Abstract
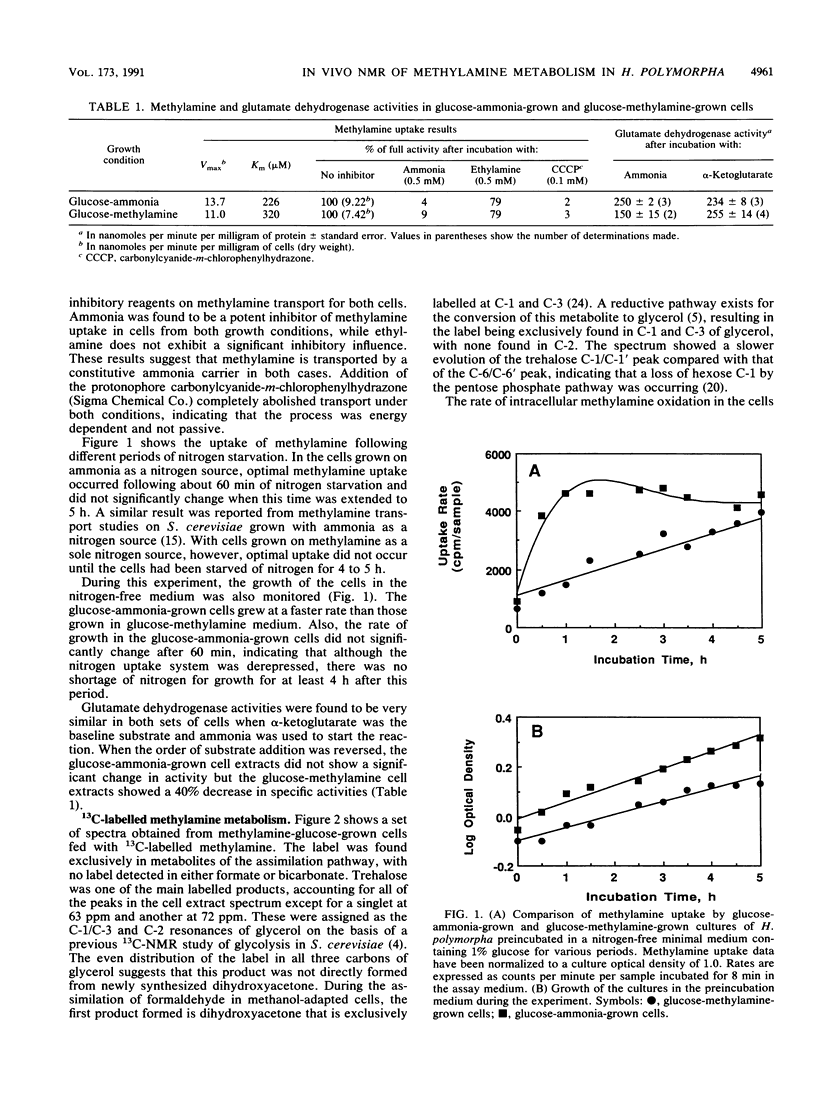
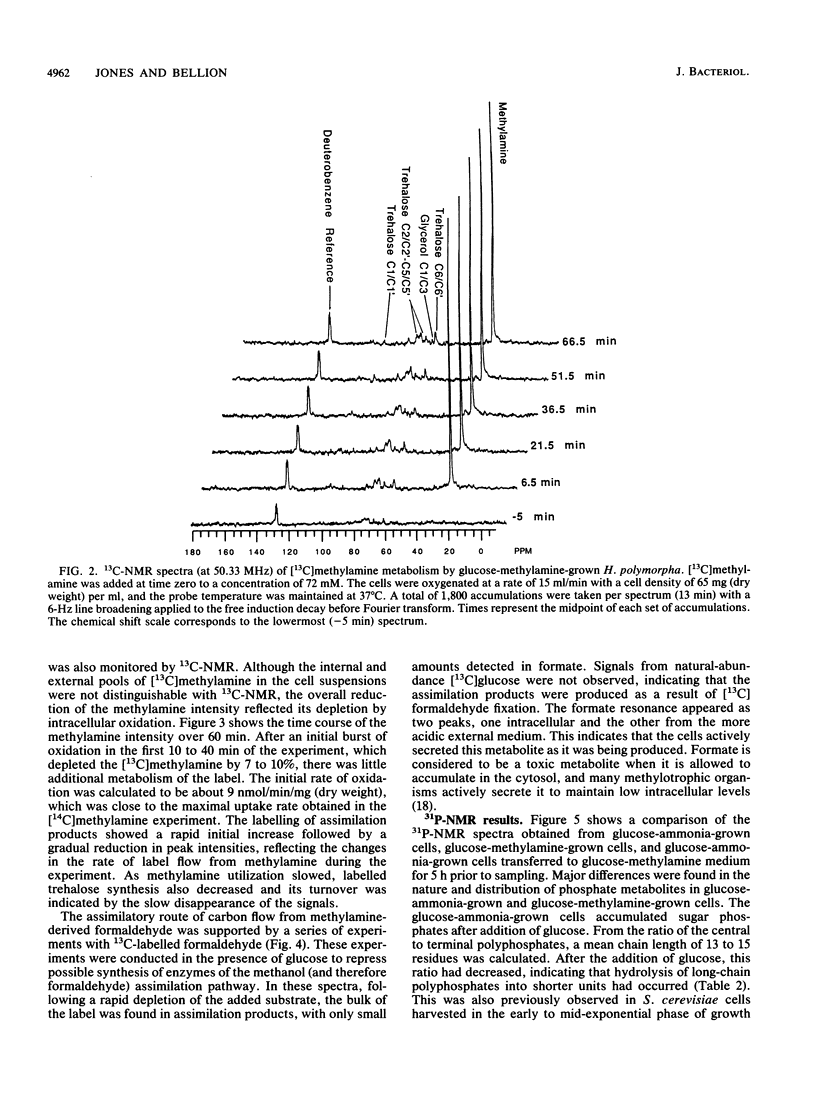
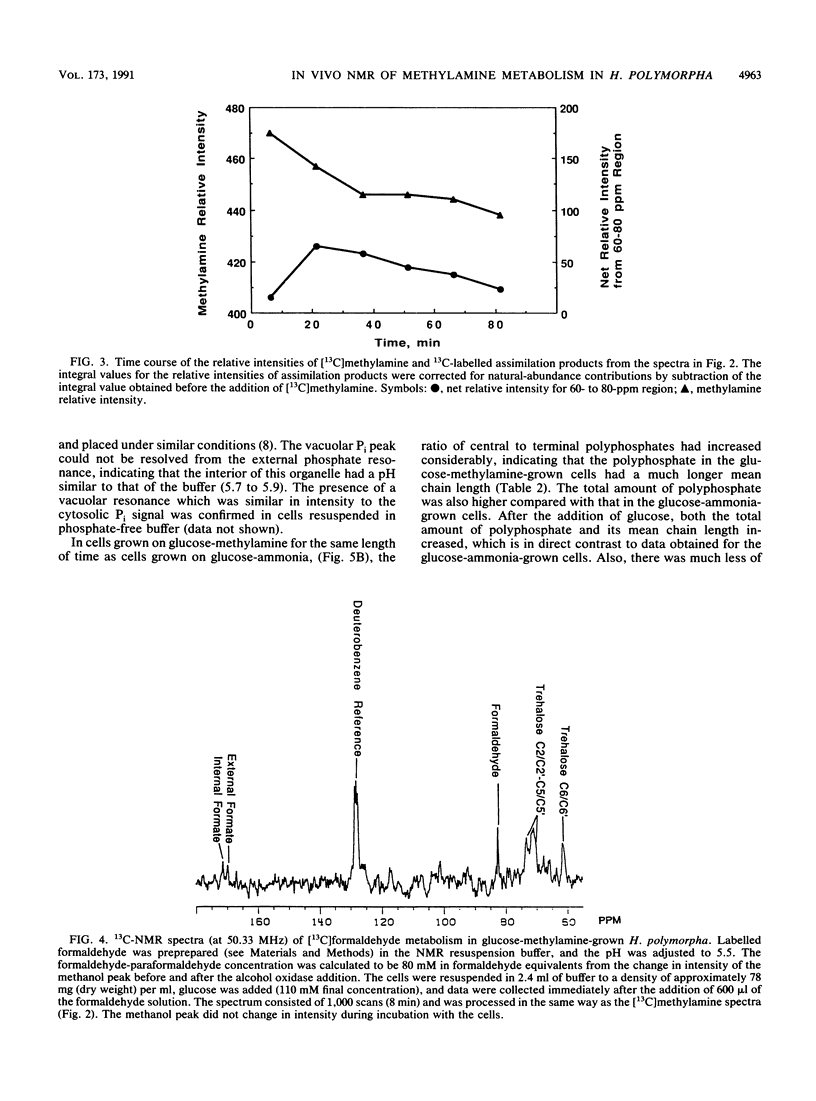
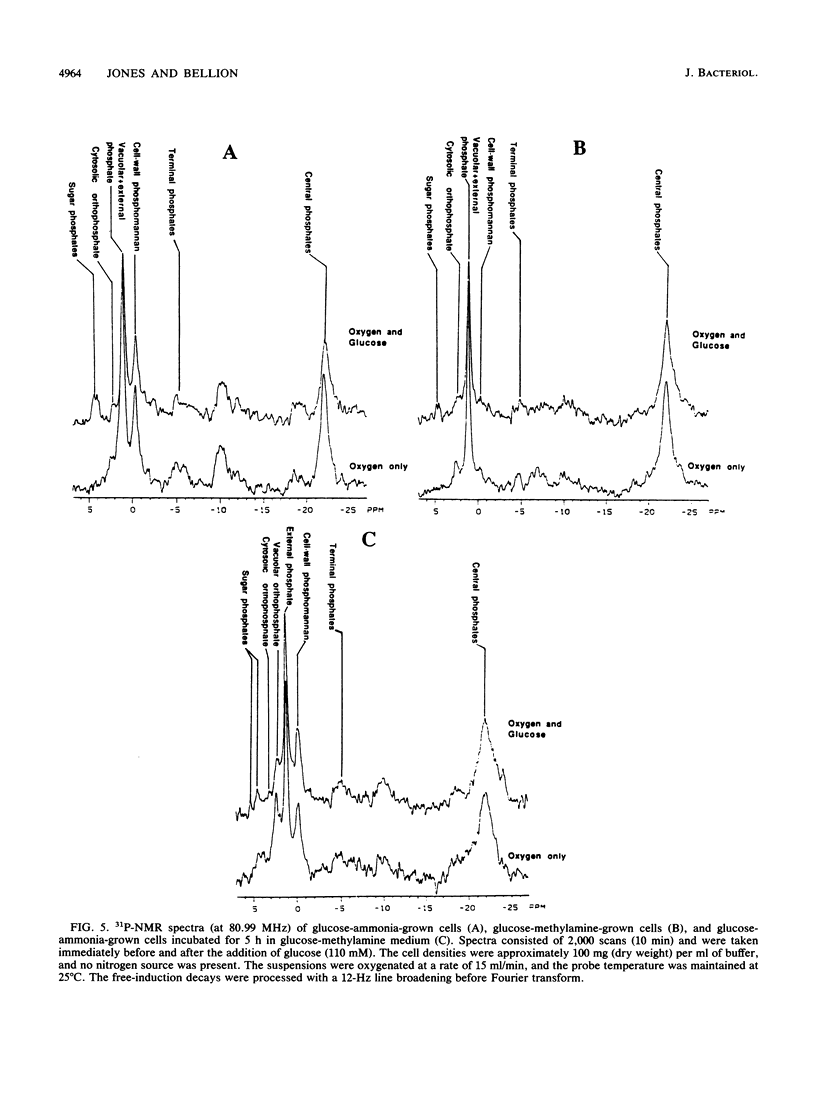
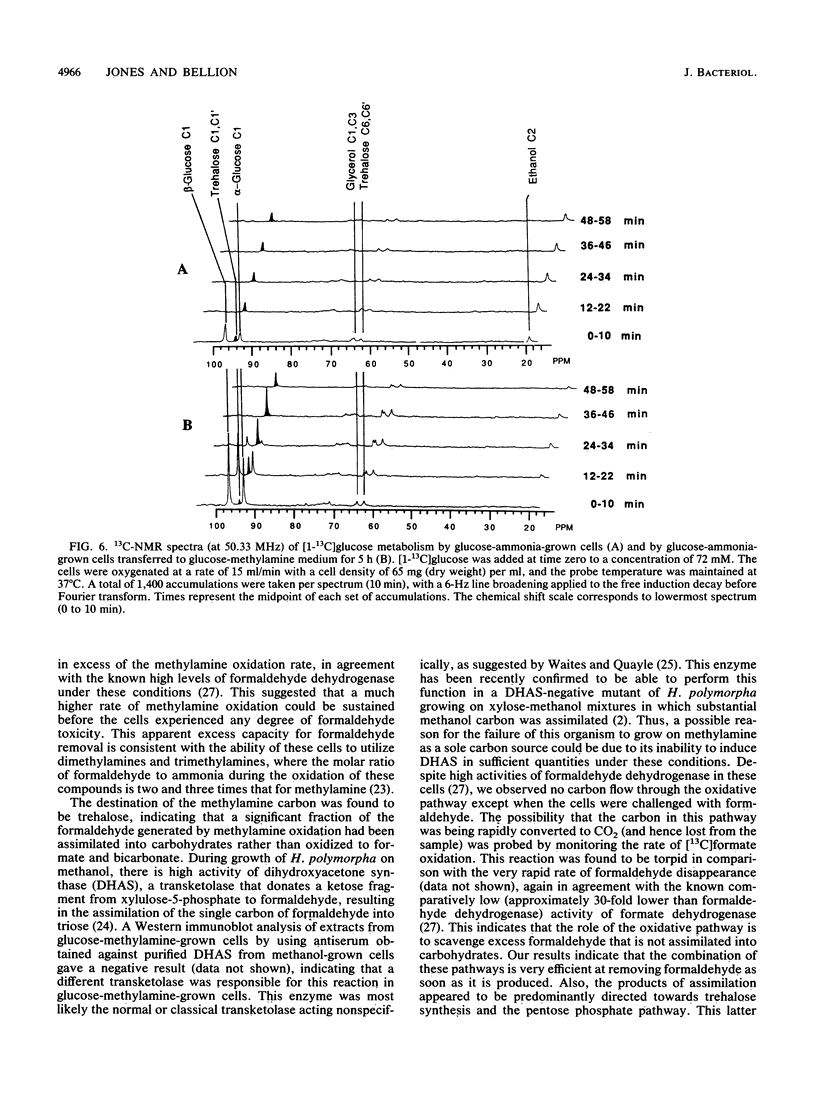
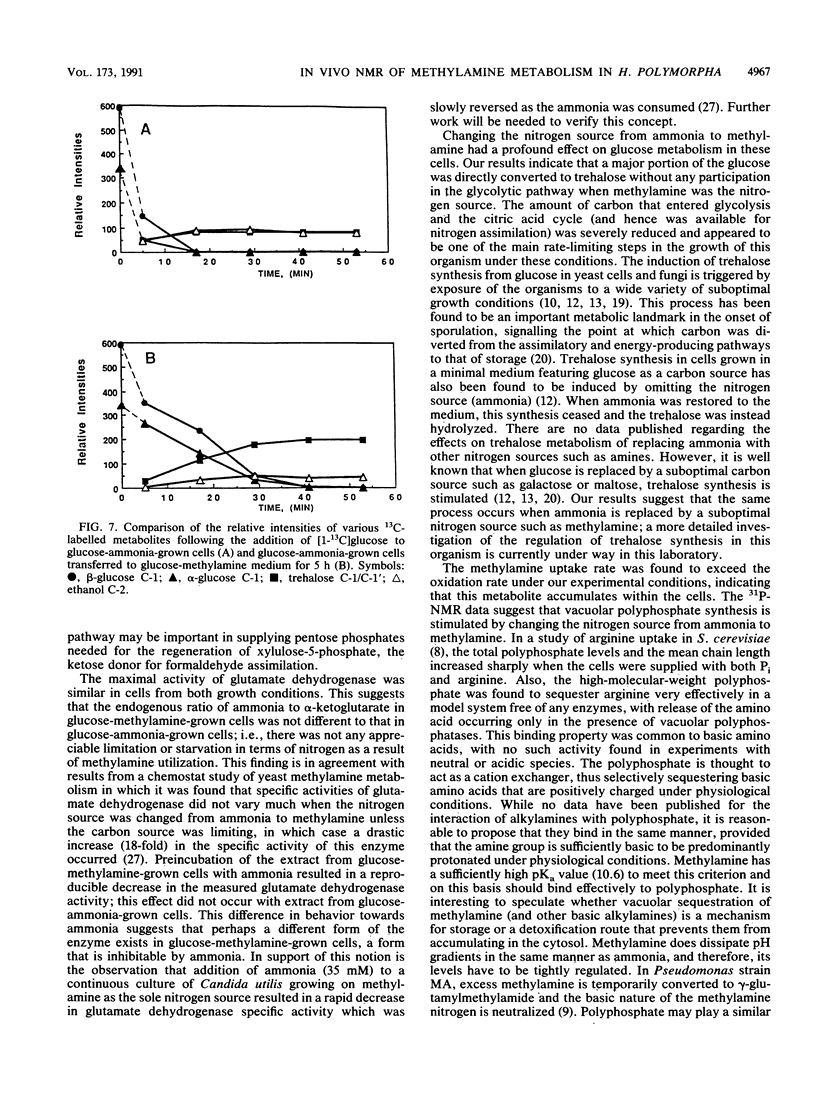
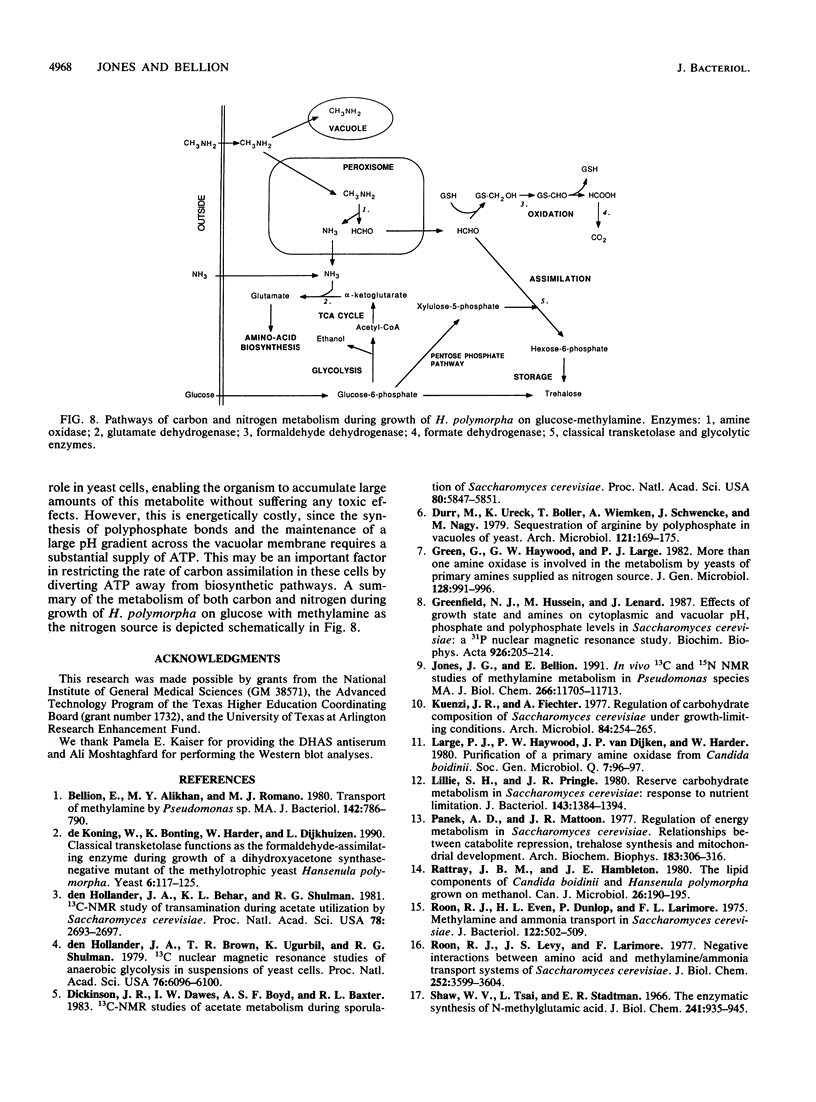
Methylamine uptake, oxidation, and assimilation were studied in Hansenula polymorpha, a methylotrophic yeast. The constitutive ammonia transport system was shown to be effective at accumulating methylamine within cells cultured with methylamine or ammonia as a nitrogen source. [13C]methylamine oxidation rates were measured in vivo in methylamine-adapted cells by 13C nuclear magnetic resonance and were found to be lower than its uptake rate into the cells. The 13C label of methylamine was found exclusively in trehalose and glycerol, and [13C]formaldehyde was also extensively assimilated, indicating the presence of an assimilation pathway for the methylamine carbon. In vivo 31P nuclear magnetic resonance analysis showed major differences in