Abstract
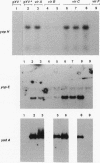
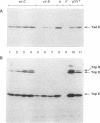
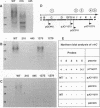
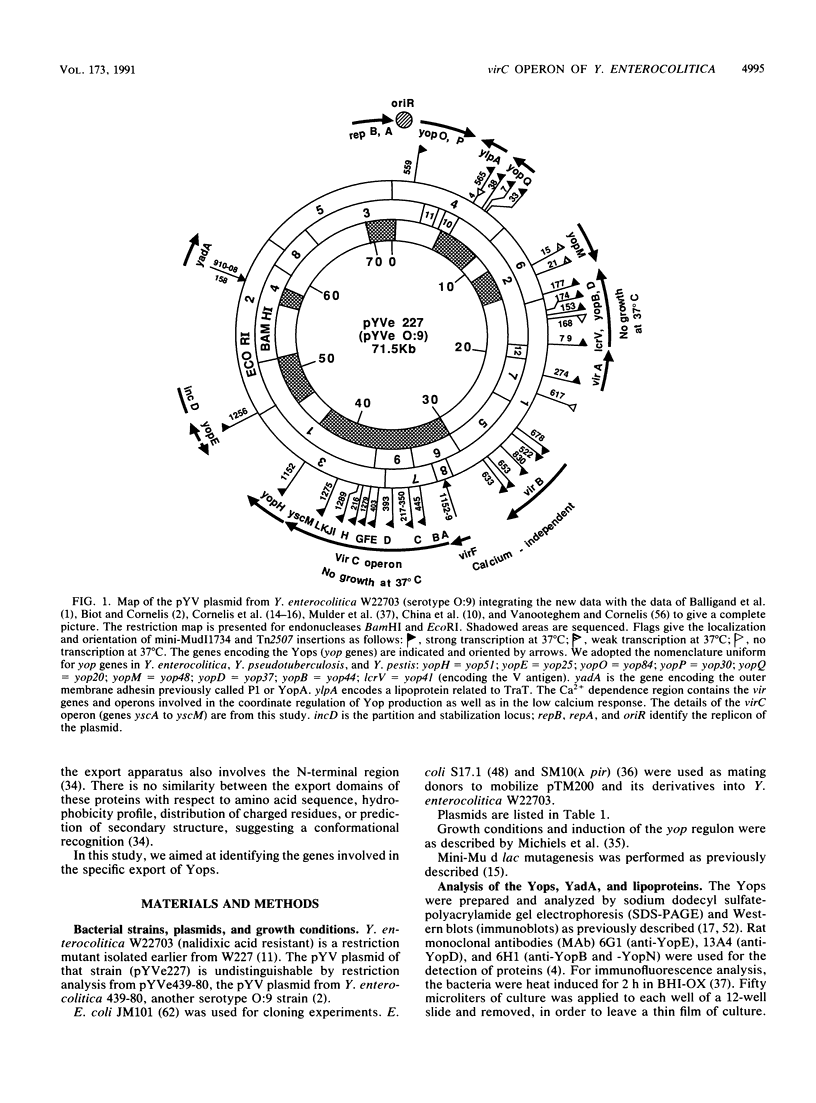
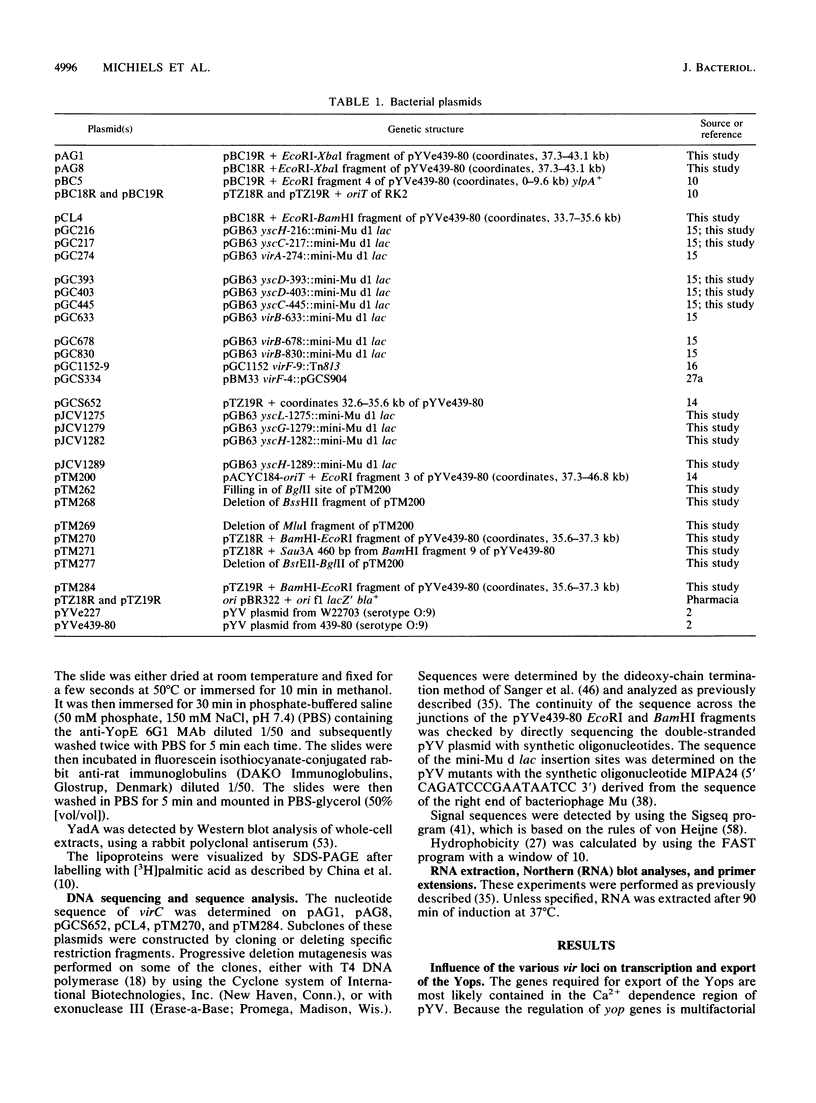
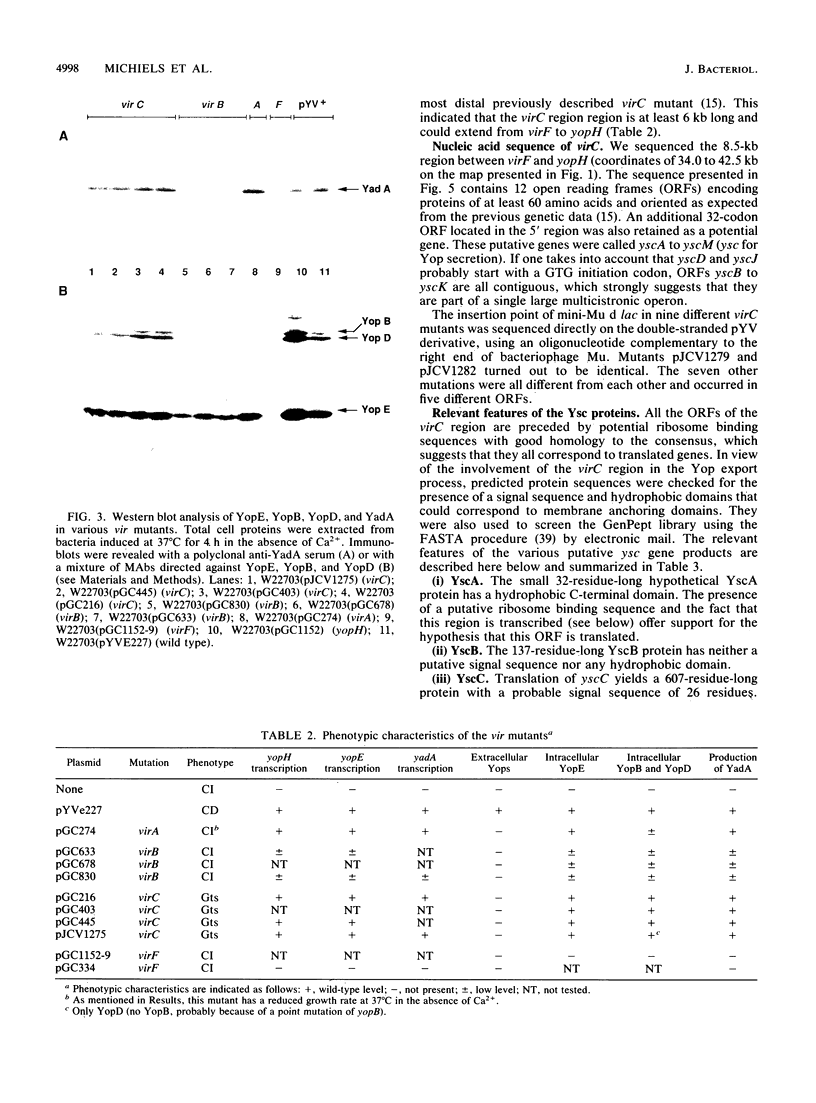
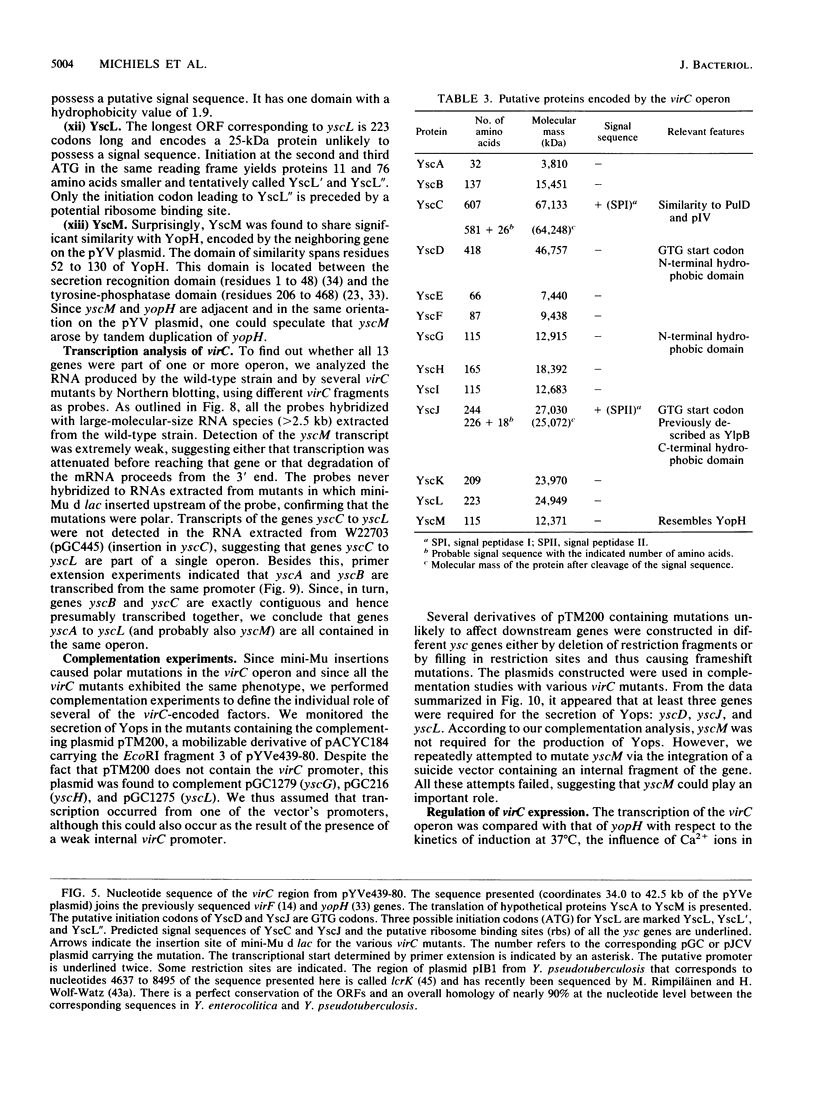
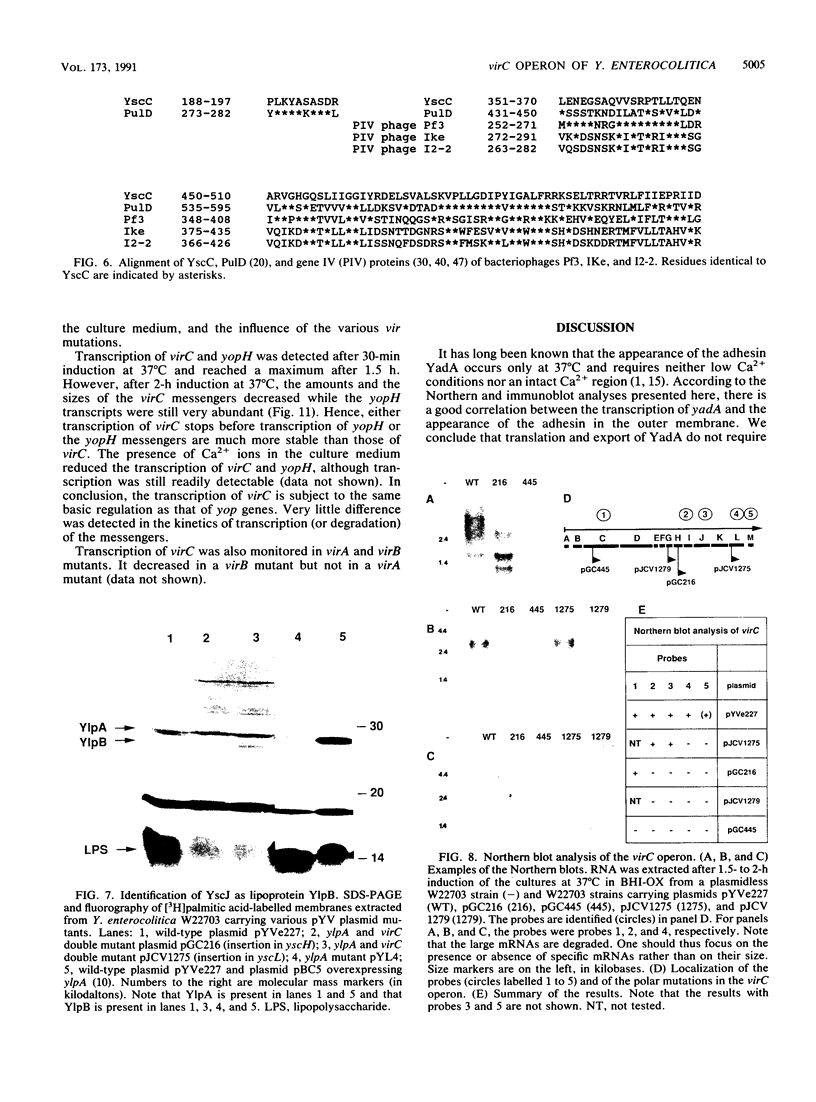
Upon incubation at 37 degrees C in the absence of Ca2+ ions, pathogenic yersiniae release large amounts of pYV plasmid-encoded proteins called Yops that are involved in pathogenesis. Yersinia enterocolitica also expresses an outer membrane protein that is considered an adhesin and called YadA (previously called P1 or YopA). The production of Yops is coordinately regulated by a 20-kb region of the plasmid referred to as the Ca2+ dependence region and containing at least four loci called virA, virB, virC, and virF. The virF gene encodes a key transcriptional activator of yop genes. We have shown here that virF is also required for transcription of yadA and that virB is necessary for full transcription of the yop and yadA genes. In contrast, mutations in genes virA and virC had only a weak influence on the transcription of yop and yadA genes. These mutations did not affect the production of YadA but they completely inhibited the translocation of Yops from the intracellular compartment to the extracellular milieu. We inferred from these data that virA and virC are involved in the specific transport of Yops. We analyzed the 8.5-kb virC region and showed that it is most probably a single operon containing 13 open reading frames called yscA to yscM (for Yop secretion). Protein YscC has a putative signal sequence and shares significant homology with outer membrane proteins involved in the secretion of pullulanase by Klebsiella pneumoniae (PulD) or in the assembly of filamentous bacteriophages (gene IV product). At least the putative products of yscD, yscJ, and yscL were shown to be required for the export of Yops. YscJ turned