Abstract
An outbreak of chronic wasting disease (CWD) in farmed elk in Saskatchewan from 1996 to 2002 was reviewed to 1, determine the progression of CWD from infection to death in farmed elk; 2, assess animal risk factors for CWD infection in farmed elk; 3, assess farm management and exposure risk factors for within herd CWD transmission; and 4, assess the suitability of the Canadian Food Inspection Agency’s (CFIA) current disease control policy for CWD in light of the findings. The results from animal movement tracing, animal testing, and a farm management questionnaire were used. The duration of CWD (time from exposure to death of a CWD test-positive animal) was between a mean minimum of 19 months and a mean maximum of 40 months. Age and sex were not associated with CWD infection, except that adult elk (≥ 2 y) were more likely to be infected than young elk (< 18 mo) (RR = 2.3, 95% CI 1.6–3.5). Elk calves born in the last 18 mo prior to the death or diagnosis of their dam were at higher risk if their dams died of CWD (RR = 4.1, 95% CI 1.5–11.4) or exhibited clinical signs of CWD (RR = 8.3, 95% CI 2.7–25.7). Significant risk factors for transmission of CWD on elk farms were the introduction from an infected farm of trace-in elk that died of CWD (RR = 13.5, 95% CI 2.0–91) or developed clinical signs of CWD (RR = 7.1, 95% CI 0.93–54) and the elapsed time in years since the incursion of CWD (OR = 5.6, 95% CI 1.8–17.4). The assumptions on which CFIA’s disease control policies were based were validated, but based on this new information, quarantine in cases where exposure to preclinical elk has occurred could be considered as an alternative to whole herd eradication.
Résumé
Épidémiologie d’une éclosion de maladie débilitante chronique dans des élevages de wapitis en Saskatchewan. Cette revue d’une éclosion de la maladie débilitante chronique (MDC) survenue en Saskatchewan entre 1996 et 2002 dans des élevages de wapitis avait pour but 1) de déterminer la progression de la MDC depuis l’infection jusqu’à la mort, 2) d’évaluer les facteurs de risques d’infection des animaux, 3) d’évaluer la gestion des élevages et l’exposition aux facteurs de risques dans la transmission de la MDC à l’intérieur des troupeaux et 4) d’évaluer à la lumière des résultats la pertinence de la politique de l’agence canadienne d’inspection des aliments (ACIA) pour le contrôle de la MDC. L’étude a été réalisée à partir des résultats de la traçabilité du déplacement des animaux, des tests fonctionnels sur les animaux ainsi que d’un questionnaire sur la gestion des fermes. La durée de la MDC (période comprise entre l’exposition et la mort de l’animal testé positif à la MDC) se situaient entre un minimum moyen de 19 mois et un maximum moyen de 40. L’âge et le sexe n’avaient pas d’incidence sur l’infection à la MDC, à l’exception des adultes (≥ 2 a) qui montraient une incidence plus grande que les jeunes (< 18 m) (RR = 2,3, 95 % IC 1,6–3,5). Les jeunes âgés de moins de 18 mois au moment de la mort ou du diagnostic de la maladie chez leurs mères avaient un risque plus élevé d’avoir la MDC si leurs mères en étaient mortes (RR = 4,1, 95 % IC 1,5–11,4) ou montraient des signes cliniques de MDC (RR = 8,3, 95 % IC 2,7–25,7). Les facteurs significatifs de risques de transmission de la MDC dans les élevages de wapitis comprenaient l’introduction d’un wapiti retraçable, provenant d’un élevage contaminé, mort du MDC (RR = 13,5, 95 % IC 2,0–91) ou qui en avait développé les signes cliniques (RR = 7,1, 95 % IC 0,93–54) et le temps écoulé (en années) depuis l’apparition de la maladie (OR = 5,6, 95 % IC 1,8–17,4). Les hypothèses sur lesquelles se basaient les politiques ou l’ACIA pour le contrôle de la maladie ont été validées. En se basant sur les nouvelles données recueillies, une alternative à l’éradication complète des troupeaux pourrait être l’établissement d’une quarantaine dans les élevages de wapitis ayant été exposés à des cas précliniques.
(Traduit par Docteur André Blouin)
Introduction
Chronic wasting disease (CWD) is a progressive, invariably fatal neurodegenerative disease of cervids. It is a member of the group of diseases known as transmissible spongiform encephalopathies, believed to be caused by prions. While CWD was identified nearly 4 decades ago (1) and, recently, has been associated with significant disease outbreaks in free-ranging wild cervids (2), captive wild cervids (3), and commercial ranch cervids (4), much is still unknown about the mechanisms of transmission of (the agent causing) this disease (1).