the endogenous polyphosphate levels and mean chain length during adaptation of the cells from ammonia to methylamine, indicating that methylamine accumulated in the vacuole in the same manner as basic amino acids and purines. [13C]glucose metabolism was drastically altered during adaptation of the cells from ammonia to methylamine as a nitrogen source. The total rate of glucose utilization and the rate of ethanol production fell. Direct trehalose synthesis from glucose increased, indicating a switch from carbon utilization for growth to that for storage. The rate of methylamine oxidation was sufficient to support a much higher flow of carbon into central biosynthetic pathways. These results suggest that this reduction in biosynthetic carbon flow, rather than nitrogen availability, was the main factor responsible for reducing the growth rate of the yeast when ammonia was replaced by methylamine as the nitrogen source.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellion E., Khan M. Y., Romano M. J. Transport of methylamine by Pseudomonas sp. MA. J Bacteriol. 1980 Jun;142(3):786–790. doi: 10.1128/jb.142.3.786-790.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson J. R., Dawes I. W., Boyd A. S., Baxter R. L. 13C NMR studies of acetate metabolism during sporulation of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5847–5851. doi: 10.1073/pnas.80.19.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield N. J., Hussain M., Lenard J. Effects of growth state and amines on cytoplasmic and vacuolar pH, phosphate and polyphosphate levels in Saccharomyces cerevisiae: a 31P-nuclear magnetic resonance study. Biochim Biophys Acta. 1987 Dec 7;926(3):205–214. doi: 10.1016/0304-4165(87)90205-4. [DOI] [PubMed] [Google Scholar]
- Jones J. G., Bellion E. In vivo 13C and 15N NMR studies of methylamine metabolism in Pseudomonas species MA. J Biol Chem. 1991 Jun 25;266(18):11705–11713. [PubMed] [Google Scholar]
- Küenzi M. T., Fiechter A. Regulation of carbohydrate composition of Saccharomyces cerevisiae under growth limitation. Arch Mikrobiol. 1972;84(3):254–265. doi: 10.1007/BF00425203. [DOI] [PubMed] [Google Scholar]
