Abstract
The pathogenicity of the primate lentiviruses, human and simian immunodeficiency viruses, is host-specific. Previous studies indicated that the highly pathogenic human lentivirus HIV-1 has markedly reduced pathogenicity compared to the pathogenic simian lentivirus SIV in pigtail macaques (Macaca nemestrina). We therefore hypothesized that the pigtail macaque peripheral blood mononuclear cells (mPBMCs) would respond differently to infections of HIV-1 and pathogenic SIV. To elucidate the cellular responses to the infections of HIV-1 and SIV, we infected mPBMC with these two viruses. Like infections in vivo, HIV-1 and SIV demonstrated distinct replication kinetics in mPBMCs, with HIV-1 replicating at significantly lower levels. Similarly, gene expression profiling facilitated by macaque-specific oligonucleotide microarrays also revealed distinct expression patterns of genes between the HIV-1 and SIV infected mPBMCs; in particular, genes associated with the antigen presentation, T-cell receptor, ERK/MAPK signaling, Wnt/β-catenin signaling, and natural killer cell signaling pathways were differentially regulated between these two viruses. Most interestingly, despite the lower levels of replication, HIV-1 triggered a more robust regulation of immune response genes early after infection; the converse was true in SIV-infected mPBMCs. Our results therefore suggest that macaques may be controlling the infection of HIV-1 at an early stage through coordinated regulation of host defense pathways.
Keywords: HIV-1, SIV, Macaque peripheral blood mononuclear cells, Microarray, Gene expression
Introduction
The AIDS/macaque model is the most widely used experimental system for studying host responses to the infection of primate lentiviruses, especially human immunodeficiency virus-2 (HIV-2) and simian immunodeficiency virus (SIV) (Kaizu et al., 2006; Lichterfeld et al., 2005). Although human immunodeficiency virus-1 (HIV-1) and SIV share limited sequence identity (Franchini & Bosch, 1989), infection of SIV in macaques could closely resemble the infection of HIV-1 in humans, as characterized by the gradual depletion of CD4+ T cells symptomatic of AIDS, which causes a dysfunctional immune system and eventual death (Mattapallil et al., 2005). Interestingly, HIV-1, the main pathogen causing AIDS in human (Simon et al., 2006), demonstrated a minimal pathogenic phenotype in macaques (Agy et al., 1992; Agy et al., 1997; Gartner et al., 1994a; Gartner et al., 1994b).
Recent studies suggested that cellular factors may impose restrictive effects on the HIV-1 replication in macaques (Zheng & Peterlin, 2005). Macaque APOBEC3G/F inhibits HIV-1 replication by introducing hypermutations during reverse transcription (Zennou & Bieniasz, 2006). Macaque TRIM5α was able to decrease HIV-1 replication also at the step of reverse transcription (Wu et al., 2006). Although these host factors may significantly hinder HIV-1 replication in vitro (Hatziioannou et al., 2006; Kamada et al., 2006), whether they are the sole determinants for the loss of HIV-1 pathogenicity in macaques is still elusive. Lines of evidence suggest that the dissociation of the replicative capacity and the pathogenicity of primate lentiviruses indeed occurred in nonhuman primates (Broussard et al., 2001; Pandrea et al., 2003; Silvestri et al., 2003). More importantly, primate lentiviruses replicating at low levels can become highly pathogenic during infections in macaques (Beer et al., 2005). Therefore, it would be reasonable to speculate that the loss of pathogenicity of HIV-1 in macaques is not solely attributed to its decreased replicative capacity in macaque cells.
Increasing evidence indicates that the patterns of cellular response to the infections of a variety of viruses could well reflect their pathogenic properties (Kash et al., 2004; Kash et al., 2006b; Kash et al., 2006a; Kobasa et al., 2007; Ploquin et al., 2006; Wang et al., 2005). Distinct responses by TGF-β signaling were observed between infections of pathogenic and nonpathogenic SIV strains in nonhuman primates (Ploquin et al., 2006). Proinflammatory and cell death pathways were found to be differentially regulated in mice infected by influenza viruses with various pathogenic properties (Kash et al., 2004; Kash et al., 2006b). Similarly, the interferon response and immune evasion pathways were seen to be regulated differently in human as well as mouse cells during the infections by highly pathogenic and lowly pathogenic filoviruses (Kash et al., 2006a) and rabies viruses (Wang et al., 2005), respectively. Therefore, we hypothesized that the infections of HIV-1 and pathogenic SIV would induce distinct cellular responses in macaque peripheral blood mononuclear cells (mPBMCs).
A comparison of the cellular responses of mPBMCs to the infections of primate lentiviruses with different pathogenic properties would greatly improve our understanding of the pathogenesis of primate lentiviruses. To gain further insights into the cellular responses to the HIV-1 and pathogenic SIV, we infected pigtail macaque PBMCs with a HIV-1 strain and a highly pathogenic SIV strain (Batten et al., 2006). By using macaque specific oligonucleotide microarrays, we were able to elucidate the cellular gene expression changes in mPBMCs in response to HIV-1 and SIV infections. Our results suggested that an enhanced early immune response distinguished the HIV-1 infection from the SIV infection in mPBMCs and could be a major contributing factor to reduced HIV-1 pathogenicity in macaques.
