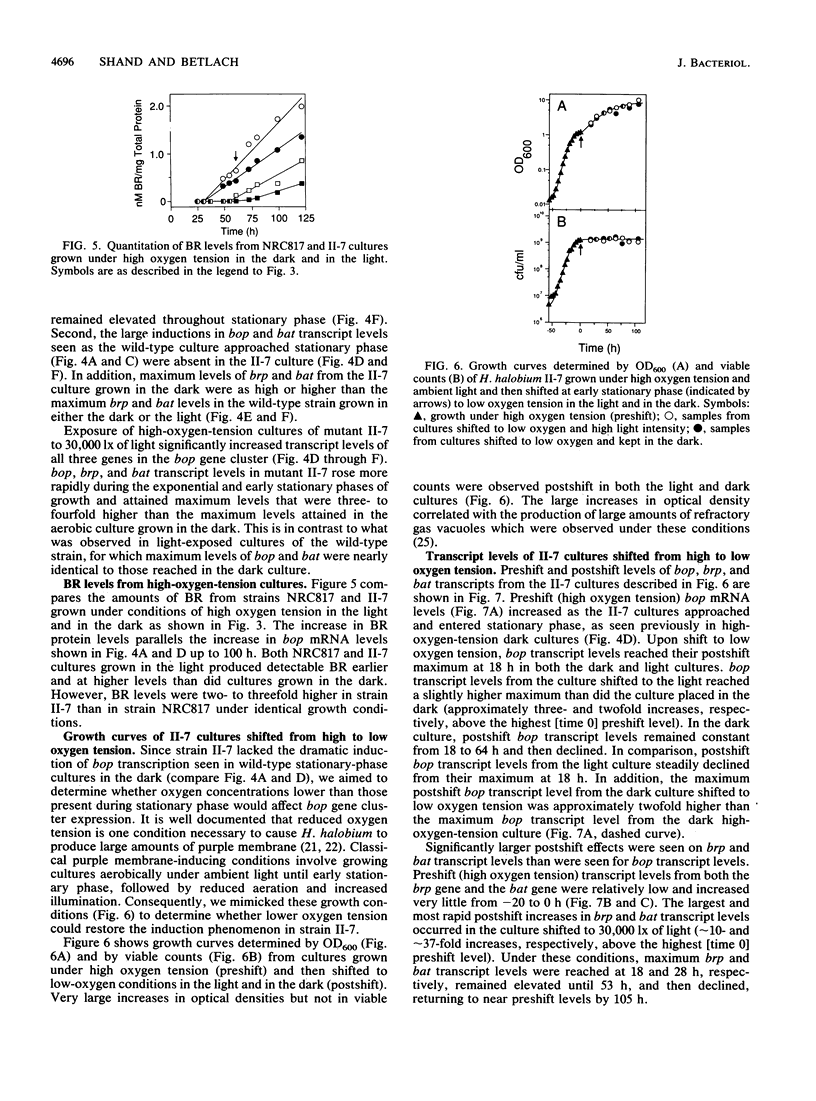
The bop gene cluster consists of at least three genes: bop (bacterio-opsin), brp (bacterio-opsin-related protein), and bat (bacterio-opsin activator). We have quantitated transcript levels from these genes in a wild-type and bacterioruberin-deficient mutant of Halobacterium halobium under conditions which affect purple membrane synthesis. In wild-type cultures grown under high oxygen tension in the dark, bop and bat transcript levels were low during steady-state growth and then increased approximately 29- and approximately 45-fold, respectively, upon entry into stationary phase. brp gene transcription remained very low and essentially unchanged under these conditions. In addition, exposure of wild-type cultures growing under high oxygen tension to 30,000 lx of light stimulated expression of all three genes, especially brp. In contrast to the wild-type, transcription from all three genes in the bacterioruberin mutant was very high during steady-state growth under high oxygen tension in the dark. Cultures of the bacterioruberin mutant were shifted at early stationary phase to low oxygen tension to determine whether oxygen concentrations lower than those present in stationary phase would induce transcription of the bop gene cluster in this strain. Indeed, transcription was induced, suggesting that the bop gene cluster is not completely uncoupled from regulation by oxygen tension in the bacterioruberin mutant. From these data, we propose a regulatory model involving two different mechanisms: (i) bat gene expression is induced under conditions of low oxygen tension and the bat gene product activates bop gene expression and (ii) light induces brp transcription, which stimulates or modulates bat transcription.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betlach M. C., Shand R. F., Leong D. M. Regulation of the bacterio-opsin gene of a halophilic archaebacterium. Can J Microbiol. 1989 Jan;35(1):134–140. doi: 10.1139/m89-020. [DOI] [PubMed] [Google Scholar]
- Betlach M., Friedman J., Boyer H. W., Pfeifer F. Characterization of a halobacterial gene affecting bacterio-opsin gene expression. Nucleic Acids Res. 1984 Oct 25;12(20):7949–7959. doi: 10.1093/nar/12.20.7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betlach M., Pfeifer F., Friedman J., Boyer H. W. Bacterio-opsin mutants of Halobacterium halobium. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1416–1420. doi: 10.1073/pnas.80.5.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braiman M., Bubis J., Doi T., Chen H. B., Flitsch S. L., Franke R. R., Gilles-Gonzalez M. A., Graham R. M., Karnik S. S., Khorana H. G. Studies on light transduction by bacteriorhodopsin and rhodopsin. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):355–364. doi: 10.1101/sqb.1988.053.01.043. [DOI] [PubMed] [Google Scholar]
- Danon A., Stoeckenius W. Photophosphorylation in Halobacterium halobium. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1234–1238. doi: 10.1073/pnas.71.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasSarma S., RajBhandary U. L., Khorana H. G. High-frequency spontaneous mutation in the bacterio-opsin gene in Halobacterium halobium is mediated by transposable elements. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2201–2205. doi: 10.1073/pnas.80.8.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond M. H., Wootton J. C. Sequence of nifL from Klebsiella pneumoniae: mode of action and relationship to two families of regulatory proteins. Mol Microbiol. 1987 Jul;1(1):37–44. doi: 10.1111/j.1365-2958.1987.tb00524.x. [DOI] [PubMed] [Google Scholar]