The natural course of CWD in infected animals is incompletely understood. Infected animals experience a latent period after exposure, followed by an infectious period during which clinical signs appear and progress until death. Simulation modelling, using estimates of 1.0 y for the latent period and 1.05 y for the infectious period, successfully reproduced the conditions observed in an epidemic in a wild deer population in Colorado and Wyoming (2). In captive elk, the incubation period was observed to be 18 to 36 mo, followed by a clinical period of less than 12 mo (3).
It has been suggested that CWD prion can be transmitted laterally, maternally, or through environmental contamination. The primary route of transmission is believed to be lateral, with infection acquired by oral exposure to secretions or excretions of animals in the infectious phase (5–7). Transmission of CWD prion by saliva and blood of infected deer has recently been demonstrated experimentally (8).
Maternal transmission was not necessary in a disease simulation model (2) and was not apparent in a captive herd (3). Increased occurence of CWD in a cohort born to dams that were later shown to be infected was not demonstrated in data from captive elk (9). Concentrating animals in captivity and artificial feeding may increase the probability of direct and indirect transmission (7). Infection has been experimentally demonstrated in mule deer that were exposed to infected animals and to environments contaminated by decomposed carcasses or excreta of infected deer (10).
The effect of genotype on susceptibility to CWD is not known, but overrepresentation of homozygotes for methionine at codon 132 of the prion protein gene has been demonstrated in infected free-ranging and farmed elk (11). Ongoing research suggests that certain elk genotypes may confer resistance to CWD or result in a prolonged incubation period (12). Genetic effects on susceptibility and incubation period have also been demonstrated for mule deer (13) and white-tailed deer (14).
Between 1996 and 2002, chronic wasting disease was diagnosed in 39 herds of farmed elk in Saskatchewan in a single epidemic. All of these herds were depopulated as part of the Canadian Food Inspection Agency’s (CFIA) disease eradication program. Animals, primarily over 12 mo of age, were tested for the presence CWD prions following euthanasia. Twenty-one of the herds were linked through movements of live animals with latent CWD from a single infected source herd in Saskatchewan, 17 through movements of animals from 7 of the secondarily infected herds. The source herd is believed to have become infected via importation of animals from a game farm in South Dakota where CWD was subsequently diagnosed (7,4). A wide range in herd prevalence of CWD at the time of herd depopulation of these herds was observed. Within-herd transmission was observed on some farms, while the disease remained confined to the introduced animals on other farms.
The CFIA’s eradication policy was developed in year 2000, based on the best available information at that time. The working assumptions were the following: CWD affects elk, mule deer, and white-tailed deer; the maximum incubation period in cervids is 36 mo; clinical cases are infectious for up to 18 mo before death; spread occurs primarily via direct contact; CWD prions are highly resistant to degradation; and the environment can be a source of infection (15). The classical disease control principles employed by the CFIA in the eradication effort included the following: quarantine and depopulation of CWD-infected herds; testing of euthanized animals > 12 mo of age; tracing of all contacts to the infected herd; euthanasia and testing of animals in contact with infected animals within 36 mo of CWD diagnosis; and surveillance of those in contact with infected animals between 36 and 60 mo (4). These assumptions and policies appear to have successfully controlled the spread of CWD in farmed elk in Canada (4).
The objectives of this study were to 1, determine the progression of CWD from infection to death in farmed elk; 2, assess animal risk factors for CWD infection in farmed elk; 3, assess farm management and exposure risk factors for within herd CWD transmission; and 4, assess the suitability of the CFIA’s current disease control policy for CWD in light of the findings.
Methods
Diagnosis of chronic wasting disease
At the time of herd depopulation on infected farms, brain samples for CWD testing were collected from all animals approximately > 1 y of age. If clinical signs of CWD were observed at the time of euthanasia, this was recorded (15). Immunohistochemical staining for PrPCWD was performed on the medulla oblongata region of the brain at the level of the obex (16,17) and the degree of staining in positive samples was graded on a scale from 1 to 3: Grade 1 (mild) — scant PrPCWD restricted to the dorsal motor nucleus of the vagus (DMNV); Grade 2 (moderate) — abundant PrPCWD in the DMNV and scant deposits around the DMNV; Grade 3 (marked) — intense PrPCWD deposits in DMNV and all surrounding nuclei.
Duration of chronic wasting disease
Each animal on an infected farm was classified as a resident, trace-in, or trace-out. Residents were those animals that were either born on the farm or acquired from a farm not involved in the CWD outbreak. Residents that tested positive for CWD were assumed to have become infected on their home farm. Trace-ins were those animals moved in to the farm of interest from a CWD-infected farm within the 36 mo preceding the diagnosis of CWD. These animals were also considered trace-outs from their infected farm of origin. If the trace-ins were found to be positive, they were assumed to have become infected at their farm of origin and to have introduced CWD to their destination farm.