Results and Discussion
HIV-1 and SIV demonstrate distinct infection kinetics in the pigtail macaque PBMC cultures
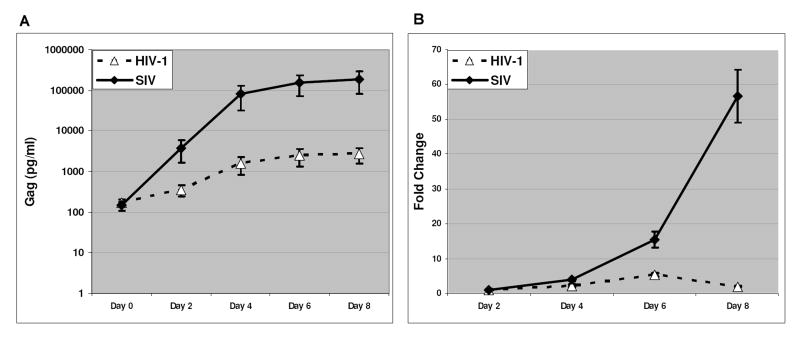
To evaluate the infections of HIV-1 and SIV in mPBMCs, we infected mPBMCs with a nonpathogenic HIV-1 strain along with a highly pathogenic SIV strain at the same multiplicity of infection (1 TCID50/ cell). Both HIV-1 and SIV strains used in this study were T-tropic viruses that primarily infect CD4+ T cells (Kestler et al., 1990; Peden et al., 1991). We examined the cell distributions of mPBMCs prior to infections using flow cytometry. mPBMCs from 3 individual macaques showed comparable compositions in term of cell types present (Supplemental Table 1) and were used in the infection experiments. During the infections in mPBMCs, HIV-1 and SIV demonstrated distinct replication kinetics. Although mPBMCs can be infected by both viruses, only a small portion of cells were actually infected in HIV-1 or SIV cultures (Fig. 1A). Infected cells were not noticeable in either HIV-1 or SIV infected mPBMC cultures until day 4 post infection (p.i.) (data not shown). Even with a higher percentage of infected cells in the mPBMC cultures on day 6 p.i., less than 1% of the cells were estimated to be infected by HIV-1 and under 2% of the cells were infected by SIV (Fig. 1B). Through the course of infections, cell viability of neither virus-infected mPBMC cultures differed from mock-infected mPBMC cultures, until a surge in cell death seen in the SIV infected cultures on day 8 p.i. (Fig 1C). As a well-accepted parameter for the replication status of HIV/SIV, we measured Gag protein levels in HIV-1 and SIV infected mPBMCs (Fig. 2A). Given that the SIV Gag level was more than 50-fold higher than that of HIV-1 Gag on both days 4 and 6 p.i., and that there was not a significantly higher number of cells infected by SIV than infected by HIV-1 in the cultures (Fig. 1), SIV replication appeared to be more robust than HIV-1 replication in mPBMCs (Kamada et al., 2006). The relative abundance of the viral genome RNA measured by real-time PCR assays also indicated that the SIV genome RNA increased more robustly than the HIV-1 genome RNA during the infection in mPBMCs (Fig. 2B). The abundance of the HIV-1 genome temporally increased during HIV-1 infection and peaked on day 6 p.i., then decreased on day 8 p.i.. In contrast, the abundance of the SIV genome steadily increased through the course of infection. Collectively, our data suggested that HIV-1 replicated at significantly lower levels compared to SIV in mPBMCs, consistent with the in vivo results reported previously (Batten et al., 2006), possibly owing in part to the presence of host restriction factors (Hatziioannou et al., 2006; Kamada et al., 2006; Rodrigo et al., 1997).
Figure 1.
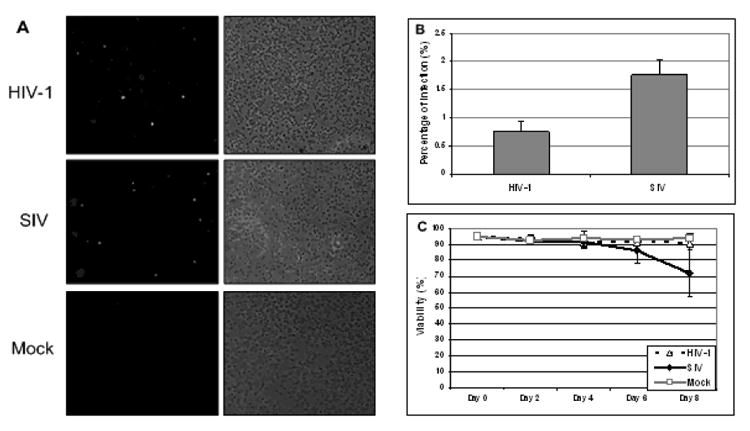
A: Indirect Immunofluorescence Assay (IFA) in mPBMCs infected for 6 days with HIV-1 and SIV. On the right are phase contrast micrographs of virus-infected cells in the experiments. On the left are the immunofluorescence analyses of the identical microscopic field. B: The HIV-1-infected culture exhibited an infection rate of under 1%, while the infection rate for the SIV-infected culture was under 2% on day 6 p.i.. There were approximately the same numbers of cells counted in both HIV-1 infected and SIV infected cultures. The data shown here is representative of mPBMCs infection from 3 animals. C: The viabilities of mPBMC cultures infected with HIV-1 and SIV, along with the mock. The data shown here is a summary of mPBMCs infections from 3 animals.
Figure 2.
A: Levels of gag protein (Gag p24-HIV-1 and Gagp27-SIV) reflect the progression of viral infection. The graph tracks the average gag levels for PBMCs from all three infected animals through 8 days post-infection, with the error bars reflecting individual variations. Both HIV-1- and SIV-infected cultures showed progressing virus replication in the infected cultures. B: Levels of HIV-1 or SIV viral genomic RNA reflect the progression of viral replication. The graph tracks the increase in the quantity the virus RNA genomes relative to the quantity of the corresponding virus genomes on day 2 p.i.. Data shown here is a summary of mPBMCs infections from 3 animals.
HIV-1 and SIV induce cellular gene expression changes via distinct kinetics
To profile the global cellular gene expression changes in response to the infections of HIV-1 and SIV in mPBMCs, we performed differential gene expression analysis using macaque-specific oligonucleotide microarrays. Taking advantage of the marked specificity of the macaque-specific oligonucleotide microarrays, we adopted a 1% false-positive rate (p ≤ 0.01) as the threshold for selecting differentially expressed genes. We profiled HIV-1-infected and SIV-infected cells from two time points, days 4 and 6 p.i.; we excluded day 8 p.i. or later due to the noticeable surge in cell death in the SIV-infected culture after day 6 p.i. (Fig 1C).
