Abstract
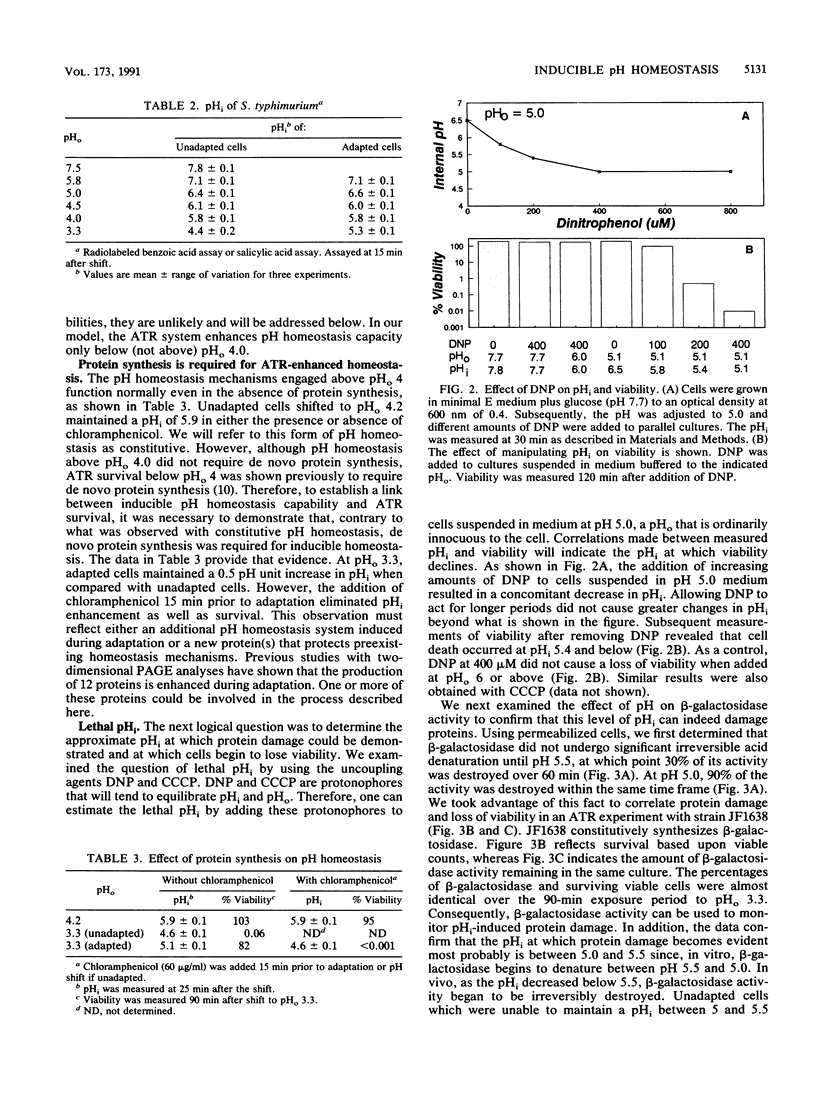
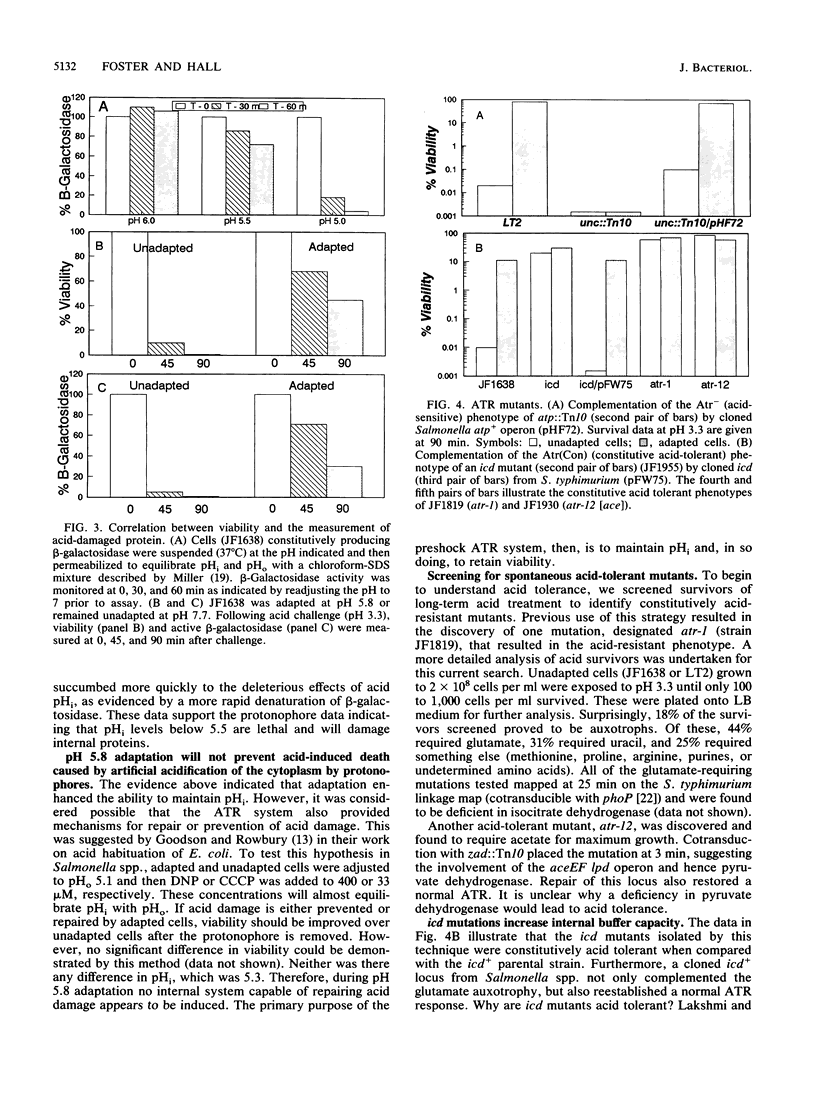
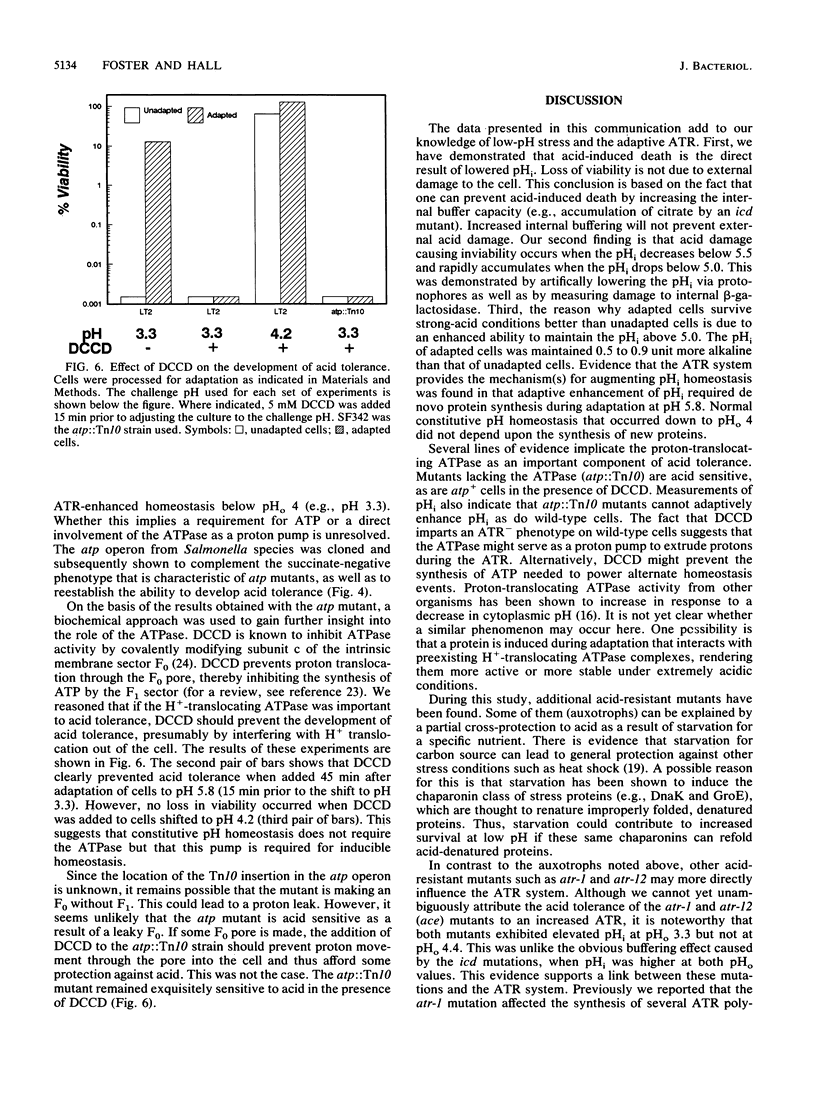
The acid tolerance response (ATR) is an adaptive system triggered at external pH (pHo) values of 5.5 to 6.0 that will protect cells from more severe acid stress (J. Foster and H. Hall, J. Bacteriol. 172:771-778, 1990). Correlations between the internal pH (pHi) of adapted versus unadapted cells at pHo of 3.3 indicate that the ATR system produces an inducible pH-homeostatic function. This function serves to maintain the pHi above 5 to 5.5. Below this range, cells rapidly lose viability. Development of this pH homeostasis mechanism was sensitive to protein synthesis inhibitors and operated only to augment the pHi at pHo values below 4. In contrast, classical constitutive pH homeostasis was insensitive to protein synthesis inhibitors and was efficient only at pHo values above 4. Physiological studies indicated an important role for the Mg(2+)-dependent proton-translocating ATPase in affording ATR-associated survival during exposure to severe acid challenges. Along with being acid intolerant, cells deficient in this ATPase did not exhibit inducible pH homeostasis. We speculate that adaptive acid tolerance is important to Salmonella species in surviving acid encounters in both the environment and the infected host.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aliabadi Z., Park Y. K., Slonczewski J. L., Foster J. W. Novel regulatory loci controlling oxygen- and pH-regulated gene expression in Salmonella typhimurium. J Bacteriol. 1988 Feb;170(2):842–851. doi: 10.1128/jb.170.2.842-851.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliabadi Z., Warren F., Mya S., Foster J. W. Oxygen-regulated stimulons of Salmonella typhimurium identified by Mu d(Ap lac) operon fusions. J Bacteriol. 1986 Mar;165(3):780–786. doi: 10.1128/jb.165.3.780-786.