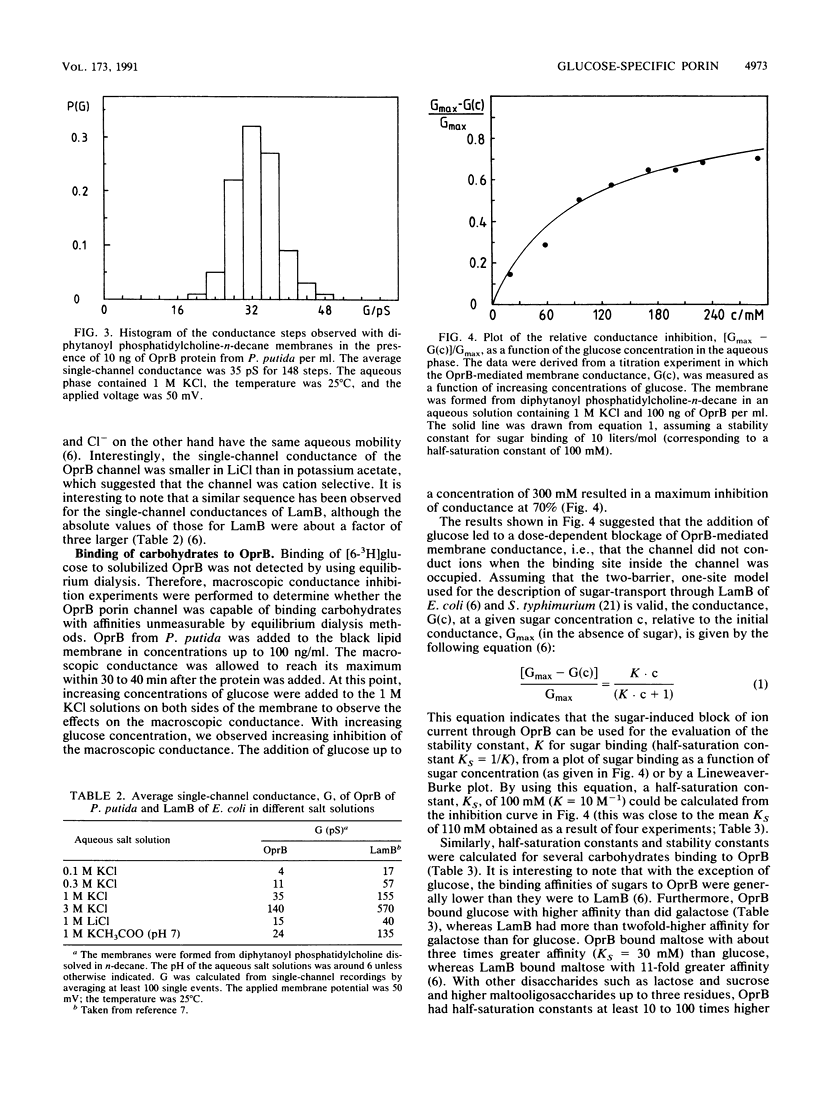
Abstract
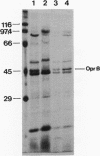
A 43,000 molecular-weight, glucose-inducible, organic acid-repressible protein (OprB) was identified in the outer membrane of Pseudomonas putida. OprB was surface expressed in whole cells, had a high beta-sheet content, and was heat modifiable, as demonstrated by 125I-labeling, circular dichroism spectroscopy, and mobility on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. OprB was extracted from outer membrane preparations by using 2% Lubrol PX with 10 mM EDTA and purified by DEAE-Sephacel ion exchange chromatography following ammonium sulfate precipitation. Reconstitution experiments with black lipid membranes showed that OprB formed small, cation-selective pores which bound glucose (KS = 110 mM) and other carbohydrates. However, the binding site of OprB appeared to be distinct from that of the maltodextrin-specific porin LamB from Escherichia coli.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agbanyo F., Taylor N. F. The active transport of 2-keto-D-gluconate in vesicles prepared from Pseudomonas purida. Biochem J. 1985 May 15;228(1):257–262. doi: 10.1042/bj2280257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Janko K., Boos W., Läuger P. Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1978 Aug 17;511(3):305–319. doi: 10.1016/0005-2736(78)90269-9. [DOI] [PubMed] [Google Scholar]
- Benz R., Schmid A., Maier C., Bremer E. Characterization of the nucleoside-binding site inside the Tsx channel of Escherichia coli outer membrane. Reconstitution experiments with lipid bilayer membranes. Eur J Biochem. 1988 Oct 1;176(3):699–705. doi: 10.1111/j.1432-1033.1988.tb14333.x. [DOI] [PubMed] [Google Scholar]
- Benz R., Schmid A., Nakae T., Vos-Scheperkeuter G. H. Pore formation by LamB of Escherichia coli in lipid bilayer membranes. J Bacteriol. 1986 Mar;165(3):978–986. doi: 10.1128/jb.165.3.978-986.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Schmid A., Vos-Scheperkeuter G. H. Mechanism of sugar transport through the sugar-specific LamB channel of Escherichia coli outer membrane. J Membr Biol. 1987;100(1):21–29. doi: 10.1007/BF02209137. [DOI] [PubMed] [Google Scholar]
- Clément J. M., Hofnung M. Gene sequence of the lambda receptor, an outer membrane protein of E. coli K12. Cell. 1981 Dec;27(3 Pt 2):507–514. doi: 10.1016/0092-8674(81)90392-5. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guymon L. F., Eagon R. G. Transport of glucose, gluconate, and methyl alpha-D-glucoside by Pseudomonas aeruginosa. J Bacteriol. 1974 Mar;117(3):1261–1269. doi: 10.1128/jb.117.3.1261-1269.