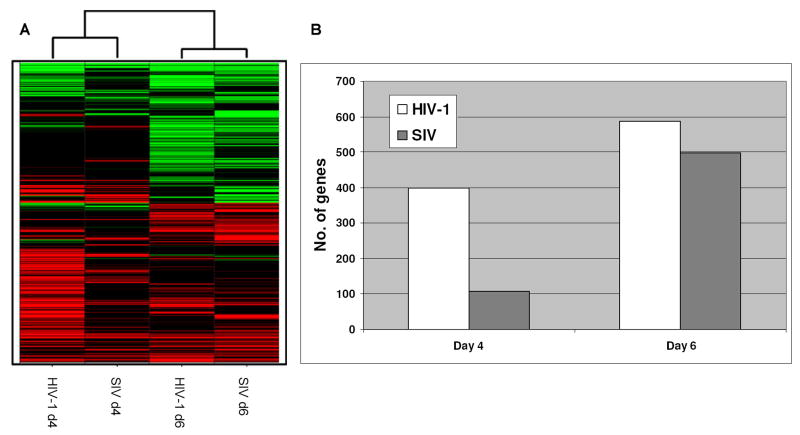
The global gene expression patterns of HIV-1 and SIV infected mPBMCs clearly indicated that despite less robust replication, the HIV-1 infection induced a stronger cellular response than the SIV infection at the earlier time point (Fig. 3A). Among ∼1300 cellular genes that had expression changes in 1 out of 4 data points (Supplemental Table 4), 399 genes in HIV-1 d4 and 107 genes in SIV d4 showed expression changes (Fig. 3B), even though the HIV-1 replication was at significantly lower levels than the SIV replication on day 4 p.i.. On day 6 p.i., the number of changed genes increased to 497 in the SIV infected mPBMCs but still fewer than the 587 genes in HIV-1 infected mPBMCs. Thus, in spite of more subdued virus replication, HIV-1 infection appeared to be able to induce a similar, if not stronger, host response when compared to the relatively more active SIV-1 infection.
Figure 3.
A: Hierarchical cluster of global gene expression patterns of mPBMC infected with HIV-1 and SIV. Each gene in the cluster passed the statistical cutoff (p≤ 0.01) at least once in 4 data points based on the Rosetta error model in Resolver™. Every expression value shown in this report is the average expression value from 3 individual animals. Genes having increased expression values compared to time matched mock were shown as red while the genes having decreased expression values compared to time matched mock were shown as green. B: The number of signature genes that have expression changes (p≤ 0.01) in HIV-1 infected or SIV infected mPBMC on days 4 or 6 p.i.. The two histograms represent data from signature genes, defined as having a p ≤ 0.01. The p-value is derived from both the errors in microarray measurements and the estimated variance among the 3 animals.
Pathways analyses of the cellular gene expression profiles of mPBMCs infected with HIV-1 and SIV
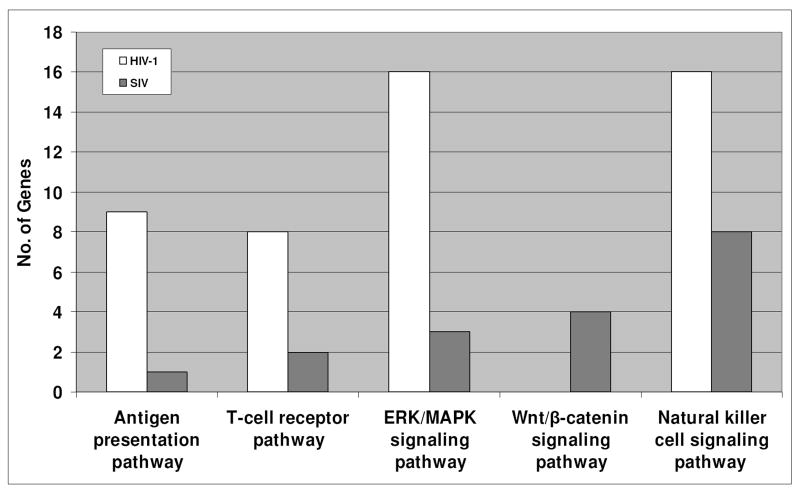
To characterize the different cellular responses induced by the infection of HIV-1 or SIV in mPBMCs, we analyzed the lists of differentially expressed genes during the infections of HIV-1 and SIV by Ingenuity Pathway Analysis (IPA), a knowledge based pathway analysis tool used in previous studies (Bowick et al., 2006; Li et al., 2005; Pasieka et al., 2006; Thomas et al., 2006). In brief, functions of cellular genes are mined from peer-reviewed literatures and manually curated into the knowledgebase. A network analysis of the knowledgebase is used to construct interaction-based relationships between proteins in the knowledgebase. Furthermore, while our differentially expressed genes were scattered across most of the networks in the knowledgebase, IPA ranks the networks based on the number of differentially expressed genes in one network as a percentage of the total number of genes in the network. In the discussion below, we focused on the top-ranking networks, as they represented cellular pathways with an overrepresentation of differentially expressed genes. In other words, the IPA analyses suggested that these pathways, as described below, exhibited differential expression upon HIV-1 and SIV infection in mPBMCs, with some being unique to HIV-1 or SIV infection (Fig. 4).
Figure 4.
Pathways that were affected in the HIV-1 infected or SIV infected mPBMCs. The Ingenuity Pathway analysis software was used to generate lists of pathways. The IPA annotation designates the differentially expressed genes in HIV-1 infected or SIV infected mPBMCs into different pathways. The antigen presentation, T-cell receptor, ERK/MAPK signaling, Wnt/β-catenin signaling and the natural killer cell signaling pathways were seen to be significantly impacted by HIV-1 or SIV infections in mPBMCs. The two histograms represent data from signature genes, defined as having a p ≤ 0.01. The p-value is derived from both the errors in microarray measurements and the estimated variance among the 3 animals.
