Abstract
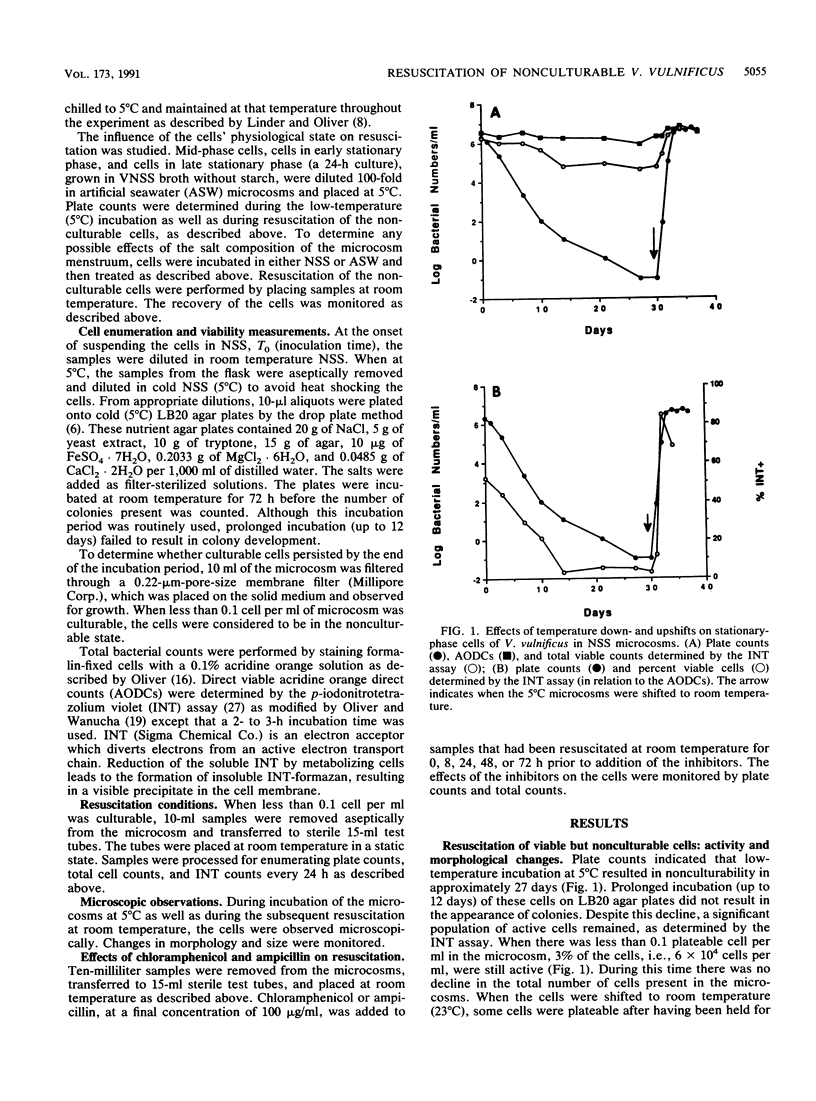
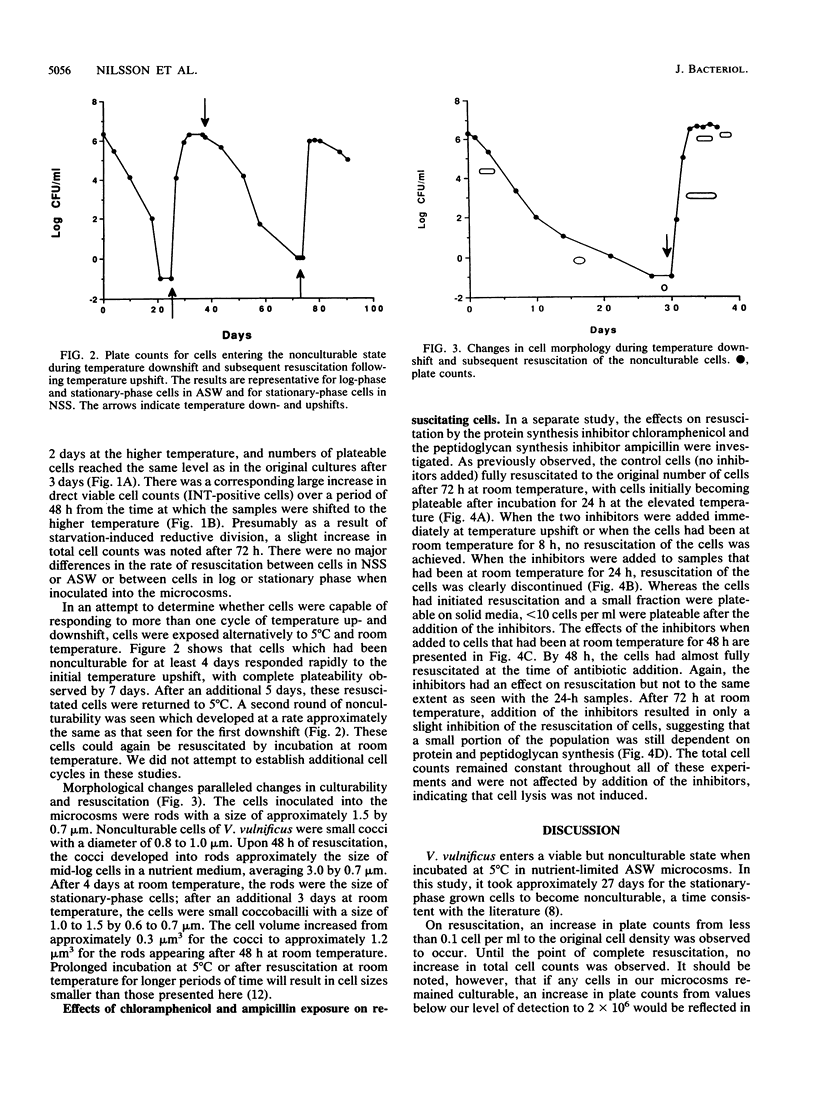
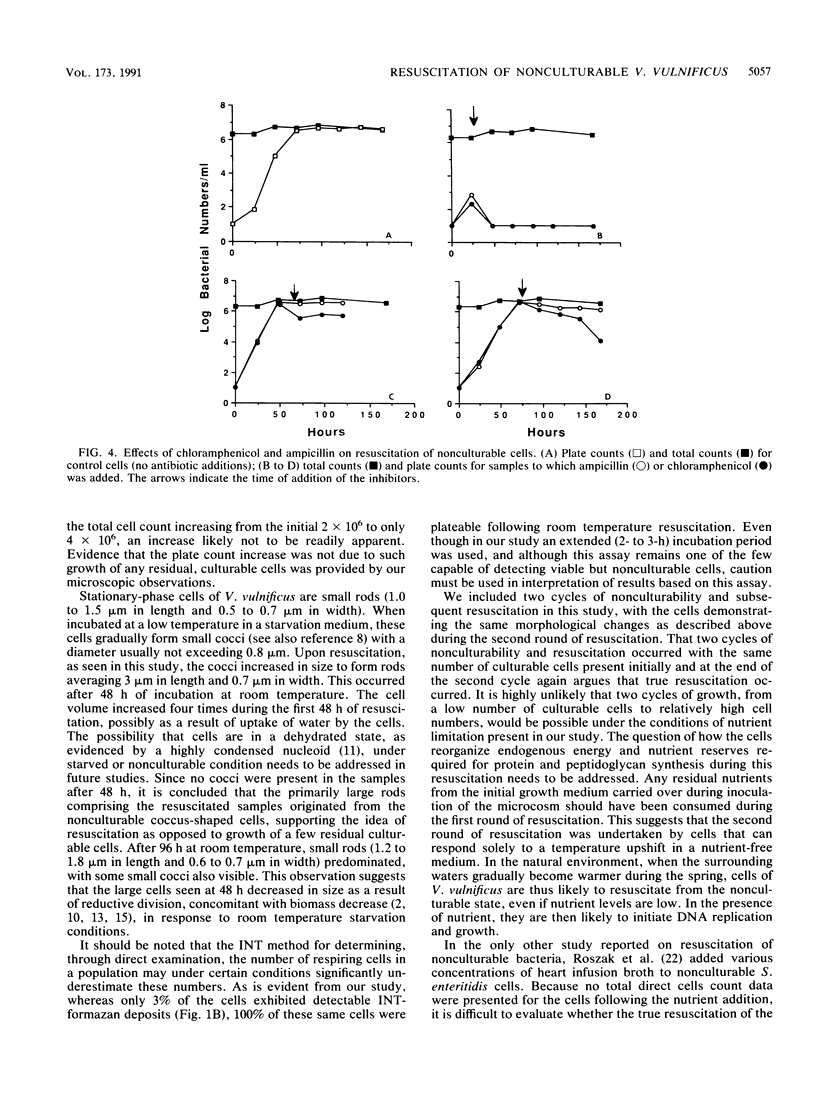
Stationary-phase-grown cells of the estuarine bacterium Vibrio vulnificus became nonculturable in nutrient-limited artificial seawater microcosms after 27 days at 5 degrees C. When the nonculturable cells were subjected to temperature upshift by being placed at room temperature, the original bacterial numbers were detectable by plate counts after 3 days, with a corresponding increase in the direct viable counts from 3% to over 80% of the total cell count. No increase in the total cell count was observed during resuscitation, indicating that the plate count increases were not due to growth of a few culturable cells. Chloramphenicol and ampicillin totally inhibited resuscitation of the nonculturable cells when added to samples that had been at room temperature for up to 24 h. After 72 h of resuscitation, the inhibitors had an easily detectable but reduced effect on the resuscitated cells, indicating that protein and peptidoglycan synthesis were still ongoing. Major changes in the morphology of the cells were discovered. Nonculturable cells of V. vulnificus were small cocci (approximately 1.0 micron in diameter). Upon resuscitation, the cells became large rods with a size of mid-log-phase cells (3.0 microns in length). Four days after the cells had become fully resuscitated, the cell size had decreased to approximately 1.5 micron in length and 0.7 micron in width. The cells were able to go through at least two cycles of nonculturability and subsequent resuscitation without changes in the total cell count. This is the first report of resuscitation, without the addition of nutrient, of nonculturable cells, and it is suggested that temperature may be the determining factor in the resuscitation from this survival, or adaptation, state of certain species in estuarine environments.