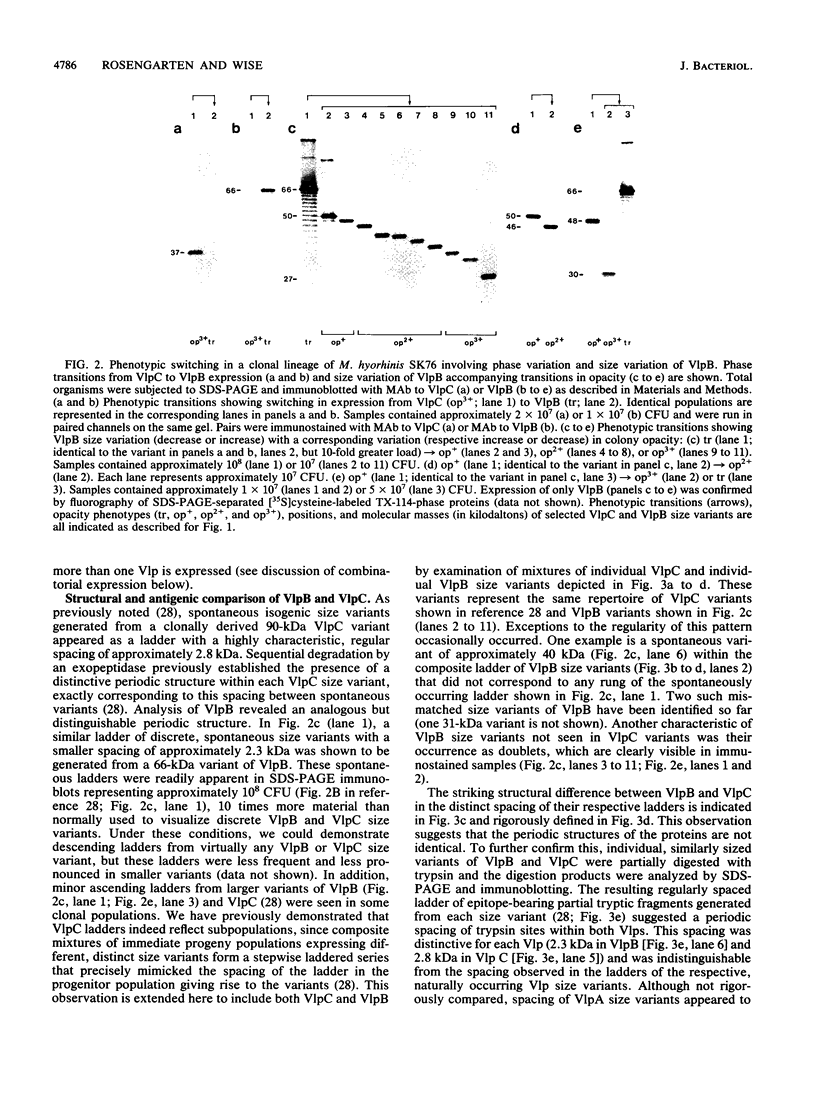
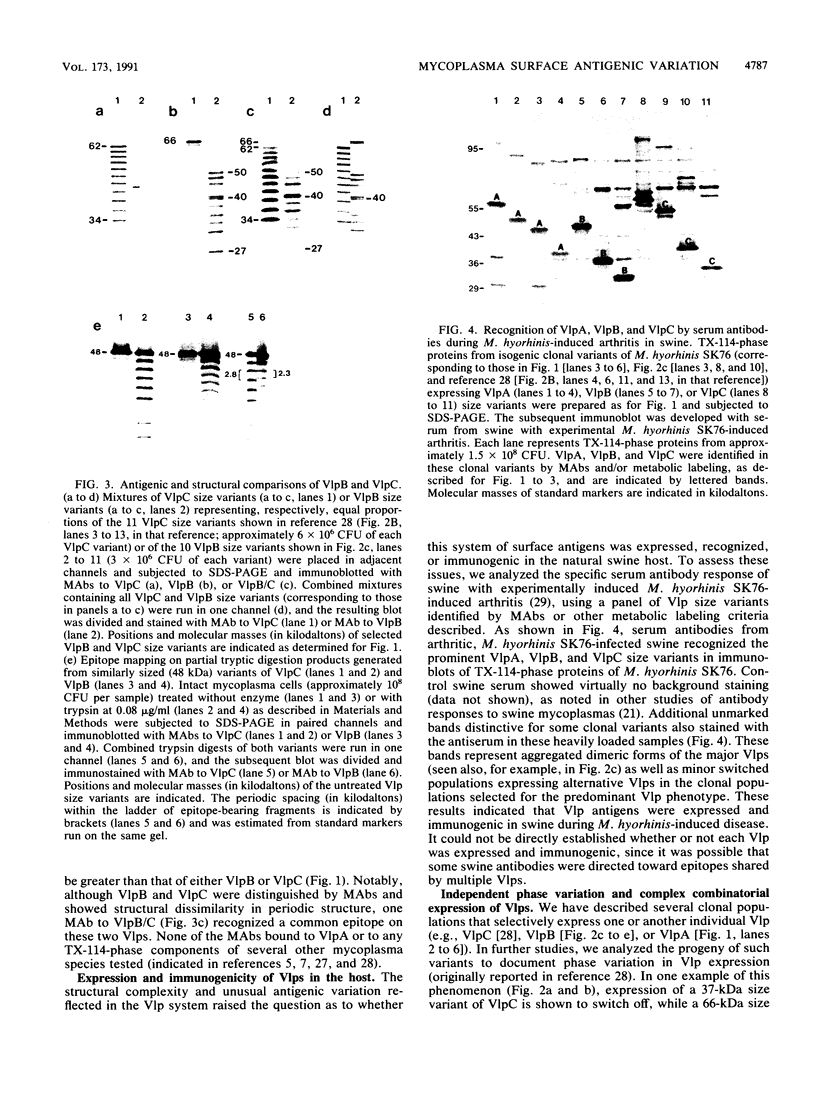
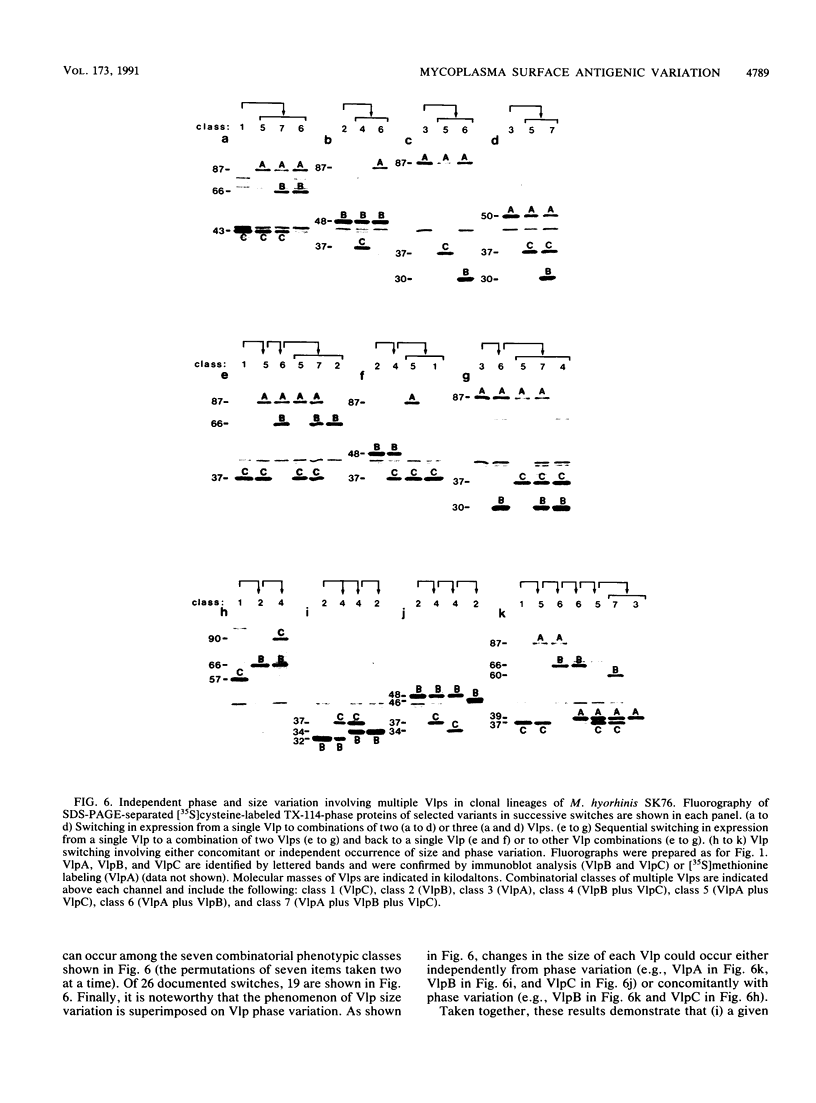
Abstract
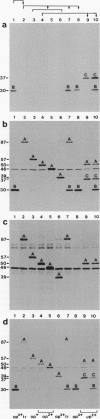
Isogenic populations of Mycoplasma hyorhinis undergo in vitro high-frequency phase variation in the expression of surface lipoproteins; these products also vary markedly in size through changes in periodic protein structure (R. Rosengarten and K.S. Wise, Science 247:315-318, 1990). In this report, we rigorously define three distinct translation products comprising the Vlp (variable lipoprotein) system of M. hyorhinis SK76 and establish parameters of Vlp structural diversity and expression that distinguish the Vlp system from previously described examples of antigenic variation. VlpA, VlpB, and VlpC are prominent amphiphilic membrane lipoproteins characterized by detergent-phase fractionation and metabolic labeling with [35S]cysteine and [3H]palmitate. VlpA is distinguished from VlpB and VlpC by its selective labeling with [35S]methionine; VlpB and VlpC are distinguished by specific epitopes defined by surface-binding monoclonal antibodies (MAbs); a third MAb defines a surface epitope shared by VlpB and VlpC (but absent from VlpA). Each Vlp displays 12 to 30 spontaneous size variant forms comprising a periodic ladder that could also be generated by partial trypsin digestion of individual Vlp size variants. Different periodic intervals within VlpB and VlpC further distinguish these two products structurally. Mycoplasma colony opacity correlates inversely with Vlp size. Each Vlp undergoes independent, oscillating high-frequency phase variation in isogenic populations and can be expressed individually or concomitantly with other Vlps in a noncoordinate manner. All seven possible combinations of these three products were observed; however, no variants were found that lacked a Vlp. High-frequency size variation of each Vlp superimposed on combinatorial diversity in Vlp expression yields greater than 10(4) possible structurally distinct Vlp mosaics, of which 104 were documented along with 24 of 42 possible transitions among the seven Vlp combinations. In addition to these features, VlpA, VlpB, and VlpC were specifically recognized by serum antibodies from swine with experimental M. hyorhinis SK76-induced arthritis, indicating expression and immunogenicity of Vlps in the natural host. The structure and variation of Vlps and their known involvement in MAb-mediated modulation of mycoplasma-infected host cell properties and mycoplasma killing are discussed in relation to the surface architecture and adaptive potential of the wall-less mycoplasmas.