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth I. R., Mitchell W. J., Hamilton W. A. Quantitative analysis of proton-linked transport systems. The lactose permease of Escherichia coli. Biochem J. 1979 Sep 15;182(3):687–696. doi: 10.1042/bj1820687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth I. R. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985 Dec;49(4):359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Salmonella as an intracellular parasite. Mol Microbiol. 1989 Dec;3(12):1833–1841. doi: 10.1111/j.1365-2958.1989.tb00170.x. [DOI] [PubMed] [Google Scholar]
- Foster J. W., Aliabadi Z. pH-regulated gene expression in Salmonella: genetic analysis of aniG and cloning of the earA regulator. Mol Microbiol. 1989 Nov;3(11):1605–1615. doi: 10.1111/j.1365-2958.1989.tb00146.x. [DOI] [PubMed] [Google Scholar]
- Foster J. W., Hall H. K. Adaptive acidification tolerance response of Salmonella typhimurium. J Bacteriol. 1990 Feb;172(2):771–778. doi: 10.1128/jb.172.2.771-778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson M., Rowbury R. J. RecA-independent resistance to irradiation with u.v. light in acid-habituated Escherichia coli. J Appl Bacteriol. 1991 Feb;70(2):177–180. doi: 10.1111/j.1365-2672.1991.tb04445.x. [DOI] [PubMed] [Google Scholar]
- Heyde M., Portalier R. Acid shock proteins of Escherichia coli. FEMS Microbiol Lett. 1990 May;57(1-2):19–26. doi: 10.1016/0378-1097(90)90406-g. [DOI] [PubMed] [Google Scholar]
- Hickey E. W., Hirshfield I. N. Low-pH-induced effects on patterns of protein synthesis and on internal pH in Escherichia coli and Salmonella typhimurium. Appl Environ Microbiol. 1990 Apr;56(4):1038–1045. doi: 10.1128/aem.56.4.1038-1045.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Suzuki T., Kinoshita N., Unemoto T. Amplification of the Streptococcus faecalis proton-translocating ATPase by a decrease in cytoplasmic pH. J Bacteriol. 1984 Jun;158(3):1157–1160. doi: 10.1128/jb.158.3.1157-1160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmi T. M., Helling R. B. Selection for citrate synthase deficiency in icd mutants of Escherichia coli. J Bacteriol. 