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker R. M., Singleton F. L., Hood M. A. Effects of nutrient deprivation on Vibrio cholerae. Appl Environ Microbiol. 1983 Oct;46(4):930–940. doi: 10.1128/aem.46.4.930-940.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury M. A., Yamanaka H., Miyoshi S., Aziz K. M., Shinoda S. Ecology of Vibrio mimicus in aquatic environments. Appl Environ Microbiol. 1989 Aug;55(8):2073–2078. doi: 10.1128/aem.55.8.2073-2078.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoben H. J., Somasegaran P. Comparison of the Pour, Spread, and Drop Plate Methods for Enumeration of Rhizobium spp. in Inoculants Made from Presterilized Peat. Appl Environ Microbiol. 1982 Nov;44(5):1246–1247. doi: 10.1128/aem.44.5.1246-1247.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. G., VanBogelen R. A., Neidhardt F. C. Induction of proteins in response to low temperature in Escherichia coli. J Bacteriol. 1987 May;169(5):2092–2095. doi: 10.1128/jb.169.5.2092-2095.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder K., Oliver J. D. Membrane fatty acid and virulence changes in the viable but nonculturable state of Vibrio vulnificus. Appl Environ Microbiol. 1989 Nov;55(11):2837–2842. doi: 10.1128/aem.55.11.2837-2842.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer C. L., Morita R. Y. Effect of growth rate and starvation-survival on the viability and stability of a psychrophilic marine bacterium. Appl Environ Microbiol. 