Pathway analyses of the cellular gene expression profiles in HIV-1-infected mPBMCs
Antigen presentation pathway
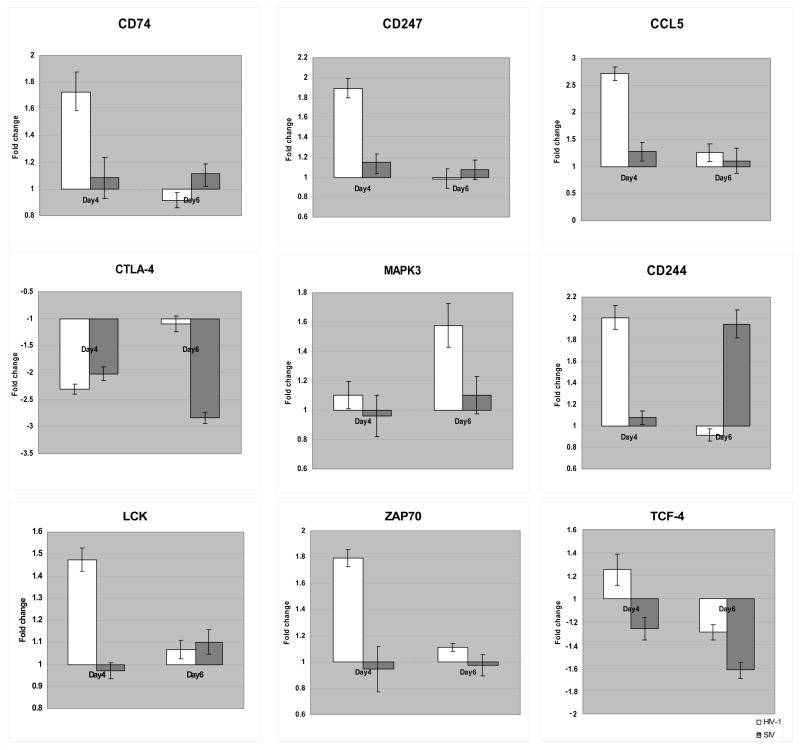
9 genes associated with the antigen presentation pathway (Table 1a) showed expression changes in the HIV-1-infected mPBMCs. Among these 9 genes, 6 genes belonged to the MHC class II family, including CD74, HLA-DMA, HLA-DMB, HLA-DRA, HLA-DRB1, and HLA-DRB5 (Fig. 5A). HLA-DM molecules (HLA-DMA and HLA-DMB) are known to be necessary for the formation of the antigen presentation complex (Fling et al., 1994) and to enhance the MHC/antigen binding (Sherman et al., 1995). The interaction between HLA-DR (HLA-DRA, HLA-DRB1 and HLA-DRB5) and HLA-DM (HLA-DMA and HLA-DMB) was shown to be crucial in preventing immune evasion (Zwart et al., 2005). The up-regulation of all these genes suggested that the MHC class II mediated antigen presentation may be enhanced in the HIV-1-infected mPBMCs. Furthermore, an MHC class I gene (HLA-A) was also up-regulated only in the HIV-1 infected mPBMCs. HLA-A presents endogenous antigens to the CD8+ T cells, which in turn play crucial roles in the anti-viral immune responses against HIV-1 infection in human cells (Levy, 2003; Lichterfeld et al., 2005). In addition, the interaction between MHC I and CD74 play is possibly involved in antigen presentation (Powis, 2006). By TaqMan qRT-PCR, we also confirmed the up-regulation of CD74 by HIV, but not SIV (Fig. 7). Collectively, the up-regulation of MHC-class I/II molecules may point to an up-regulation of antigen presentations during the HIV-1 infection compared to the SIV infection in macaques, which may in turn enhance immune surveillance and account for the subdued pathogenicity of HIV.
Table 1.
The expression pattern of the genes associated with the pathways affected in HIV-1 infected and SIV infected mPBMCs. A: The expression pattern of genes associated with the pathways unique in the HIV-1 infected mPBMCs. B: The expression pattern of genes associated the Wnt/β-catenin signaling pathway. C: The expression pattern of genes associated with the NK cell signaling pathway in both HIV-1 infected and SIV infected mPBMCs. The gene expression changes that passed a cutoff p≤ 0.01 were shown as Up-regulation or Down-regulation. Otherwise, were shown as Unch.(unchanged). All the gene expression changes were revealed by macaque-specific oligonucleotide microarrays.
| Table 1a | |||
|
| |||
| The antigen presentation pathway | |||
|
| |||
| Accession # | Primary sequence name | HIV-1 | SIV |
|
| |||
| NM_004355 | CD74 | Up-regulated | Unch. |
| NM_002116 | HLA-A | Up-regulated | Unch. |
| NM_006120 | HLA-DMA | Up-regulated | Unch. |
| NM_002118 | HLA-DMB | Up-regulated | Unch. |
| NM_019111 | HLA-DRA | Up-regulated | Unch. |
| NM_002124 | HLA-DRB1 | Up-regulated | Unch. |
| NM_002125 | HLA-DRB5 | Up-regulated | Unch. |
| NM_002798 | PSMB6 | Down-regulated | Unch. |
| NM_004159 | PSMB8 | Down-regulated | Down-regulated |
|
| |||
| The T-cell receptor pathway | |||
|
| |||
| Accession # | Primary sequence name | HIV-1 | SIV |
|
| |||
| NM_198053 | CD247 | Up-regulated | Unch. |
| NM_001768 | CD8A | Up-regulated | Unch. |
| NM_004931 | CD8B | Up-regulated | Up-regulated |
| NM_005214 | CTLA4 | Down-regulated | Down-regulated |
| NM_002037 | FYN | Up-regulated | Unch. |
| NM_005356 | LCK | Up-regulated | Unch. |
| NM_002746 | MAPK3 | Up-regulated | Unch. |
| NM_001079 | ZAP70 | Up-regulated | Unch. |
|
| |||
| The ERK/MAPK signaling pathway | |||
|
| |||
| Accession # | Primary sequence name | HIV-1 | SIV |
|
| |||
| NM_004418 | DUSP2 | Up-regulated | Unch. |
| NM_001946 | DUSP6 | Up-regulated | Unch. |