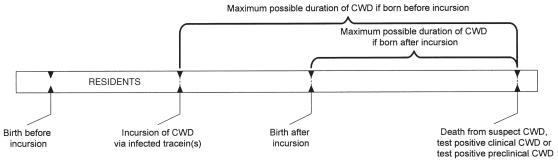
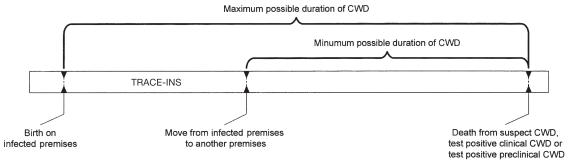
For resident animals on farms where CWD was introduced by trace-ins, the maximum possible duration of the disease, assuming initial contact could have resulted in transmission, was from the date of earliest possible exposure to an infected trace-in to the date of euthanasia (Figure 1). For trace-in animals, the minimum duration of the disease was from the date of the latest possible exposure at their infected farm of origin to the date of death or euthanasia at their destination farm. For these animals, the maximum duration of disease was from the date of earliest possible exposure at birth on the infected farm to the date of death or euthanasia (Figure 2).
Figure 1.
Method of calculating possible duration of chronic wasting disease (CWD) in resident elk.
Figure 2.
Method of calculating possible duration of chronic wasting disease (CWD) in trace-in elk.
Positive animals were considered to be in the preclinical phase if clinical signs of CWD were not observed by caretakers or CFIA staff prior to euthanasia. If signs were noted, animals were in the clinical phase. An animal was classified as a suspect CWD death, if it met all the following criteria: 1. it originated from an infected farm and died on a subsequently infected farm; 2. it died under circumstances consistent with CWD; 3. it exhibited secondary postmortem findings consistent with CWD; and 4. the brain was not tested.
Animal risk factors
Prevalence of transmitted CWD on farms was based on the disease status of resident and trace-out animals that had left the farm in the previous 36 mo. The status of trace-in animals was not considered in the calculation of prevalence, because these animals were assumed to have become infected (or not) at their farm of origin. Therefore, farms that had CWD cases only in trace-in animals and had no cases in resident or trace-out animals were assigned a transmitted CWD prevalence of zero. Farms with prevalence greater than zero experienced cases in resident (or trace-out) animals, and, therefore, within-herd transmission had occurred.
For analysis of the effect of maternal transmission, the cohort of calves included all animals that were ≤ 18 mo when euthanized. The dam and her CWD status were known for 27 of the 31 CWD-positive calves. The unknown dams of the remaining 4 calves were assumed to be negative. The stage of disease of the calf in 4 positive dam:calf pairs was inconsistent with maternal transmission, so they were also ascribed to negative dams. There were 115 CWD-positive female elk ≥ 2 y in the last calving season before depopulation. When their calves were not identified, the conservative assumption was made that the positive cows had produced negative calves in the previous 18 mo, in lieu of the alternative assumption that they had produced no calves. The remainder of the negative calves in the cohort were then attributed to negative dams. These data were classified by whether the positive dam was in the preclinical or clinical phase of the disease, or had died of suspected CWD.
Farm management risk factors
Farm management risk factors were assessed by a questionnaire to the producer or farm manager and from reports by the CFIA staff involved in the investigation on the farm. Management practice risk factors included comanagement with other cervid farms and the sharing of equipment; methods of providing feed, water, and medication; whether the herd was primarily used for breeding or some other purpose, such as velvet production or hunting; and descriptors of the farm itself.
Exposure risk factors
Chronic wasting disease was introduced onto farms by the movement of infected animals. These introduced animals then suffered a number of possible fates: they died of unrecognized and undetected CWD, they showed clinical signs of CWD resulting in euthanasia or death and tested positive for CWD, or they were euthanized as asymptomatic trace-ins from an infected farm during the preclinical phase of disease and tested positive for CWD.
The number of trace-ins introducing CWD, their stage of disease (died, clinical, or preclinical), and the date of their introduction to the farm were tabulated. The elapsed time between the date of CWD incursion and the date of depopulation of the herd was calculated. For analysis, each type of trace-in was expressed as a dichotomous variable and each farm was classified by the ‘worst’ type of exposure experienced (suspect death > clinical > preclinical), if it had more than 1 type of trace-in.
Statistical analysis
Statistical analysis was accomplished by using statistical software (Stata, Version 8; StataCorp, College Station, Texas, USA). Risk ratios were calculated for each dichotomous variable and tested for significance by Fisher’s exact test. Odds ratios were calculated for continuous variables by logistic regression and tested for significance by the likelihood ratio test. Risk ratio is also known as relative risk and is the ratio of the risk of disease in the exposed group to the risk of disease in the nonexposed group (18). The odds ratio is the ratio of the odds of disease in the exposed group to the odds of disease in the nonexposed group (18). Both measures represent the degree of association between the factor and the outcome of interest.
