In the summer of 2007, the Prairie Diagnostic Services (PDS) laboratory in Saskatoon had a substantial increase in the number of confirmed cases of West Nile virus (WNV) disease. For the 4 western provinces and Ontario, the number of tests for the presence of WNV increased from 107 in 2005 and 88 in 2006 to 132 in 2007 by August 28. Between January 1 and August 28, 2007, 79 animals tested positive (60% of submissions), surpassing the 23 positive animals in 2005 (21% of all submissions) and the 18 positive animals in 2006 (20% of all submissions). Along with the increased numbers of positive cases, a wider variety of animals succumbing to the disease has been observed. Previously, unreported species at the PDS included mountain goat, sheep, snowy owl, great gray owl, captive lesser scaup, and the domestic duck.
This report provides an account of the WNV outbreak in a flock of 17-week-old, Saskatchewan hatched and raised, Rouen ducks. The flock consisted of 44 ducks kept permanently outdoors in a chain-link pen. A small flock of young geese of the same age was also kept nearby.
The first 2 ducks were found dead on August 4, 2007. At this point, 2 live ducks (a male and a female) were submitted to the PDS. Clinically, the ducks were unable to maintain balance and had difficulty in standing. The birds attempted to use their wings to right themselves, but generally the wings were held down as if paralyzed. The heads and necks performed repetitive and aimless gyrations, punctuated by tossing the heads over the backs. In addition, the male appeared to have lost its voice. However, it still opened its bill to produce a quiet, barely audible, raspy noise. Prime differential diagnoses for these clinical signs are botulism, and toxic, metabolic, degenerative, traumatic, and infectious processes. Both ducks were euthanized with a barbiturate overdose and necropsied.
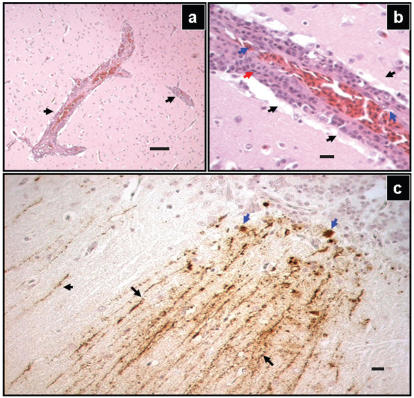
The birds were in good nutritional condition, but the gastrointestinal tracts were empty, due to a few days of anorexia. There were no gross lesions. On microscopic examination, significant lesions were observed in the brain (Figure 1) and heart (Figure 2). Numerous vessels in all parts of the brain were surrounded by mononuclear cuffs consisting primarily of lymphocytes and fewer plasma cells. The endothelia of affected vessels were hypertrophic. There were several widely scattered foci of gliosis. In 1 bird, the cerebellar meninges were diffusely infiltrated with moderate numbers of lymphocytes.
Figure 1.
Representative lesions in the brain tissue of a duck naturally infected by the West Nile virus. Throughout the brain tissue, there were numerous vessels with perivascular inflammatory infiltrate (a, arrows). Hematoxylin and eosin (H&E) stain, Bar = 50 μm. Higher magnification of vascular cuff (b) revealed perivascular mononuclear infiltration (red arrows). Thick layers of lymphocytes was peripherally surrounded by edema (b, black arrows). The endothelial nuclei (blue arrows) were hypertrophied. H&E stain, Bar = 10 μm. Immune staining with specific antibodies showed positive reaction within the entire brain tissue, but the most prominent lesions were apparent in the cerebellum (c). The molecular layer (black arrows) and the Purkinje cell layer (blue arrows) show abundant presence of WNV antigen. The detection was performed by using a peroxidase labeled polymer with diamine benzydine (DAB) as chromophore. The presence of the antigen was identified by specific dark brown staining, Bar = 10 μm.
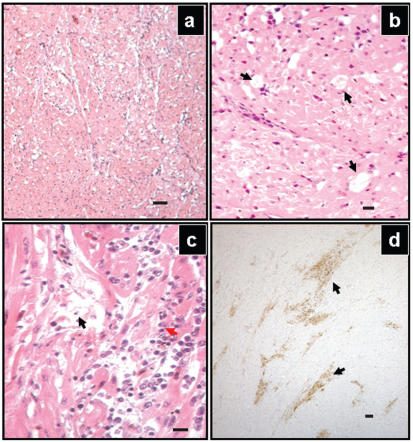
Figure 2.
Representative lesions in the heart tissue of a duck naturally infected by West Nile virus. The myocardium of the left ventricle (a) appears “moth-eaten” due to extensive dropout of the myocytes. Hematoxylin and eosin (H&E) stain, Bar = 50 μm. On higher magnification of the left ventricular myocardium (b), significant dropout of cardiomyocyes was apparent (black arrows). H&E stain, Bar = 10 μm. Multifocally the myocardium was edematous (black arrows) and infiltrated with mononuclear inflammatory cells (red arrows) (c). H&E stain, Bar = 10 μm. Large areas of the myocardium showed positive staining for WNV antigen (d, arrows), using a peroxidase labeled polymer with DAB as chromophore. The presence of the antigen was identified by specific dark brown staining, Bar = 50 μm.
The lesions in the heart spanned the entire left ventricle and consisted of large patches of edema multifocally infiltrated with lymphocytes, plasma cells, and macrophages. The involved myocytes were either absent, degenerate, or undergoing coagulative necrosis. Most of the periportal areas of the liver were infiltrated with aggregates of lymphocytes.
Based on the microscopic findings, WNV infection was suspected and pooled sections of brain, spleen, and kidney were submitted to the polymerase chain reaction (PCR) and immuno histochemical (IHC) laboratories. The primary antibody used was a rabbit antiserum to WNV (1:1000, BioReliance Corporation, Rockville, Maryland, USA). Binding of the primary antibody was detected by using goat antimouse and anti-rabbit immunoglobulins conjugated to a peroxidase-labelled polymer (EnVision + Dual Link; Dako Canada Corporation, Mississauga, Ontario). Both PCR and IHC staining were positive for WNV. Results from IHC staining for Newcastle disease virus were negative.
After the initial loss of 2 ducks and over the course of the following 12 d, 13 more ducks died. The last death occurred on August 16. In total, the outbreak claimed 17 birds (38.7% of the flock). The geese kept in the neighboring pen did not show any clinical signs of disease. This in itself is unusual, as geese have traditionally been considered very susceptible to infection with WNV. Both ducks and geese were not vaccinated against any diseases. The enclosure did not have any specific mosquito control measures, but the grass had been kept short and no pools of standing water were permitted.
To the best of our knowledge, this is the 1st report of WNV infection in domestic ducks in Canada; it makes us aware about the growing number of species susceptible to the disease and illustrates the economic seriousness of such an outbreak for both small and large duck operations. Cumulative mortality shows a gradually increasing prevalence of the disease. The approach of fall leaves us with an uneasy anticipation regarding future outbreaks in yet to be determined species.
Acknowledgment
The authors gratefully acknowledge the assistance of Dr. Sandra Stephens (CFIA) in gathering of epidemiological information on WNV infections.