- Lillie S. H., Pringle J. R. Reserve carbohydrate metabolism in Saccharomyces cerevisiae: responses to nutrient limitation. J Bacteriol. 1980 Sep;143(3):1384–1394. doi: 10.1128/jb.143.3.1384-1394.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panek A. D., Mattoon J. R. Regulation of energy metabolism in Saccharomyces cerevisiae. Relationships between catabolite repression, trehalose synthesis, and mitochondrial development. Arch Biochem Biophys. 1977 Sep;183(1):306–316. doi: 10.1016/0003-9861(77)90444-1. [DOI] [PubMed] [Google Scholar]
- Rattray J. B., Hambleton J. E. The lipid components of Candida boidinii and Hansenula polymorpha grown on methanol. Can J Microbiol. 1980 Feb;26(2):190–195. doi: 10.1139/m80-029. [DOI] [PubMed] [Google Scholar]
- Roon R. J., Even H. L., Dunlop P., Larimore F. L. Methylamine and ammonia transport in Saccharomyces cerevisiae. J Bacteriol. 1975 May;122(2):502–509. doi: 10.1128/jb.122.2.502-509.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roon R. J., Levy J. S., Larimore F. Negative interactions between amino acid and methylamine/ammonia transport systems of Saccharomyces cerevisiae. J Biol Chem. 1977 Jun 10;252(11):3599–3604. [PubMed] [Google Scholar]
- Shaw W. V., Tsai L., Stadtman E. R. The enzymatic synthesis of N-methylglutamic acid. J Biol Chem. 1966 Feb 25;241(4):935–945. [PubMed] [Google Scholar]
- Sibirny A. A., Titorenko V. I., Gonchar M. V., Ubiyvovk V. M., Ksheminskaya G. P., Vitvitskaya O. P. Genetic control of methanol utilization in yeasts. J Basic Microbiol. 1988;28(5):293–319. doi: 10.1002/jobm.3620280503. [DOI] [PubMed] [Google Scholar]
- Thevelein J. M. Regulation of trehalose mobilization in fungi. Microbiol Rev. 1984 Mar;48(1):42–59. doi: 10.1128/mr.48.1.42-59.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. H., Baxter R. L. Analysis of mutational lesions of acetate metabolism in Neurospora crassa by 13C nuclear magnetic resonance. J Bacteriol. 1987 Jan;169(1):359–366. doi: 10.1128/jb.169.1.359-366.