out to be YlpB, a lipoprotein that we had detected previously. The yscM gene shares homology with yopH, the adjacent gene on the pYV plasmid. Its product does not appear to be necessary for the production of Yops. Transcription of the virC operon was subjected to the same regulation as the yop genes.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balligand G., Laroche Y., Cornelis G. Genetic analysis of virulence plasmid from a serogroup 9 Yersinia enterocolitica strain: role of outer membrane protein P1 in resistance to human serum and autoagglutination. Infect Immun. 1985 Jun;48(3):782–786. doi: 10.1128/iai.48.3.782-786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biot T., Cornelis G. R. The replication, partition and yop regulation of the pYV plasmids are highly conserved in Yersinia enterocolitica and Y. pseudotuberculosis. J Gen Microbiol. 1988 Jun;134(6):1525–1534. doi: 10.1099/00221287-134-6-1525. [DOI] [PubMed] [Google Scholar]
- Blight M. A., Holland I. B. Structure and function of haemolysin B,P-glycoprotein and other members of a novel family of membrane translocators. Mol Microbiol. 1990 Jun;4(6):873–880. doi: 10.1111/j.1365-2958.1990.tb00660.x. [DOI] [PubMed] [Google Scholar]
- Boyd D., Beckwith J. The role of charged amino acids in the localization of secreted and membrane proteins. Cell. 1990 Sep 21;62(6):1031–1033. doi: 10.1016/0092-8674(90)90378-r. [DOI] [PubMed] [Google Scholar]
- Brissette J. L., Russel M. Secretion and membrane integration of a filamentous phage-encoded morphogenetic protein. J Mol Biol. 1990 Feb 5;211(3):565–580. doi: 10.1016/0022-2836(90)90266-O. [DOI] [PubMed] [Google Scholar]
- Bölin I., Portnoy D. A., Wolf-Watz H. Expression of the temperature-inducible outer membrane proteins of yersiniae. Infect Immun. 1985 Apr;48(1):234–240. doi: 10.1128/iai.48.1.234-240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölin I., Wolf-Watz H. The plasmid-encoded Yop2b protein of Yersinia pseudotuberculosis is a virulence determinant regulated by calcium and temperature at the level of transcription. Mol Microbiol. 1988 Mar;2(2):237–245. doi: 10.1111/j.1365-2958.1988.tb00025.x. [DOI] [PubMed] [Google Scholar]