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Benz R. Demonstration and chemical modification of a specific phosphate binding site in the phosphate-starvation-inducible outer membrane porin protein P of Pseudomonas aeruginosa. Biochim Biophys Acta. 1986 Sep 11;860(3):699–707. doi: 10.1016/0005-2736(86)90569-9. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Siehnel R., Martin N. Outer membrane proteins of Pseudomonas. Mol Microbiol. 1990 Jul;4(7):1069–1075. doi: 10.1111/j.1365-2958.1990.tb00680.x. [DOI] [PubMed] [Google Scholar]
- Lessie T. G., Phibbs P. V., Jr Alternative pathways of carbohydrate utilization in pseudomonads. Annu Rev Microbiol. 1984;38:359–388. doi: 10.1146/annurev.mi.38.100184.002043. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Maier C., Bremer E., Schmid A., Benz R. Pore-forming activity of the Tsx protein from the outer membrane of Escherichia coli. Demonstration of a nucleoside-specific binding site. J Biol Chem. 1988 Feb 15;263(5):2493–2499. [PubMed] [Google Scholar]
- Richardson K., Parker C. D. Identification and characterization of Vibrio cholerae surface proteins by radioiodination. Infect Immun. 1985 Apr;48(1):87–93. doi: 10.1128/iai.48.1.87-93.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schülein K., Benz R. LamB (maltoporin) of Salmonella typhimurium: isolation, purification and comparison of sugar binding with LamB of Escherichia coli. Mol Microbiol. 1990 Apr;4(4):625–632. doi: 10.1111/j.1365-2958.1990.tb00631.x. [DOI] [PubMed] [Google Scholar]
- Sokol P. A., Woods D. E. Antibody inhibition of ferripyochelin binding to Pseudomonas aeruginosa cell envelopes. Biochemistry. 1984 Oct 9;23(21):5076–5080. doi: 10.1021/bi00316a038. [DOI] [PubMed] [Google Scholar]
- Stinson M. W., Cohen M. A., Merrick J. M. Isolation of dicarboxylic acid- and glucose-binding proteins from Pseudomonas aeruginosa. J Bacteriol. 1976 Nov;128(2):573–579. doi: 10.1128/jb.128.2.573-579.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trias J., Nikaido H. Protein D2 channel of the Pseudomonas aeruginosa outer membrane has a binding site for basic amino acids and peptides. J Biol Chem. 1990 Sep 15;265(26):15680–15684. [PubMed] [Google Scholar]
- Trias J., Rosenberg E. Y., Nikaido H. Specificity of the glucose channel formed by protein D1 of Pseudomonas aeruginosa. Biochim Biophys Acta. 1988 Mar 3;938(3):493–496. doi: 10.1016/0005-2736(88)90148-4. [DOI] [PubMed] [Google Scholar]
- de Weger L. A., van Boxtel R., van der Burg B., Gruters R. A., Geels F. P., Schippers B., Lugtenberg B. Siderophores and outer membrane proteins of antagonistic, plant-growth-stimulating, root-colonizing Pseudomonas spp. J Bacteriol. 1986 Feb;165(2):585–594. doi: 10.1128/jb.165.2.585-594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]