Results
Diagnosis of chronic wasting disease
On the 39 farms analyzed in this study, 6213 animals had negative results on immunohistochemical staining, 226 had positive results, and 53 were retrospectively classified as suspect CWD deaths without a positive test. The degree of staining was available for 223 of the positive animals. The presence of clinical signs consistent with CWD was recorded at the time of euthanasia for 33 elk. During this outbreak retention of the winter hair coat during the summer months was an observed clinical sign of CWD that has not previously been reported. There was a highly significant association between the presence of clinical signs of CWD and an increased immunohistochemical staining grade (P < 0.001) (Table 1).
Table 1.
The immunohistochemical staining grade of elk in the preclinical and clinical stages of chronic wasting disease (CWD)
| Clinical status
|
|||
|---|---|---|---|
| Staining grade | Preclinical | Clinical | Total |
| 1 | 49 | 2 | 51 |
| 2 | 128 | 5 | 133 |
| 3 | 16 | 23 | 39 |
| Total | 193 | 30a | 223 |
Staining grade was not available for 3 elk that expressed clinical signs of CWD
Duration of chronic wasting disease
The mean maximum and mean minimum possible durations of CWD for elk, from exposure to euthanasia or death, are grouped according to whether they were residents or trace-ins and their disease stage (preclinical, clinical or suspect death), as shown in Table 2. The weighted mean of the minimum durations was 19.2 mo and the weighted mean of the maximum durations was 40.0 mo.
Table 2.
Maximum and minimum possible durations of chronic wasting disease (CWD) measured in various groups of infected and suspect elk on game farms in Saskatchewan
| Group | Clinical status | Observations | Mean (months) | Standard deviation (months) | Range (months) |
|---|---|---|---|---|---|
| Maximum durations | |||||
| Residentsa | Preclinical | 155 | 36.0 | 23.0 | 4–137 |
| Trace-insb | Preclinical | 36 | 52.3 | 31.8 | 17–149 |
| Residents | Clinical | 21 | 46.7 | 33.8 | 22–135 |
| Trace-ins | Clinical | 11 | 42.8 | 15.3 | 21–79 |
| Minimum durations | |||||
| Trace-ins | Preclinical | 37 | 18.1 | 4.9 | 7–27 |
| Trace-ins | Clinical | 11 | 23.5 | 10.8 | 1–39 |
| Trace-ins | Suspected | ||||
| CWD death | 25 | 18.8 | 13.1 | 0–57 | |
Resident animals were either born on the farm or acquired from a farm not involved in the CWD outbreak and were believed to have become infected on their home farm
Trace-ins were moved from an infected farm to a previously uninfected farm, where they were diagnosed as CWD-positive and believed to have become infected on their farm of origin
Animal risk factors
The prevalence of transmitted CWD ranged from zero (20 farms) to 100% (1 farm with 1 animal). The median prevalence of CWD on 18 farms where transmission occurred was 4.4% (range 0.7% to 100%, interquartile range [25% to75%] 2.5% to 12.7%). The prevalence of CWD was significantly different among farms (P < 0.001).
Following the finding of a CWD-positive animal, the entire exposed herd was depopulated. The mean age of the CWD-negative animals in depopulated herds was 3.4 y (95% CI 3.3–3.4) and the mean age of CWD-positive and suspect animals was 3.7 y (95% CI 3.4–4.0). The difference in age was not significant (P = 0.09).
Two-year-olds were more likely to be diagnosed with CWD than were calves, with a risk ratio (RR) of 2.3 (95% CI 1.6–3.5, P < 0.001). No other age groups were significantly different from 2-year-olds (P > 0.05).
Females (4.6%) were no more likely than males (3.6%) to be diagnosed with CWD (RR = 1.3; 95% CI 0.96–1.6; P = 0.07). The odds ratios (OR) for CWD for each age group stratified by sex were not significantly different from those for the whole group (P = 0.38).
Chronic wasting disease was more likely to be found in calves born within the previous 18 mo to positive dams with clinical signs of CWD (12.5%) and dams that were suspected to have died from CWD (6.2%) than in calves born to dams that were negative before euthanasia on infected farms (1.5%) (Table 3). There was no significant increase in risk to calves born to dams in the preclinical phase of the disease.
Table 3.
Risk ratios (RR) for chronic wasting disease (CWD) causal agent infection of calves born within 18 months of death or positive diagnosis to elk cows with differing CWD status on infected farms
Farm management risk factors
Table 4 shows that none of the farm management factors resulted in a significant increase in the risk or odds of within-herd CWD transmission occurring. The preceding was true for all the farms and for the subset of farms where CWD was introduced by an animal that died or exhibited clinical signs of CWD (data not shown).
Table 4.