- Chin D. T., Goff S. A., Webster T., Smith T., Goldberg A. L. Sequence of the lon gene in Escherichia coli. A heat-shock gene which encodes the ATP-dependent protease La. J Biol Chem. 1988 Aug 25;263(24):11718–11728. [PubMed] [Google Scholar]
- China B., Michiels T., Cornelis G. R. The pYV plasmid of Yersinia encodes a lipoprotein, YlpA, related to TraT. Mol Microbiol. 1990 Sep;4(9):1585–1593. doi: 10.1111/j.1365-2958.1990.tb02070.x. [DOI] [PubMed] [Google Scholar]
- Cornelis G. R., Biot T., Lambert de Rouvroit C., Michiels T., Mulder B., Sluiters C., Sory M. P., Van Bouchaute M., Vanooteghem J. C. The Yersinia yop regulon. Mol Microbiol. 1989 Oct;3(10):1455–1459. doi: 10.1111/j.1365-2958.1989.tb00129.x. [DOI] [PubMed] [Google Scholar]
- Cornelis G. R., Sluiters C., Delor I., Geib D., Kaniga K., Lambert de Rouvroit C., Sory M. P., Vanooteghem J. C., Michiels T. ymoA, a Yersinia enterocolitica chromosomal gene modulating the expression of virulence functions. Mol Microbiol. 1991 May;5(5):1023–1034. doi: 10.1111/j.1365-2958.1991.tb01875.x. [DOI] [PubMed] [Google Scholar]
- Cornelis G., Colson C. Restriction of DNA in Yersinia enterocolitica detected by recipient ability for a derepressed R factor from Escherichia coli. J Gen Microbiol. 1975 Apr;87(2):285–291. doi: 10.1099/00221287-87-2-285. [DOI] [PubMed] [Google Scholar]
- Cornelis G., Laroche Y., Balligand G., Sory M. P., Wauters G. Yersinia enterocolitica, a primary model for bacterial invasiveness. Rev Infect Dis. 1987 Jan-Feb;9(1):64–87. doi: 10.1093/clinids/9.1.64. [DOI] [PubMed] [Google Scholar]