1976 Jul;127(1):76–83. doi: 10.1128/jb.127.1.76-83.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M., Castle A. M. A variable stoichiometry model for pH homeostasis in bacteria. Biophys J. 1987 Oct;52(4):637–647. doi: 10.1016/S0006-3495(87)83255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A. The molecular basis of carbon-starvation-induced general resistance in Escherichia coli. Mol Microbiol. 1991 Jan;5(1):3–10. doi: 10.1111/j.1365-2958.1991.tb01819.x. [DOI] [PubMed] [Google Scholar]
- Padan E., Schuldiner S. Intracellular pH and membrane potential as regulators in the prokaryotic cell. J Membr Biol. 1987;95(3):189–198. doi: 10.1007/BF01869481. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, edition VII. Microbiol Rev. 1988 Dec;52(4):485–532. doi: 10.1128/mr.52.4.485-532.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E., Altendorf K. Bacterial adenosine 5'-triphosphate synthase (F1F0): purification and reconstitution of F0 complexes and biochemical and functional characterization of their subunits. Microbiol Rev. 1987 Dec;51(4):477–497. doi: 10.1128/mr.51.4.477-497.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebald W., Friedl P., Schairer H. U., Hoppe J. Structure and genetics of the H+-conducting F0 portion of the ATP synthase. Ann N Y Acad Sci. 1982;402:28–44. doi: 10.1111/j.1749-6632.1982.tb25730.x. [DOI] [PubMed] [Google Scholar]
- Slonczewski J. L., Gonzalez T. N., Bartholomew F. M., Holt N. J. Mu d-directed lacZ fusions regulated by low pH in Escherichia coli. J Bacteriol. 1987 Jul;169(7):3001–3006. doi: 10.1128/jb.169.7.3001-3006.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector M. P., Aliabadi Z., Gonzalez T., Foster J. W. Global control in Salmonella typhimurium: two-dimensional electrophoretic analysis of starvation-, anaerobiosis-, and heat shock-inducible proteins. J Bacteriol. 1986 Oct;168(1):420–424. doi: 10.1128/jb.168.1.420-424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]