1989 May;55(5):1122–1127. doi: 10.1128/aem.55.5.1122-1127.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström T., Flärdh K., Kjelleberg S. Responses to multiple-nutrient starvation in marine Vibrio sp. strain CCUG 15956. J Bacteriol. 1990 Dec;172(12):7085–7097. doi: 10.1128/jb.172.12.7085-7097.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. The survival of starved bacteria. J Gen Microbiol. 1962 Oct;29:233–263. doi: 10.1099/00221287-29-2-233. [DOI] [PubMed] [Google Scholar]
- Rollins D. M., Colwell R. R. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl Environ Microbiol. 1986 Sep;52(3):531–538. doi: 10.1128/aem.52.3.531-538.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Grimes D. J., Colwell R. R. Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can J Microbiol. 1984 Mar;30(3):334–338. doi: 10.1139/m84-049. [DOI] [PubMed] [Google Scholar]
- Simpson L. M., White V. K., Zane S. F., Oliver J. D. Correlation between virulence and colony morphology in Vibrio vulnificus. Infect Immun. 1987 Jan;55(1):269–272. doi: 10.1128/iai.55.1.269-272.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E., Markiewicz Z., Tomasz A. Autolysis-resistant peptidoglycan of anomalous composition in amino-acid-starved Escherichia coli. J Bacteriol. 1988 Mar;170(3):1373–1376. doi: 10.1128/jb.170.3.1373-1376.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R., Iturriaga R., Becker-Birck J. Simultaneous determination of the total number of aquatic bacteria and the number thereof involved in respiration. Appl Environ Microbiol. 1978 Dec;36(6):926–935. doi: 10.1128/aem.36.6.926-935.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