- Cornelis G., Sluiters C., de Rouvroit C. L., Michiels T. Homology between virF, the transcriptional activator of the Yersinia virulence regulon, and AraC, the Escherichia coli arabinose operon regulator. J Bacteriol. 1989 Jan;171(1):254–262. doi: 10.1128/jb.171.1.254-262.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G., Sory M. P., Laroche Y., Derclaye I. Genetic analysis of the plasmid region controlling virulence in Yersinia enterocolitica 0:9 by Mini-Mu insertions and lac gene fusions. Microb Pathog. 1986 Aug;1(4):349–359. doi: 10.1016/0882-4010(86)90067-7. [DOI] [PubMed] [Google Scholar]
- Cornelis G., Vanootegem J. C., Sluiters C. Transcription of the yop regulon from Y. enterocolitica requires trans acting pYV and chromosomal genes. Microb Pathog. 1987 May;2(5):367–379. doi: 10.1016/0882-4010(87)90078-7. [DOI] [PubMed] [Google Scholar]
- D'Enfert C., Pugsley A. P. Klebsiella pneumoniae pulS gene encodes an outer membrane lipoprotein required for pullulanase secretion. J Bacteriol. 1989 Jul;171(7):3673–3679. doi: 10.1128/jb.171.7.3673-3679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Forsberg A., Viitanen A. M., Skurnik M., Wolf-Watz H. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol Microbiol. 1991 Apr;5(4):977–986. doi: 10.1111/j.1365-2958.1991.tb00773.x. [DOI] [PubMed] [Google Scholar]
- Forsberg A., Wolf-Watz H. The virulence protein Yop5 of Yersinia pseudotuberculosis is regulated at transcriptional level by plasmid-plB1-encoded trans-acting elements controlled by temperature and calcium. Mol Microbiol. 1988 Jan;2(1):121–133. [PubMed] [Google Scholar]
- Goguen J. D., Yother J., Straley S. C. Genetic analysis of the low calcium response in Yersinia pestis mu d1(Ap lac) insertion mutants. J Bacteriol. 1984 Dec;160(3):842–848. doi: 10.1128/jb.160.3.842-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science. 1990 Aug 3;249(4968):553–556. doi: 10.1126/science.2166336. [DOI] [PubMed] [Google Scholar]