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andachi Y., Yamao F., Muto A., Osawa S. Codon recognition patterns as deduced from sequences of the complete set of transfer RNA species in Mycoplasma capricolum. Resemblance to mitochondria. J Mol Biol. 1989 Sep 5;209(1):37–54. doi: 10.1016/0022-2836(89)90168-x. [DOI] [PubMed] [Google Scholar]
- Bergström S., Bundoc V. G., Barbour A. G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989 Apr;3(4):479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Boyer M. J., Wise K. S. Lipid-modified surface protein antigens expressing size variation within the species Mycoplasma hyorhinis. Infect Immun. 1989 Jan;57(1):245–254. doi: 10.1128/iai.57.1.245-254.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt M. E., Riley B. S., Radolf J. D., Norgard M. V. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect Immun. 1990 Apr;58(4):983–991. doi: 10.1128/iai.58.4.983-991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker T. M., Boyer M. J., Keith J., Watson-McKown R., Wise K. S. Association of lipids with integral membrane surface proteins of Mycoplasma hyorhinis. Infect Immun. 1988 Feb;56(2):295–301. doi: 10.1128/iai.56.2.295-301.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRita V. J., Mekalanos J. J. Genetic regulation of bacterial virulence. Annu Rev Genet. 1989;23:455–482. doi: 10.1146/annurev.ge.23.120189.002323. [DOI] [PubMed] [Google Scholar]
- Dudler R., Schmidhauser C., Parish R. W., Wettenhall R. E., Schmidt T. A mycoplasma high-affinity transport system and the in vitro invasiveness of mouse sarcoma cells. EMBO J. 1988 Dec 1;7(12):3963–3970. doi: 10.1002/j.1460-2075.1988.tb03283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybvig K., Simecka J. W., Watson H. L., Cassell G. H. High-frequency variation in Mycoplasma pulmonis colony size. J Bacteriol. 1989 Sep;171(9):5165–5168. doi: 10.1128/jb.171.9.5165-5168.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell R. V., Heidari M. B., Wise K. S., McIntosh M. A. A Mycoplasma genetic element resembling prokaryotic insertion sequences. Mol Microbiol. 