Risk ratios (RR) for within-herd transmission of chronic wasting disease (CWD) causal agent for farms with different management factors
| Farm management factor | RR | P | 95% Confidence interval |
|---|---|---|---|
| Comanagement | 0.78 | 0.701 | 0.29–2.1 |
| Oral medications | 1.4 | 0.675 | 0.67–3.0 |
| Shared equipment | 1.7 | 0.179 | 0.84–3.4 |
| Forage in feeders | 1.5 | 0.462 | 0.55–4.1 |
| Forage on ground | 0.61 | 0.188 | 0.31–1.2 |
| Any feed on ground | 0.77 | 0.703 | 0.37–1.6 |
| Waterpond | 1.1 | 1.00 | 0.56–2.2 |
| Waterbowl | 0.73 | 0.471 | 0.37–1.4 |
| Watertub | 0.94 | 1.00 | 0.47–1.9 |
| Breeding herd | 1.5 | 0.663 | 0.46–4.9 |
| Years establisheda | 1.2 | 0.123 | 0.96–1.4 |
| Herd size(00’s)a | 1.3 | 0.274 | 0.83–2.0 |
Odds ratios for continuous variables
Exposure risk factors
Chronic wasting disease was much more likely to be transmitted on farms where the disease was introduced by a trace-in that died of suspected CWD or exhibited clinical signs of CWD than on farms where the disease was introduced by a trace-in that was destroyed when it was in the preclinical stage (this includes 1 farm where animals were exposed to an infected herd where an elk with preclinical CWD had previously resided) (Table 5). The odds of CWD transmission also increased with increasing elapsed time between the introduction of infected elk and depopulation (Table 5). The elapsed time on farms where CWD was introduced by trace-ins in the preclinical stage was significantly less than on farms where CWD was introduced by trace-ins with clinical disease (P < 0.001) and farms where CWD was introduced by trace-ins that died of suspected CWD (P = 0.001) (Table 6).
Table 5.
Risk ratios (RR) for within-herd transmission of chronic wasting disease (CWD) causal agent on farms with different types of exposure to CWD
| Exposure | RR | P | 95% Confidence interval |
|---|---|---|---|
| Infected elk that did not express clinical signs or co-mingling with exposed elk | 1.00 | — | — |
| Infected elk that expressed clinical CWD | 7.1 | 0.040 | 0.93–54 |
| Elk that died of suspected CWD | 13.5 | <0.001 | 2.0–91 |
| Time from introduction of infected animals to herd depopulation (years)a | 5.6 | 0.003 | 1.8–17 |
Odds ratios for continuous variables
Table 6.
Mean elapsed time from the introduction of elk infected with chronic wasting disease (CWD) to depopulation on farms with different types of exposure to CWD
| Exposure | Observations | Elapsed time (months) | Standard deviation (months) | Range (months) |
|---|---|---|---|---|
| Infected elk that did not express clinical signs or co-mingling with exposed elk | 15 | 17.1 | 4.2 | 9–24 |
| Infected elk that expressed clinical CWD | 7 | 28.7 | 7.8 | 18–39 |
| Elk that died of suspected CWD | 17 | 45.4 | 30.2 | 15–138 |
Discussion
Diagnosis of chronic wasting disease
Higher grades of immunohistochemical staining of the obex were associated with the presence of clinical signs of CWD. Grade 3 staining could therefore be used as an indicator for advanced disease, where observation and reporting of clinical signs might be incomplete. If control actions in exposed herds were dependent on the stage of the disease in introduced animals, the use of the results of clinical examination and the staining grade, together, could increase the sensitivity of detection of animals with advanced disease.
Duration of chronic wasting disease
The duration of a case of CWD includes a latent period following exposure, that is, when the disease is undetectable by clinical examination or currently available laboratory testing methods. The animal’s status becomes detectable by immunohistochemical staining during the subsequent preclinical phase. The latent phase and the preclinical phase comprise the incubation period. Following the incubation period, spongiform degeneration occurs in the brain and clinical signs are expressed, culminating in death. Sometime during the preclinical or clinical phase, the elk becomes infectious to other animals. The precise order of events following the latent period is unknown, but it has been suggested that the shedding of prions begins early, precedes the onset of clinical signs, and is progressive throughout the course of the disease (7). In experimentally infected elk, CWD is detectable by positive immunohistochemical staining on brain tissue 6 mo earlier than the onset of clinical signs or development of spongiform change, which are believed to occur simultaneously (7).
From observations of elk involved in the CWD outbreak in Saskatchewan, the maximum possible disease duration was determined for 223 animals. The mean maximum duration for resident elk not exhibiting clinical signs of CWD was observed to be equal to the 36-month incubation period assumed by the CFIA during policy development. Residents exhibiting clinical signs had a mean maximum disease duration of 46 mo, reflecting an additional 10-month clinical period. This is similar to the 12-month maximum duration of the clinical period proposed by Miller et al (3).
