Abstract
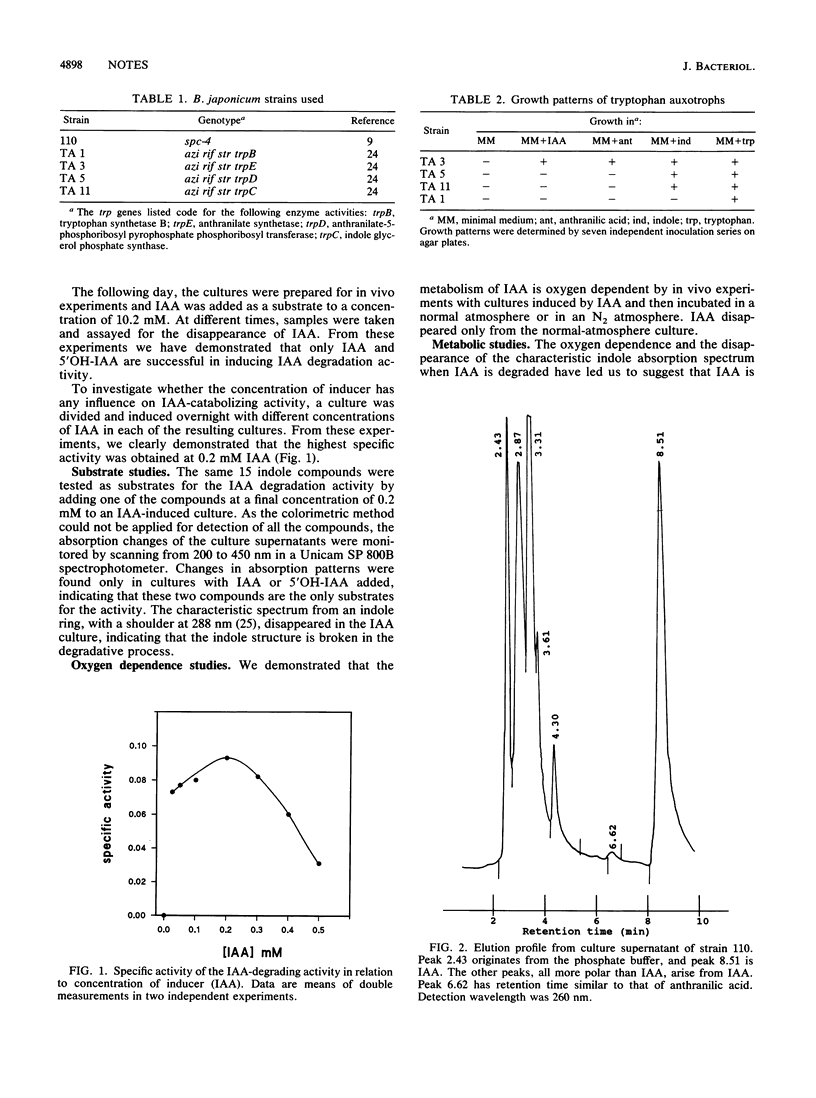
Some strains of Bradyrhizobium japonicum have the ability to catabolize indole-3-acetic acid (IAA). Examination of this catabolism in strain 110 by in vivo experiments has revealed an enzymatic activity catalyzing the degradation of IAA and 5-hydroxy-indole-3-acetic acid. The activity requires addition of the substrates for induction and is oxygen dependent. The highest activity is obtained when the concentration of inducer is 0.2 mM. Spectrophotometric data are consistent with the suggestion that the indole ring is broken during degradation of IAA. We hypothesize that the enzyme catalyzes an oxygen-consuming opening of the indole ring analogous to the one catalyzed by tryptophan 2,3-dioxygenase. The pattern of metabolite usage by known tryptophan-auxotrophic mutants and studies of metabolites by high-performance liquid chromatography indicate that anthranilic acid is a terminal degradation product in the proposed pathway.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badenoch-Jones J., Rolfe B. G., Letham D. S. Phytohormones, Rhizobium Mutants, and Nodulation in Legumes : III. Auxin Metabolism in Effective and Ineffective Pea Root Nodules. Plant Physiol. 1983 Oct;73(2):347–352. doi: 10.1104/pp.73.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. M., Dilworth M. J. Ammonia assimilation by rhizobium cultures and bacteroids. J Gen Microbiol. 1975 Jan;86(1):39–48. doi: 10.1099/00221287-86-1-39. [DOI] [PubMed] [Google Scholar]
- Gordon S. A., Weber R. P. COLORIMETRIC ESTIMATION OF INDOLEACETIC ACID. Plant Physiol. 1951 Jan;26(1):192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter W. J. Influence of 5-Methyltryptophan-Resistant Bradyrhizobium japonicum on Soybean Root Nodule Indole-3-Acetic Acid Content. Appl Environ Microbiol. 1987 May;53(5):1051–1055. doi: 10.1128/aem.53.5.1051-1055.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutzinger O., Kosuge T. Microbial synthesis and degradation of indole-3-acetic acid. 3. The isolation and characterization of indole-3-acetyl-epsilon-L-lysine. Biochemistry. 1968 Feb;7(2):601–605. doi: 10.1021/bi00842a013. [DOI] [PubMed] [Google Scholar]
- Nonhebel H. M., Bandurski R. S. Oxidation of indole-3-acetic acid and oxindole-3-acetic acid to 2,3-dihydro-7-hydroxy-2-oxo-1H indole-3-acetic acid-7'-O-beta-D-glucopyranoside in Zea mays seedlings. Plant Physiol. 1984;76:979–983. doi: 10.1104/pp.76.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonhebel H. M., Kruse L. I., Bandurski R. S. Indole-3-acetic acid catabolism in Zea mays seedlings. Metabolic conversion of oxindole-3-acetic acid to 7-hydroxy-2-oxindole-3-acetic acid 7'-O-beta-D-glucopyranoside. J Biol Chem. 1985 Oct 15;260(23):12685–12689. [PubMed] [Google Scholar]
- PROCTOR M. H. Bacterial dissimilation of indoleacetic acid: a new route of breakdown of the indole nucleus. Nature. 1958 May 10;181(4619):1345–1345. doi: 10.1038/1811345a0. [DOI] [PubMed] [Google Scholar]
- Reinecke D. M., Bandurski R. S. Oxindole-3-acetic Acid, an Indole-3-acetic Acid Catabolite in Zea mays. Plant Physiol. 1983 Jan;71(1):211–213. doi: 10.1104/pp.71.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann K. V. On the Physiology of the Formation of Nodules on Legume Roots. Proc Natl Acad Sci U S A. 1936 Aug;22(8):511–514. doi: 10.1073/pnas.22.8.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells S. E., Kuykendall L. D. Tryptophan auxotrophs of Rhizobium japonicum. J Bacteriol. 1983 Dec;156(3):1356–1358. doi: 10.1128/jb.156.3.1356-1358.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