| NM_002037 | FYN | Up-regulated | Unch. |
| NM_002107 | H3F3A | Up-regulated | Unch. |
| NM_002746 | MAPK3 | Up-regulated | Unch. |
| NM_002661 | PLCG2 | Up-regulated | Unch. |
| NM_005167 | PPM1J | Up-regulated | Up-regulated |
| NM_001008709 | PPP1CA | Up-regulated | Unch. |
| NM_002730 | PRKACA | Up-regulated | Unch. |
| NM_002732 | PRKACG | Up-regulated | Up-regulated |
| NM_001010935 | RAP1A | Down-regulated | Unch. |
| NM_001006665 | RPS6KA1 | Up-regulated | Unch. |
| NM_003131 | SRF | Down-regulated | Unch. |
| NM_007315 | STAT1 | Down-regulated | Unch. |
| NM_006289 | TLN1 | Up-regulated | Unch. |
| NM_003405 | YWHAH | Up-regulated | Unch. |
|
| |||
| Table 1b | |||
|
| |||
| The Wnt/β-catenin signaling pathway | |||
|
| |||
| Accession # | Primary sequence name | SIV | HIV-1 |
|
| |||
| NM_145259 | ACVR1C | Down-regulated | Unch. |
| NM_016269 | LEF1 | Down-regulated | Unch. |
| NM_181674 | PPP2R2B | Up-regulated | Unch. |
| NM_003199 | TCF4 | Down-regulated | Unch. |
|
| |||
| Table 1c | |||
|
| |||
| The Natual killer cell pathway | |||
|
| |||
| Accession # | Primary sequence name | HIV-1 | SIV |
|
| |||
| NM_002983 | CCL3 | Down-regulated | Up/Down-regulated |
| NM_002984 | CCL4 | Up-regulated | Unch. |
| NM_002985 | CCL5 | Up-regulated | Unch. |
| NM_016382 | CD244 | Up-regulated | Up-regulated |
| NM_000569 | FCGR3A | Up-regulated | Unch. |
| NM_005541 | INPP5D | Up-regulated | Unch. |
| NM_014218 | KIR2DL1 | Up-regulated | Up-regulated |
| NM_014219 | KIR2DL2 | Up-regulated | Unch. |
| NM_013289 | KIR3DL1 | Up-regulated | Up-regulated |
| NM_006737 | KIR3DL2 | Up-regulated | Unch. |
| NM_002259 | KLRC1 | Up-regulated | Up-regulated |
| NM_002260 | KLRC2 | Up-regulated | Up-regulated |
| NM_002262 | KLRD1 | Up-regulated | Unch. |
| NM_002831 | PTPN6 | Down-regulated | Unch. |
| NM_003177 | SYK | Up-regulated | Up-regulated |
| NM_003332 | TYROBP | Up-regulated | Up-regulated |
Figure 5.
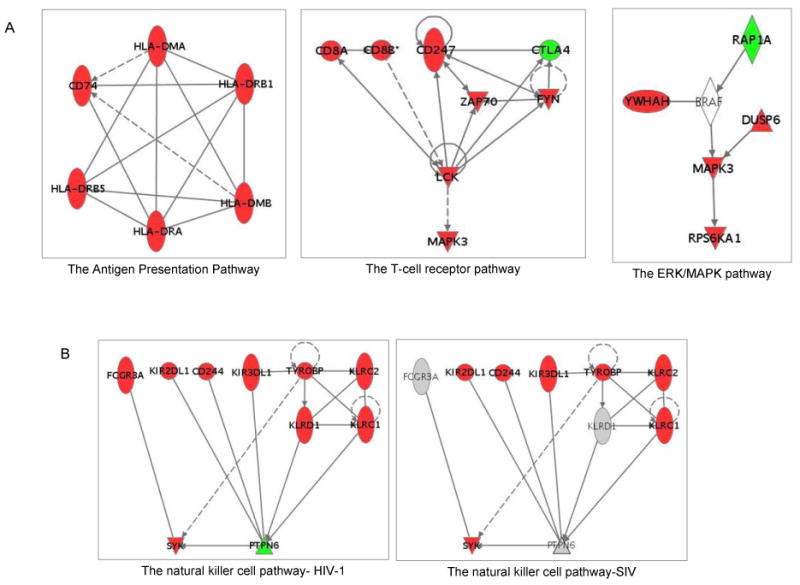
A: Networks of pathways unique in the HIV-1 regulated mPBMCs. The networks showing the relationship of genes in the antigen presentation, the T-cell receptor and the ERK/MAPK pathways are shown. B: Relationship networks of genes associated with the NK cell signaling pathway in both HIV-1- and SIV-infected mPBMCs. The networks were built by Ingenuity pathway analysis. Each dot represents a gene, with green indicating down-regulation of the gene, and red indicating up-regulation of the gene. Each line connecting two genes indicates a relationship between the gene products as reported in literature.
Figure 7.
qRT-PCR confirmation of the microarray results. The expression changes of selected genes were also quantified by qRT-PCR assay. The expression changes in the virus infected cells relative to the time-matched mock infected cells were calculated.
T-cell receptor pathway
MHC class I/II molecules primarily present antigens to CD4+ or CD8+ T cells through coupling with the T-cell receptor complex (TCR/CD3). It may consequently induce the T-cell-mediated immune responses (Carreno et al., 2006). Exogenous signals from MHC could be transduced into T cells via CD247 (CD3-zeta) (Sancho et al., 1992) and CD247-associated molecules, including ZAP-70, LCK and FYN (Molina et al., 1992; Sloan-Lancaster et al., 1994; Sugie et al., 2004). In the HIV-1-infected mPBMCs, we observed the expression changes of 8 genes in the T-cell receptor pathway. Notably, the expression of CD247, ZAP-70, LCK and FYN was up-regulated in the HIV-1 infected mPBMCs (Fig. 5A, Table 1a); the up-regulation of CD247, LCK and ZAP70 was also confirmed by qRT-PCR (Fig. 7).