The maximum disease duration for trace-in elk exhibiting clinical signs was less than for animals that did not exhibit clinical signs. This could reflect a faster disease progression in these animals because they were exposed at a younger age. All the trace-in elk in the preclinical stage had minimum durations less than the 36-month incubation period proposed by CFIA policy.
The high values in the ranges for each of the maximum measurements reflect the inclusion of the time between earliest possible exposure and actual exposure, which was not part of the incubation period, but cannot be quantified and excluded from the measured interval. Similarly, the low values in the ranges for the minimum measurements reflect the omission of the time between exposure and movement, which was part of the incubation period, but cannot be quantified and is not included in the measured interval.
All infected animals that were still in the incubation period in this outbreak were identified and euthanized by following the 36-month tracing policy adopted by the CFIA. While it is unlikely that an animal still healthy at 36 mo following removal from an infected premises could be incubating CWD, based on these data, clinical signs or death would be expected to occur well within the additional 24-month surveillance period.
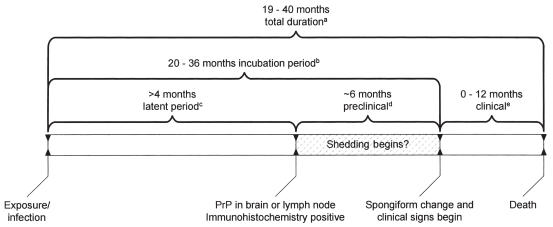
Based on this study and supplemented with values from the literature, a proposed timeline for CWD in elk from exposure to death is illustrated in Figure 3.
Figure 3.
Proposed course of chronic wasting disease (CWD) in farmed elk
aCalculated mean minimum and maximum durations in Saskatchewan farmed elk
b Observed youngest clinical elk and longest incubation in a preclinical elk
c Observed youngest positive elk
Animal risk factors
Farm of origin was a highly significant risk factor for CWD for elk in this outbreak. This may be associated with the way that CWD was introduced to the farm and how quickly it was detected.
Similar prevalence estimates in free-ranging male and female elk, mule deer, and white-tailed deer have previously been reported (2). More recently, however, an increased risk in males and an effect of age in males has been reported for free-ranging mule deer (19). The decreased risk seen in calves in this study probably reflects the length of the incubation period and the inability of current tests to detect infection early in the incubation period. Chronic wasting disease was detected in 7 elk < 12 mo and 24 elk between 12 and 18 mo of age, but most elk less than 12 mo of age were not tested. It is impossible to determine if some of these animals were in the latent phase and would have become positive had they been tested at a later date. This finding, therefore, does not suggest a decrease in susceptibility in younger elk.
Calves born to dams with clinical signs of CWD or dams that died of CWD experienced a risk of CWD greater than their cohort born to negative dams. As immunohistochemical examination of the placentas of CWD-positive pregnant elk has not revealed the presence of the CWD prion (Aru Balachandran, unpublished observations), this likely represents a special case of lateral transmission due to close association, rather than true maternal transmission. Miller et al (2) reported the relative lack of preclinical disease in 16- to 17-month-old deer as evidence that maternal transmission is not important in epidemics in free-ranging populations, but for individual farmed elk, having a CWD-positive dam was a significant risk. The higher risk in calves of dams that exhibited clinical disease or died from suspected CWD may reflect increased shedding of prion by dams that are in later stages of CWD.
The mechanism and significance of finding increased risk in calves of CWD-infected elk requires further investigation. Cohort studies undertaken to determine the role of maternally associated transmission of bovine spongiform encephalopathy (BSE) suggest that bovine calves born within the last 6 mo of the incubation period of BSE-infected cows may experience a slightly higher risk of developing BSE than others of their birth cohort (20), but modelling based on these results predicts a higher incidence of BSE due to maternal transmission than has been observed in the UK. Scrapie has long been believed to be maternally transmitted, and prions have been demonstrated in the placentas of infected ewes (21,22); however, recent work indicates that lambs are infected by peri- or post-natal lateral transmission, and that infection is no more common in the lambs of infected ewes than in others of their lambing cohort (21,22).
Farm management risk factors
None of the farm management factors assessed were significantly associated with the occurrence of CWD transmission. This may reflect lack of power in the study due to the small number of infected farms, or it may be that none of the factors studied influenced the transmission of prion disease.
This study suggests that sharing equipment and the method of feeding could influence the transmission of CWD. If shared equipment were demonstrated to be a risk factor, it would suggest that fomites could be a mechanism of transmission. Fomites encountered in this study included saws used for removing velvet antler and equipment used to administer oral medication. This may indicate blood- or saliva-borne infectivity. The tendency for feeding on the ground to be protective and the use of feeders to be a risk factor, suggests that close animal contact and contamination by saliva may be modes of transmission, while urine or fecal contamination of feed may be less important. This is consistent with recent experimental work demonstrating that CWD is transmitted by saliva and blood (8).