- Heesemann J., Algermissen B., Laufs R. Genetically manipulated virulence of Yersinia enterocolitica. Infect Immun. 1984 Oct;46(1):105–110. doi: 10.1128/iai.46.1.105-110.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesemann J., Gross U., Schmidt N., Laufs R. Immunochemical analysis of plasmid-encoded proteins released by enteropathogenic Yersinia sp. grown in calcium-deficient media. Infect Immun. 1986 Nov;54(2):561–567. doi: 10.1128/iai.54.2.561-567.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapperud G., Namork E., Skurnik M., Nesbakken T. Plasmid-mediated surface fibrillae of Yersinia pseudotuberculosis and Yersinia enterocolitica: relationship to the outer membrane protein YOP1 and possible importance for pathogenesis. Infect Immun. 1987 Sep;55(9):2247–2254. doi: 10.1128/iai.55.9.2247-2254.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Leung K. Y., Reisner B. S., Straley S. C. YopM inhibits platelet aggregation and is necessary for virulence of Yersinia pestis in mice. Infect Immun. 1990 Oct;58(10):3262–3271. doi: 10.1128/iai.58.10.3262-3271.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K. Y., Straley S. C. The yopM gene of Yersinia pestis encodes a released protein having homology with the human platelet surface protein GPIb alpha. J Bacteriol. 1989 Sep;171(9):4623–4632. doi: 10.1128/jb.171.9.4623-4632.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luiten R. G., Putterman D. G., Schoenmakers J. G., Konings R. N., Day L. A. Nucleotide sequence of the genome of Pf3, an IncP-1 plasmid-specific filamentous bacteriophage of Pseudomonas aeruginosa. J Virol. 1985 Oct;56(1):268–276. doi: 10.1128/jvi.56.1.268-276.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackman N., Nicaud J. M., Gray L., Holland I. B. Genetical and functional organisation of the Escherichia coli haemolysin determinant 2001. Mol Gen Genet. 1985;201(2):282–288. doi: 10.1007/BF00425672. [DOI] [PubMed] [Google Scholar]