The increased presence of T-cell receptor associated molecules may potentiate T cells for activation or lead to anergy, depending on the availability of co-stimulation from the CD28 pathway (Kane et al., 2000). Although we did not observe the expression changes of the CD28 signaling pathway, the up-regulation of JUND in the HIV-1 infected mPBMC suggested that the HIV-1 infected mPBMCs may not be in a state of anergy, the immuno-nonresponsive status (Supplemental Table 2) (Heisel & Keown, 2001). Thus, a heightened state of T-cell activation and the transcription programs it triggered would in turn be in agreement with the increased gene expression regulation exhibited by HIV-1-infected PMBCs relative to SIV-infected ones, in spite of the lower replication levels of HIV-1. Therefore, our results suggested that T-cell activation may be enhanced in HIV-1 infected mPBMCs. Activated T cells may be more effective in mediating host responses against HIV-1 infection, thereby contributing to the lower pathogenicity of HIV-1 in macaques.
ERK/MAPK signaling pathway
The activation of the T-cell receptor pathway would in turn affect a number of down stream signaling pathways including the ERK/MAPK pathway (Michie et al., 1999). Interestingly, a few genes associated with the ERK/MAPK pathway were differentially expressed only in the HIV-1 infected mPBMCs, including YWHAH (14-3-3), RAP1A, and MAPK3 (ERK1). The 14-3-3 protein plays an inhibitory role in apoptosis by differentially regulating the activities of the MAPK pathways (Xing et al., 2000). An up-regulation of YWHAH may confer protective effects on the immune cells during the HIV-1 infection in macaques. The RAP1A protein is important in mediating T cell anergy by blocking the TCR signals to ERKs (Boussiotis et al., 1997; Carey et al., 2000). The down-regulation of RAP1A supported that the HIV-1 infected mPBMC may not be in an anergy status. Furthermore, the MAPK3 gene, known to play important roles in T cell development (Fischer et al., 2005), was up-regulated only in HIV-1 infected mPBMCs and as confirmed by qRT-PCR (Fig. 7); this up-regulation could be the direct result of the activation of the T-cell receptor pathway (Haks et al., 2005). Given that ERK pathway plays a critical role during the development of T cells and the immune selection (berola-Ila & Hernandez-Hoyos, 2003), up-regulation of the ERK signaling pathway early on during the HIV-1 infection in macaques may enhance the functions of T cells and consequently induce stronger anti-viral responses, in turn accounting for the subdued pathogenicity of the virus in comparison to SIV.
Taken together, our data suggested that the signaling cascade from the MHC/TCR interaction to the downstream ERK/MAPK signaling pathway was up-regulated in the HIV-1-infected mPBMCs compared to SIV infected ones. The heightened state of immune activation may be an indication of the host's success in warding off HIV-1 infection, resulting in reduced pathogenicity in macaques. However, many genes in the T-cell receptor and the ERK/MAPK pathways, for example CD247 (CD3-zeta), PLCG2 and MAPK3 (ERK1) itself, are not exclusively expressed in T cells but also in other cell types such as B cells and NK cells (Arase et al., 2003; Chiu et al., 2005; Yu et al., 2000). Therefore, it is possible that other cell populations in the mPBMCs, besides T cells, were activated during the HIV-1 infections as well. Given the dominance of immune cells in PBMC cultures, our results implicated the activation of different subsets of immune cells. The activation of these immune cells may be capable of mediating stronger antiviral immune responses during the HIV-1 infection compared to the SIV infection in macaques.
Pathway analyses of cellular gene expression profiles in SIV infected mPBMCs
Wnt/β-catenin signaling pathway
The Wnt/β-catenin signaling pathway plays important roles in the survival of T cells (Goux et al., 2005). Our results indicated that the Wnt/β-catenin signaling pathway may be repressed in the SIV infected mPBMCs, as characterized by the expression changes of 3 key genes in the Wnt/β-catenin signaling pathway, TCF-4, LEF-1 and PPP2R2B (Hovanes et al., 2001; Li et al., 2001; Miller & Moon, 1996) (Table 1b). TCF-4 was shown to have repressive effects on the HIV-1 replication in human cells (Wortman et al., 2002). A down-regulation of TCF-4 in the SIV infected mPBMC, as confirmed by qRT-PCR (Figure 7), may contribute to a more robust replication of SIV than HIV-1 in mPBMCs. LEF-1, shown to play a critical role in the survival of T cells (Ioannidis et al., 2001), was also down-regulated in the SIV infected mPBMCs. Given that a down-regulation of LEF-1 may impair the CD8+ T-cell development and its functions (Okamura et al., 1998), SIV may be able to compromise the antiviral functions of the CD8+ T cells by down-regulating the expression of LEF-1 during the infection in macaques. Moreover, PPP2R2B (PP2A), known to inhibit the Wnt/β-catenin pathway (Li et al., 2001), was up-regulated only in the SIV infected mPBMCs. Our data therefore suggested that Wnt/β-catenin signaling pathway may be repressed in the SIV infected mPBMCs. Given that the pathogenic SIV infection in macaques was characterized by increased T cell death (Batten et al., 2006), the concerted down-regulation of Wnt/β-catenin signaling pathway, characterized by the down-regulation of LEF-1 and TCF-4 and the up-regulation of PPP2R2B, deals a double blow to T-cell functions and survival and may contribute to the pathogenicity of SIV in macaques.
Natural killer cell signaling pathway
Natural killer (NK) cells play important roles in restraining the infection of HIV-1 in humans (Fauci et al., 2005; Mavilio et al., 2003). By extension, we speculated that the NK cell signaling may be different in the HIV-1 infected and SIV infected mPBMCs. Interestingly, we observed the regulation of the NK signaling pathway in both HIV-1 infected and SIV infected mPBMCs, albeit with different patterns (Fig. 5B and Table 1c). A few NK cell signaling-associated genes were commonly regulated in both HIV-1 and SIV infected mPBMCs. CD244 (2B4), a NK cell receptor that stimulate NK cell cytotoxicity (Messmer et al., 2006), and SYK, a key gene to NK cell functions (Jiang et al., 2002), were up-regulated in both HIV-1 and SIV infected mPBMCs; the up-regulation of CD244 by both viruses was confirmed by qRT-PCR (Fig. 7). However, the NK cell marker FCGR3A (CD16), CCL4 and CCL5 (Fig. 7) were up-regulated in mPBMCs infected with HIV-1, but not with SIV, suggesting that NK cells may be at a heightened activation status in the HIV-1 infected mPBMCs (Arase et al., 2003; Dorner et al., 2004).