Exposure risk factors
Introduction of CWD onto a farm via an animal that subsequently died of the disease was a highly significant risk factor for subsequent within-herd transmission. Animals that eventually died of CWD probably shed greater amounts of prion, compared with animals that were euthanized, and contributed to greater contamination of the environment and greater opportunity for direct transmission to other animals. It is possible that the carcasses of elk that died of CWD may have decomposed in elk enclosures, thus serving as an environmental source of infection to other elk, as experimentally demonstrated in mule deer (10).
The introduction of an animal that subsequently exhibited clinical signs of CWD was also a significant risk factor for within-herd transmission. If shedding of prions is coincident with clinical signs, elk that were euthanized would not have shed as much of the infectious agent as those that were allowed to progress to death.
The introduction of a positive animal that did not express clinical signs of CWD before it was euthanized was not associated with transmission of CWD. In future, it might be reasonable to maintain such exposed animals in quarantine to determine whether or not they would eventually succumb to CWD. Substantial savings to producers and the public could be realized, and many animals spared, if herd depopulation was not required in such cases.
The elapsed time between the introduction of CWD-infected animals and the depopulation of the farm was highly associated with transmission. Shorter elapsed times were associated with farms where the trace-ins had not yet developed clinical signs, reflecting tracing activity and detection of the positive animal occurring earlier in the incubation of the trace-in. Early detection and removal of clinically affected elk in a captive herd infected with CWD has reportedly led to decreased prevalence of CWD in the herd, compared with a previous outbreak where this strategy was not employed (3).
Conclusions
The CFIA’s policies for eradication of CWD in farmed cervids were successful in controlling the disease on farms in Canada (4). This analysis of the outbreak of CWD in farmed elk in Saskatchewan in 1996 to 2002 provides additional information that validates the assumptions used to develop the policy.
Immunohistochemical staining is a highly reliable method to detect CWD prions (4) and could also provide information on the stage of the disease, based on grading of lesions. While animals under 12 mo of age have a low risk of infection and, therefore, may reasonably be exempted from testing, this is not true of calves of positive dams, and testing of these animals should be reconsidered.
This analysis suggests that the maximum clinical period of CWD is 12 mo and that animals are infectious during the clinical stage. Analysis of this outbreak failed to provide evidence that elk are infectious prior to the development of clinical signs of CWD. Farms that were exposed to trace-ins that did not express clinical disease did not experience transmission of CWD, and calves of dams with preclinical disease were not at increased risk of CWD. The conclusion that elk are not infectious during the preclinical phase of CWD cannot be made definitively, because the shorter elapsed time between incursion and depopulation on farms exposed to such elk may have precluded the detection of infected animals that were early in the incubation period. In future, in cases of herd exposure to infected elk with preclinical CWD, maintenance of the herd in quarantine for 4 y with surveillance for clinical signs of CWD could be used to determine whether transmission occurs in this situation. If it could be confirmed that elk do not transmit CWD during the preclinical phase, substantial reduction in eradication costs, hardship to producers, and loss of animal life could be realized. CVJ
Footnotes
Reprints will not be available from the authors.
References
- 1.Williams ES, Miller MW, Kreeger TJ, Kahn RH, Thorne ET. Chronic wasting disease of deer and elk: a review with recommendations for management. J Wildl Manage. 2002;66:551–563. [Google Scholar]
- 2.Miller MW, Williams ES, McCarty CW, et al. Epizootiology of chronic wasting disease in free-ranging cervids in Colorado and Wyoming. J Wildl Dis. 2000;36:676–689. doi: 10.7589/0090-3558-36.4.676. [DOI] [PubMed] [Google Scholar]
- 3.Miller MW, Wild MA, Williams ES. Epidemiology of chronic wasting disease in captive Rocky Mountain elk. J Wildl Dis. 1998;34:532–538. doi: 10.7589/0090-3558-34.3.532. [DOI] [PubMed] [Google Scholar]