- Martinez R. J. Thermoregulation-dependent expression of Yersinia enterocolitica protein 1 imparts serum resistance to Escherichia coli K-12. J Bacteriol. 1989 Jul;171(7):3732–3739. doi: 10.1128/jb.171.7.3732-3739.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels T., Cornelis G. R. Secretion of hybrid proteins by the Yersinia Yop export system. J Bacteriol. 1991 Mar;173(5):1677–1685. doi: 10.1128/jb.173.5.1677-1685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels T., Cornelis G. Nucleotide sequence and transcription analysis of yop51 from Yersinia enterocolitica W22703. Microb Pathog. 1988 Dec;5(6):449–459. doi: 10.1016/0882-4010(88)90006-x. [DOI] [PubMed] [Google Scholar]
- Michiels T., Wattiau P., Brasseur R., Ruysschaert J. M., Cornelis G. Secretion of Yop proteins by Yersiniae. Infect Immun. 1990 Sep;58(9):2840–2849. doi: 10.1128/iai.58.9.2840-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988 Jun;170(6):2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder B., Michiels T., Simonet M., Sory M. P., Cornelis G. Identification of additional virulence determinants on the pYV plasmid of Yersinia enterocolitica W227. Infect Immun. 1989 Aug;57(8):2534–2541. doi: 10.1128/iai.57.8.2534-2541.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson T. A., Court D. L., Dubuc G., Michniewicz J. J., Goodchild J., Bukhari A. I., Narang S. A. Transposition studies of mini-Mu plasmids constructed from the chemically synthesized ends of bacteriophage Mu. Gene. 1986;50(1-3):101–109. doi: 10.1016/0378-1119(86)90314-8. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters B. P., Peters R. M., Schoenmakers J. G., Konings R. N. Nucleotide sequence and genetic organization of the genome of the N-specific filamentous bacteriophage IKe. Comparison with the genome of the F-specific filamentous phages M13, fd and f1. J Mol Biol. 1985 Jan 5;181(1):27–39. doi: 10.1016/0022-2836(85)90322-5. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., d'Enfert C., Reyss I., Kornacker M. G. Genetics of extracellular protein secretion by gram-negative bacteria. Annu Rev Genet. 1990;24:67–90. doi: 10.1146/annurev.ge.24.120190.000435. [DOI] [PubMed] [Google Scholar]