Reportedly, NK cells are able to repress the HIV-1 infection in human cells by secreting large amounts of CCL4 and CCL5 to block the entry of HIV-1 (Fehniger et al., 1998). However, given that CCL5 only blocks the infection of the M-tropic HIV-1 but not the T-tropic HIV-1 used in this study, blocking the entry of HIV-1 by CCL5 may not entirely account for the low pathogenicity of this specific HIV-1 strain in macaques. Recent studies suggested that NK cells play crucial roles in connecting the innate and adaptive immune responses (Cooper et al., 2004; gli-Esposti & Smyth, 2005; Moretta, 2002). Therefore, activated NK cells may play roles in coordinating other immune cells to effectively repress the HIV-1 infection in macaques and contribute to the reduced HIV-1 pathogenicity indirectly (Gerosa et al., 2002; Groot et al., 2006; Piccioli et al., 2002).
HIV-1 infection induces earlier and stronger immune response than the SIV infection in mPBMCs
The pathway analyses described above revealed that the infections of HIV-1 and SIV induced changes in largely distinct sets of pathways. It should be noted that the expression changes of these pathways could take place in more than one cell population. For instance, the antigen presentation pathways could appear in different types of dendritic cells (DCs). The T cell receptor pathway may be affected in CD4+ T cells and/or CD8+ T cells. Furthermore, the intracellular signaling pathways such as the ERK and the Wnt/β-catenin signaling pathways are functional not only in T cells (Tan et al., 2006) but also in B cells and other immune cells (Guo & Rothstein, 2005; Yu et al., 2000). By design, gene expression profiling does not single out individual cell populations that are affected by the expression of these pathways; instead, the analysis reveals changes in the transcriptional program associated with the virus infections. Notably, using IPA, we observed differential expression predominantly among genes involved in immune response. Thus, we attempted to examine the expression pattern of the immune response genes as a whole between the HIV-1 infected and SIV infected mPBMCs.
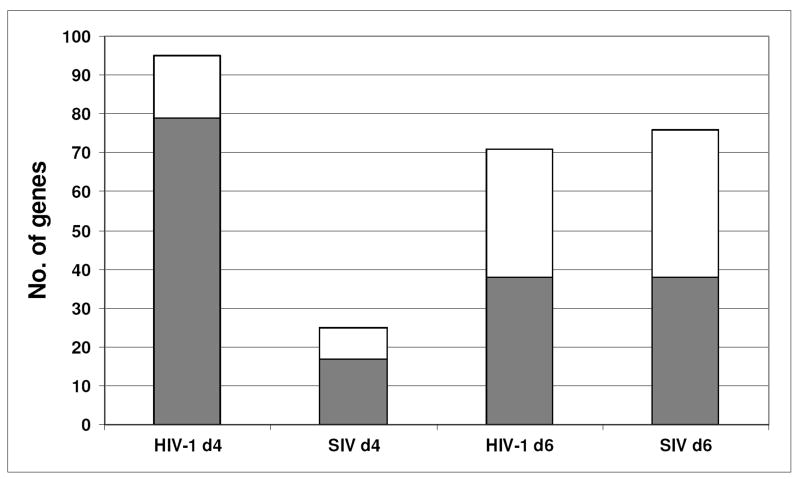
Interestingly, our results suggested that HIV-1 infection induced expression changes of immune response genes earlier on and to a greater extent than SIV infection in mPBMCs (Fig. 6). Based on annotations in the IPA knowledgebase, we identified 187 immune response genes (Supplemental Table 2) from our list of ∼1300 differentially expressed genes in HIV-1 infected and SIV infected mPBMCs (Supplemental Table 4). Overall, 140 immune response genes had expression changes in the HIV-1 infected mPBMCs, in contrast to 91 immune response genes in the SIV infected cells. Interestingly, 95 genes were regulated by HIV-1 infection on day 4 p.i., only 25 immune genes were changed in SIV infected mPBMCs at the same time point. In other words, the HIV-1 infection induced about 4 times as many immune response genes as the SIV infection did on day 4 p.i.. On day 6 p.i., HIV-1 and SIV induced expression changes of 71 and 76 immune response genes, respectively. Given that the HIV-1 replication is significantly lower than that of SIV on both days 4 and 6 p.i., our expression profiling results of immune response genes therefore suggested that the HIV-1 infection induced a more robust and prompt immune response than the SIV infection.
Figure 6.
HIV-1 infection regulates the expression of a large number of immune response genes at day 4 p.i.. The immune response genes were defined by the knowledge based pathway analysis tool, Ingenuity Pathway Analysis. The expression changes were revealed by macaque-specific oligonucleotide microarrays. (□) indicates the down-regulated genes and (
 ) indicates the up-regulated genes. Each gene of changed passed the statistical cutoff (p≤ 0.01). The two histograms represent data from signature genes, defined as having a p ≤ 0.01. The p-value is derived from both the errors in microarray measurements and the estimated variance among the 3 animals.
) indicates the up-regulated genes. Each gene of changed passed the statistical cutoff (p≤ 0.01). The two histograms represent data from signature genes, defined as having a p ≤ 0.01. The p-value is derived from both the errors in microarray measurements and the estimated variance among the 3 animals.