- 4.Kahn S, Dubé C, Bates L, Balachandran A. Chronic wasting disease in Canada: Part 1. Can Vet J. 2004;45:397–404. [PMC free article] [PubMed] [Google Scholar]
- 5.Sigurdson CJ, Williams ES, Miller MW, Spraker TR, O’Rourke KI, Hoover EA. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus) J Gen Virol. 1999;80:2757–2764. doi: 10.1099/0022-1317-80-10-2757. [DOI] [PubMed] [Google Scholar]
- 6.Sigurdson CJ, Spraker TR, Miller MW, Oesch B, Hoover EA. PrPCWD in the myenteric plexus, vagosympathetic trunk and endocrine glands of deer with chronic wasting disease. J Gen Virol. 2001;82:2327–2334. doi: 10.1099/0022-1317-82-10-2327. [DOI] [PubMed] [Google Scholar]
- 7.Williams ES, Miller MW. Chronic wasting disease in deer and elk in North America. Rev Sci Tech Off Int Epiz. 2002;21:305–316. doi: 10.20506/rst.21.2.1340. [DOI] [PubMed] [Google Scholar]
- 8.Mathiason CK, Powers JG, Dahmes SJ, et al. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science. 2006;314:133–136. doi: 10.1126/science.1132661. [DOI] [PubMed] [Google Scholar]
- 9.Miller MW, Williams ES. Horizontal prion transmission in mule deer. Nature. 2003;425:35–36. doi: 10.1038/425035a. [DOI] [PubMed] [Google Scholar]
- 10.Miller MW, Williams ES, Hobbs T, Wolfe L. Environmental sources of prion transmission in Mule Deer. Emerg Infect Dis. 2004;10:1003–1006. doi: 10.3201/eid1006.040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Rourke KI, Besser TE, Miller MW, et al. PrP genotypes of captive and free-ranging Rocky Mountain elk (Cervus elaphus nelsoni) with chronic wasting disease. J Gen Virol. 1999;80:2765–2769. doi: 10.1099/0022-1317-80-10-2765. [DOI] [PubMed] [Google Scholar]
- 12.Hamir AN, Gidlewski T, Spraker TR, et al. Preliminary observations of genetic susceptibility of elk (Cervus elaphus nelsoni) to chronic wasting disease by experimental oral inoculation. J Vet Diagn Invest. 2006;18:110–114. doi: 10.1177/104063870601800118. [DOI] [PubMed] [Google Scholar]
- 13.Jewell JE, Conner MM, Wolfe LL, Miller MW, Williams ES. Low frequency of PrP genotype 225SF among free-ranging mule deer (Odocoileus hemionus) with chronic wasting disease. J Gen Virol. 2005;86:2127–2134. doi: 10.1099/vir.0.81077-0. [DOI] [PubMed] [Google Scholar]
- 14.O’Rourke KI, Spraker TR, Hamburg LK, Besser TE, Brayton KA, Knowles DP. Polymorphisms in the prion precursor functional gene but not the pseudogene are associated with susceptibility to chronic wasting disease in white-tailed deer. J Gen Virol. 2004;85:1339–1346. doi: 10.1099/vir.0.79785-0. [DOI] [PubMed] [Google Scholar]
- 15.Canadian Food Inspection Agency Disease Control Manual of Procedures. Chronic Wasting Disease. Ottawa: Canadian Food Inspection Agency; 2002. [Google Scholar]
- 16.Spraker TR, Balachandran A, Zhuang D, O’Rourke KI. Variable patterns of distribution of PrP(CWD) in the obex and cranial lymphoid tissues of Rocky Mountain elk (Cervus elaphus nelsoni) with subclinical chronic wasting disease. Vet Rec. 2004;155:295–302. doi: 10.1136/vr.155.10.295. [DOI] [PubMed] [Google Scholar]
- 17.Spraker TR, O’Rourke KI, Balachandran A, et al. Validation of monoclonal antibody F99/97.6.1 for immunohistochemical staining of brain and tonsil in mule deer (Odocoileus hemionus) with chronic wasting disease. J Vet Diagn Invest. 2002;12:579–582. doi: 10.1177/104063870201400102. [DOI] [PubMed] [Google Scholar]
- 18.Dohoo I, Martin W, Stryhn H. Veterinary Epidemiologic Research, Charlottetown. Prince Edward Island: AVC Inc; 2003. pp. 123–125. [Google Scholar]
- 19.Miller MW, Conner MM. Epidemiology of chronic wasting disease in free-ranging mule deer : spatial, temporal, and demographic influences on observed prevalence patterns. J Wildl Dis. 2005;41:275–290. doi: 10.7589/0090-3558-41.2.275. [DOI] [PubMed] [Google Scholar]
- 20.Prince MJ, Bailey JA, Barrowman PR, Bishop KJ, Campbell GR, Wood JM. Bovine spongiform encephalopathy. Rev Sci Tech. 2003;22:37–60. doi: 10.20506/rst.22.1.1389. [DOI] [PubMed] [Google Scholar]
- 21.Andreoletti O, Lacroux C, Chabert A, et al. PrPSc accumulation in placentas of ewes exposed to natural scrapie: influence of foetal PrP genotype and effect on ewe-to-lamb transmission. J Gen Virol. 2002;83:2607–2616. doi: 10.1099/0022-1317-83-10-2607. [DOI] [PubMed] [Google Scholar]
- 22.Ryder S, Dexter G, Bellworthy S, Tongue S. Demonstration of lateral transmission of scrapie between sheep kept under natural conditions using lymphoid tissue biopsy. Res Vet Sci. 2004;76:211–217. doi: 10.1016/j.rvsc.2003.11.007. [DOI] [PubMed] [Google Scholar]