- Rosqvist R., Bölin I., Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988 Aug;56(8):2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosqvist R., Forsberg A., Rimpiläinen M., Bergman T., Wolf-Watz H. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol Microbiol. 1990 Apr;4(4):657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- Skurnik M., Wolf-Watz H. Analysis of the yopA gene encoding the Yop1 virulence determinants of Yersinia spp. Mol Microbiol. 1989 Apr;3(4):517–529. doi: 10.1111/j.1365-2958.1989.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Smirnov G. B. Molecular biology of the factors responsible for Yersinia virulence. Biomed Sci. 1990 Mar;1(3):223–232. [PubMed] [Google Scholar]
- Sory M. P., Cornelis G. Yersinia enterocolitica O:9 as a potential live oral carrier for protective antigens. Microb Pathog. 1988 Jun;4(6):431–442. doi: 10.1016/0882-4010(88)90028-9. [DOI] [PubMed] [Google Scholar]
- Sory M. P., Hermand P., Vaerman J. P., Cornelis G. R. Oral immunization of mice with a live recombinant Yersinia enterocolitica O:9 strain that produces the cholera toxin B subunit. Infect Immun. 1990 Aug;58(8):2420–2428. doi: 10.1128/iai.58.8.2420-2428.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sory M. P., Tollenaere J., Laszlo C., Biot T., Cornelis G. R., Wauters G. Detection of pYV+ Yersinia enterocolitica isolates by P1 slide agglutination. J Clin Microbiol. 1990 Nov;28(11):2403–2408. doi: 10.1128/jcm.28.11.2403-2408.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C. The plasmid-encoded outer-membrane proteins of Yersinia pestis. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 2):S323–S326. doi: 10.1093/cid/10.supplement_2.s323. [DOI] [PubMed] [Google Scholar]