1989 Jul;3(7):957–967. doi: 10.1111/j.1365-2958.1989.tb00245.x. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Heffron F., Falkow S. Epithelial cell surfaces induce Salmonella proteins required for bacterial adherence and invasion. Science. 1989 Feb 17;243(4893):940–943. doi: 10.1126/science.2919285. [DOI] [PubMed] [Google Scholar]
- Hauschildt S., Steffens U., Wagner-Roos L., Bessler W. G. Role of proteinkinase C and phosphatidylinositol metabolism in lipopeptide-induced leukocyte activation as signal transducing mechanism. Mol Immunol. 1988 Nov;25(11):1081–1086. doi: 10.1016/0161-5890(88)90141-1. [DOI] [PubMed] [Google Scholar]
- Hayflick L., Stanbridge E. Isolation and identification of mycoplasma from human clinical materials. Ann N Y Acad Sci. 1967 Jul 28;143(1):608–621. doi: 10.1111/j.1749-6632.1967.tb27705.x. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Strominger J. L. Monoclonal antibodies reacting with immunogenic mycoplasma proteins present in human hematopoietic cell lines. J Immunol. 1982 Dec;129(6):2734–2738. [PubMed] [Google Scholar]
- Hollingshead S. K., Fischetti V. A., Scott J. R. Size variation in group A streptococcal M protein is generated by homologous recombination between intragenic repeats. Mol Gen Genet. 1987 May;207(2-3):196–203. doi: 10.1007/BF00331578. [DOI] [PubMed] [Google Scholar]
- Horowitz S. A., Garrett B., Davis J. K., Cassell G. H. Isolation of Mycoplasma pulmonis membranes and identification of surface antigens. Infect Immun. 1987 May;55(5):1314–1320. doi: 10.1128/iai.55.5.1314-1320.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. F., Heidari M. B., Stull S. J., McIntosh M. A., Wise K. S. Identification and mapping of an immunogenic region of Mycoplasma hyopneumoniae p65 surface lipoprotein expressed in Escherichia coli from a cloned genomic fragment. Infect Immun. 1990 Aug;58(8):2637–2643. doi: 10.1128/iai.58.8.2637-2643.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani H., McGarrity G. J. Identification of mycoplasma colonies by immunobinding. J Clin Microbiol. 1986 Apr;23(4):783–785. doi: 10.1128/jcm.23.4.783-785.