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurbil K., Brown T. R., den Hollander J. A., Glynn P., Shulman R. G. High-resolution 13C nuclear magnetic resonance studies of glucose metabolism in Escherichia coli. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3742–3746. doi: 10.1073/pnas.75.8.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urech K., Dürr M., Boller T., Wiemken A., Schwencke J. Localization of polyphosphate in vacuoles of Saccharomyces cerevisiae. Arch Microbiol. 1978 Mar;116(3):275–278. doi: 10.1007/BF00417851. [DOI] [PubMed] [Google Scholar]
- Waites M. J., Quayle J. R. The interrelation transketolase and dihydroxyacetone synthase activities in the methylotrophic yeast Candida boidinii. J Gen Microbiol. 1981 Jun;124(2):309–316. doi: 10.1099/00221287-124-2-309. [DOI] [PubMed] [Google Scholar]
- Walker T. E., Han C. H., Kollman V. H., London R. E., Matwiyoff N. A. 13C nuclear magnetic resonance studies of the biosynthesis by Microbacterium ammoniaphilum of L-glutamate selectively enriched with carbon-13. J Biol Chem. 1982 Feb 10;257(3):1189–1195. [PubMed] [Google Scholar]
- Zwart K., Veenhuis M., van Dijken J. P., Harder W. Development of amine oxidase-containing peroxisomes in yeasts during growth on glucose in the presence of methylamine as the sole source of nitrogen. Arch Microbiol. 1980 Jun;126(2):117–126. doi: 10.1007/BF00511216. [DOI] [PubMed] [Google Scholar]
- den Hollander J. A., Behar K. L., Shulman R. G. 13C NMR study of transamination during acetate utilization by Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 May;78(5):2693–2697. doi: 10.1073/pnas.78.5.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander J. A., Brown T. R., Ugurbil K., Shulman R. G. 13C nuclear magnetic resonance studies of anaerobic glycolysis in suspensions of yeast cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6096–6100. doi: 10.1073/pnas.76.12.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijken J. P., Bos P. Utilization of amines by yeasts. Arch Microbiol. 1981 Jan;128(3):320–324. doi: 10.1007/BF00422538. [DOI] [PubMed] [Google Scholar]