- Tokunaga M., Tokunaga H., Wu H. C. Post-translational modification and processing of Escherichia coli prolipoprotein in vitro. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2255–2259. doi: 10.1073/pnas.79.7.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanooteghem J. C., Cornelis G. R. Structural and functional similarities between the replication region of the Yersinia virulence plasmid and the RepFIIA replicons. J Bacteriol. 1990 Jul;172(7):3600–3608. doi: 10.1128/jb.172.7.3600-3608.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viitanen A. M., Toivanen P., Skurnik M. The lcrE gene is part of an operon in the lcr region of Yersinia enterocolitica O:3. J Bacteriol. 1990 Jun;172(6):3152–3162. doi: 10.1128/jb.172.6.3152-3162.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W., Vogel M., Goebel W. Transport of hemolysin across the outer membrane of Escherichia coli requires two functions. J Bacteriol. 1983 Apr;154(1):200–210. doi: 10.1128/jb.154.1.200-210.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C., Delepelaire P. TolC, an Escherichia coli outer membrane protein required for hemolysin secretion. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4776–4780. doi: 10.1073/pnas.87.12.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Tokunaga M. Biogenesis of lipoproteins in bacteria. Curr Top Microbiol Immunol. 1986;125:127–157. doi: 10.1007/978-3-642-71251-7_9. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yother J., Chamness T. W., Goguen J. D. Temperature-controlled plasmid regulon associated with low calcium response in Yersinia pestis. J Bacteriol. 1986 Feb;165(2):443–447. doi: 10.1128/jb.165.2.443-447.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yother J., Goguen J. D. Isolation and characterization of Ca2+-blind mutants of Yersinia pestis. J Bacteriol. 1985 Nov;164(2):704–711. doi: 10.1128/jb.164.2.704-711.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Enfert C., Reyss I., Wandersman C., Pugsley A. P. Protein secretion by gram-negative bacteria. Characterization of two membrane proteins required for pullulanase secretion by Escherichia coli K-12. J Biol Chem. 1989 Oct 15;264(29):17462–17468. [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]