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Mekalanos J. J., Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989 Feb 17;243(4893):916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- Miller L., Gray L., Beachey E., Kehoe M. Antigenic variation among group A streptococcal M proteins. Nucleotide sequence of the serotype 5 M protein gene and its relationship with genes encoding types 6 and 24 M proteins. J Biol Chem. 1988 Apr 25;263(12):5668–5673. [PubMed] [Google Scholar]
- Riethman H. C., Boyer M. J., Wise K. S. Triton X-114 phase fractionation of an integral membrane surface protein mediating monoclonal antibody killing of Mycoplasma hyorhinis. Infect Immun. 1987 May;55(5):1094–1100. doi: 10.1128/iai.55.5.1094-1100.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengarten R., Wise K. S. Phenotypic switching in mycoplasmas: phase variation of diverse surface lipoproteins. Science. 1990 Jan 19;247(4940):315–318. doi: 10.1126/science.1688663. [DOI] [PubMed] [Google Scholar]
- Ross R. F., Dale S. E., Duncan J. R. Experimentally induced Mycoplasma hyorhinis arthritis of swine: immune response to 26th postinoculation week. Am J Vet Res. 1973 Mar;34(3):367–372. [PubMed] [Google Scholar]
- Sasaki T., Sasaki Y., Matsumura T., Oyama N., Koshimizu K. Characterization of a strain of Mycoplasma hominis lacking 120 kDa membrane protein isolated from Vero cell culture. Microbiol Immunol. 1989;33(5):423–427. doi: 10.1111/j.1348-0421.1989.tb01990.x. [DOI] [PubMed] [Google Scholar]
- Schmidhauser C., Dudler R., Schmidt T., Parish R. W. A mycoplasmal protein influences tumour cell invasiveness and contact inhibition in vitro. J Cell Sci. 1990 Mar;95(Pt 3):499–506. doi: 10.1242/jcs.95.3.499. [DOI] [PubMed] [Google Scholar]
- Seifert H. S., So M. Genetic mechanisms of bacterial antigenic variation. Microbiol Rev. 1988 Sep;52(3):327–336. doi: 10.1128/mr.52.3.327-336.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers in procaryotes. J Bacteriol. 1988 Jul;170(7):2891–2897. doi: 10.1128/jb.170.7.2891-2897.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinemann C., Fenner M., Binz H., Parish R. W. Invasive behavior of mouse sarcoma cells is inhibited by blocking a 37,000-dalton plasma membrane glycoprotein with Fab fragments. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3747–3750. doi: 10.1073/pnas.81.12.3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. A., McIntosh M. A., Robbins J., Wise K. S. Cloned genomic DNA sequences from Mycoplasma hyorhinis encoding antigens expressed in Escherichia coli. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4154–4158. doi: 10.1073/pnas.80.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirkell D., Myles A. D., Russell W. C. Palmitoylated proteins in Ureaplasma urealyticum. Infect Immun. 1991 Mar;59(3):781–784. doi: 10.1128/iai.59.3.781-784.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn L. R., Hirsch S. Comparison of four Mycoplasma arthritidis strains by enzyme immunoassay, metabolism inhibition, one- and two-dimensional electrophoresis, and immunoblotting. J Clin Microbiol. 1990 Sep;28(9):1974–1981. doi: 10.1128/jcm.28.9.1974-1981.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson H. L., Blalock D. K., Cassell G. H. Variable antigens of Ureaplasma urealyticum containing both serovar-specific and serovar-cross-reactive epitopes. Infect Immun. 1990 Nov;58(11):3679–3688. doi: 10.1128/iai.58.11.3679-3688.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson H. L., Dybvig K., Blalock D. K., Cassell G. H. Subunit structure of the variable V-1 antigen of Mycoplasma pulmonis. Infect Immun. 1989 Jun;57(6):1684–1690. doi: 10.1128/iai.57.6.1684-1690.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson H. L., McDaniel L. S., Blalock D. K., Fallon M. T., Cassell G. H. Heterogeneity among strains and a high rate of variation within strains of a major surface antigen of Mycoplasma pulmonis. Infect Immun. 1988 May;56(5):1358–1363. doi: 10.1128/iai.56.5.1358-1363.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise K. S., Kim M. F. Major membrane surface proteins of Mycoplasma hyopneumoniae selectively modified by covalently bound lipid. J Bacteriol. 1987 Dec;169(12):5546–5555. doi: 10.1128/jb.169.12.5546-5555.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise K. S., Watson R. K. Mycoplasma hyorhinis GDL surface protein antigen p120 defined by monoclonal antibody. Infect Immun. 1983 Sep;41(3):1332–1339. doi: 10.1128/iai.41.3.1332-1339.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods J. P., Dempsey J. F., Kawula T. H., Barritt D. S., Cannon J. G. Characterization of the neisserial lipid-modified azurin bearing the H.8 epitope. Mol Microbiol. 1989 May;3(5):583–591. doi: 10.1111/j.1365-2958.1989.tb00205.x. [DOI] [PubMed] [Google Scholar]