- Dunn R., McCoy J., Simsek M., Majumdar A., Chang S. H., Rajbhandary U. L., Khorana H. G. The bacteriorhodopsin gene. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6744–6748. doi: 10.1073/pnas.78.11.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R., Oesterhelt D. Bacteriorhodopsin-mediated photophosphorylation in Halobacterium halobium. Eur J Biochem. 1977 Jul 15;77(2):325–335. doi: 10.1111/j.1432-1033.1977.tb11671.x. [DOI] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Leong D., Boyer H., Betlach M. Transcription of genes involved in bacterio-opsin gene expression in mutants of a halophilic archaebacterium. J Bacteriol. 1988 Oct;170(10):4910–4915. doi: 10.1128/jb.170.10.4910-4915.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong D., Pfeifer F., Boyer H., Betlach M. Characterization of a second gene involved in bacterio-opsin gene expression in a halophilic archaebacterium. J Bacteriol. 1988 Oct;170(10):4903–4909. doi: 10.1128/jb.170.10.4903-4909.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Auger E. A., Blum P. H., Schultz J. E. Genetic basis of starvation survival in nondifferentiating bacteria. Annu Rev Microbiol. 1989;43:293–316. doi: 10.1146/annurev.mi.43.100189.001453. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Krippahl G. Phototrophic growth of halobacteria and its use for isolation of photosynthetically-deficient mutants. Ann Microbiol (Paris) 1983 Jul-Aug;134B(1):137–150. doi: 10.1016/s0769-2609(83)80101-x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Pfeifer F. A., Boyer H. W., Betlach M. C. Restoration of bacterioopsin gene expression in a revertant of Halobacterium halobium. J Bacteriol. 1985 Oct;164(1):414–420. doi: 10.1128/jb.164.1.414-420.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehorek M., Heyn M. P. Binding of all-trans-retinal to the purple membrane. Evidence for cooperativity and determination of the extinction coefficient. Biochemistry. 1979 Oct 30;18(22):4977–4983. doi: 10.1021/bi00589a027. [DOI] [PubMed] [Google Scholar]
- Sumper M., Herrmann G. Biogenesis of purple membrane: regulation of bacterio-opsin synthesis. FEBS Lett. 1976 Oct 15;69(1):149–152. doi: 10.1016/0014-5793(76)80673-4. [DOI] [PubMed] [Google Scholar]
- Sumper M., Herrmann G. Biosynthesis of purple membrane: control of retinal synthesis by bacterio-opsin. FEBS Lett. 1976 Dec 1;71(2):333–336. doi: 10.1016/0014-5793(76)80964-7. [DOI] [PubMed] [Google Scholar]
- Sumper M., Reitmeier H., Oesterhelt D. Biosynthesis of the purple membrane of halobacteria. Angew Chem Int Ed Engl. 1976 Apr;15(4):187–194. doi: 10.1002/anie.197601871. [DOI] [PubMed] [Google Scholar]
- Yang C. F., DasSarma S. Transcriptional induction of purple membrane and gas vesicle synthesis in the archaebacterium Halobacterium halobium is blocked by a DNA gyrase inhibitor. J Bacteriol. 1990 Jul;172(7):4118–4121. doi: 10.1128/jb.172.7.4118-4121.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]