Previous studies have suggested that chronic immune activation is a major characteristic of the pathogenic HIV-1 and SIV infections in vivo (Folks et al., 1997; George et al., 2006; Giorgi et al., 1999; Onanga et al., 2006). However, our molecular profiling data from the HIV-1 infected mPBMCs described a different scenario by suggesting that the HIV-1 infection may induce a better controlled immune activation in macaques. HIV-1 infection in mPBMCs transiently induced the expression changes of a few key genes that are crucial in controlling the activation of immune cells. CD247, ZAP70 and LCK, known to be expressed in multiple immune cells, were up-regulated only at day 4 p.i. but not at the later time point(Supplemental Table 2); on the other hand, CTLA-4, a repressor of T cell activation critical to regulating immune cell activities (Schneider et al., 2006) and the overall immune response (Rudd & Schneider, 2003), was down-regulated on day 4 but not on day 6 p.i. in the HIV-1 infected mPBMCs, as confirmed by qRT-PCR (Fig. 7). The transient down-regulation of CTLA-4 in the HIV-1 infected mPBMCs at the early time point is in line with the enhanced T cell receptor signaling we observed at the same time point and provides further evidence for T cell activation at this earlier time point. On the other hand, the recovery of CTLA-4 expression on day 6 p.i., and the concurrent down-regulation of ICOS, an inducer of T cell activation (Nurieva et al., 2003), indicates that activation of these immune cells is not persistent. In contrast, SIV-infected mPBMCs did not exhibit comparable decrease in T cell activation, as the repressor CTLA-4 stayed down-regulated on both days 4 and 6 p.i., with the inducer ICOS unaffected by SIV infection. Taken together, our data indicated that HIV-1 and SIV infections were associated with distinct T cell activation states: HIV-1 appears to first induce and then suppress T cell activation, which is in line with the heightened immune response on day 4 p.i.; HIV-1 replication was subsequently subdued at later time points, as T cell activation is required for efficient HIV replication (Gowda et al., 1989); in contrast, SIV-1 does not appear to effect a similar suppression on T cells and therefore may support more robust virus replication.
The distinct pathogenic properties of HIV-1 and SIV in macaques are likely to be caused by multiple factors. Persistent activation and the activation induced cell death (AICD) of immune cells have been reported extensively as a key to the HIV-1 pathogenesis in humans (Grossman et al., 2006). Unlike the pathogenic infection in humans, HIV-1 infection may only temporally induce the activation of immune cells in macaques. The temporal immune activation could be robust enough to hinder the progression of infection by antiviral immune responses but not enough to induce massive cell death in macaques. The other reason could be a failure of the macaques to mount an effective immune response in spite of robust SIV replication at the early stage of infection. Unlike the SIV infection, a low level replication of HIV-1 would be able to trigger a robust immune response in macaque. It is conceivable that the immune activation can be triggered by both direct and indirect mechanisms. Specifically, we speculate that the temporal surge in the release of cytokines by the HIV-infected cells (Bahbouhi et al., 2004), albeit in the minority, can signal gene expression changes in bystander cells in a paracrine fashion. Evading the immune surveillance is one well adopted strategy of primate lentiviruses during pathogenic infections (Evans & Desrosiers, 2001). One well studied example is nef, a gene encoded by all primate lentiviruses. Nef plays a central role in the evasion of host immune surveillance (Fackler & Baur, 2002). The pathogenicity of SIV with defects in the nef gene is significantly compromised in macaques (Kestler et al., 1990). A recent study suggested that, unlike SIV Nef, HIV-1 Nef does not suppress the immune cell activation (Schindler et al., 2006). Given that the Nef is dominantly expressed at the very early stage during the SIV infection, the repressive role of Nef in the immune activation could be crucial to the pathogenicity of SIV in macaques. Therefore, a failure of suppressing the immune cell activation by HIV-1 Nef may allow for rapid antiviral immune response to take effect during the HIV-1 infection in macaques.
In conclusion, our results indicated that the infection of HIV-1 and SIV in mPBMCs induced distinct pattern of cellular gene expression changes. While HIV-1 and SIV exhibited similar replication rates up until day 4 p.i., noticeable differences in host gene expression observed from that point on suggested that the pathogenic potential of the viruses could be reflected in the transcriptional programs triggered by the infections. Specifically, HIV-1 infection transiently triggered enhanced antigen presentation, as well as signaling and activation of T cells and NK cells at the early time point. By comparison, SIV infection was associated repressed T cell activation via Wnt/β-catenin signaling. HIV-1 infection induced more robust cellular response, possibly able to keep HIV-1 replication in check earlier on. However, as the infection progresses, the immune response is modulated, as seen in the lessened T cell activation at the late time point, which may render the susceptible cells less permissive to virus replication. The failure to sustain robust virus production caused by the host antiviral immune responses may partially explain the low pathogenicity of HIV-1 in macaques; unlike HIV-1, SIV is likely to have acquired the necessary fitness through evolution to sustain replication via the more expedient repression of host immune response. To reveal the restrained pathogenicity of HIV-1 in macaques mechanistically, further studies should focus on the investigation of the roles of individual cell populations during the HIV-1 infection in the mPBMCs.
Material and Methods
PBMC preparation
Peripheral blood was collected from three healthy pigtail macaques at Washington National Primate Research Center. Macaque PBMCs were recovered by standard Ficoll density-gradient centrifugation. The recovered cells were then washed, counted, and placed at a density of 2 × 106 cells/ml in complete RPMI 1640 growth medium containing 10% fetal bovine serum and 5% lectin-purified IL-2 (Roche Diagnostics). Phytohemagglutinin (Sigma Chemical) was then added at a concentration of 5μg/ml, and after a 24 hour incubation period, the medium was replaced with fresh complete medium and the culture was incubated at 37 °C for 4 days prior to infections.
Virus
The SIV strain, SIVmac239, containing an open reading frame of the nef gene was produced from two plasmids p239SpSp5′ and p239SpE3′Nef open (The NIH AIDS Research and Reference Reagent Program) as described previously (Gibbs et al., 1994). The HIV-1 strain, HIV-1 Lai, was the lab stock originally obtained from the Institute Pasteur, France (Peden et al., 1991). The SIV stock was grown in CEMx174 cells and the HIV-1 stock grown in CEMss cells. Both virus stocks were filtered through 0.22μm filters to remove cell derbies prior to the infections. The infectious titers of HIV-1 and SIV were determined in CEMx174 cells by the end-point dilution assays (Rodrigo et al., 1997).
Virus infection
Unfractionated mPBMCs (10 × 106 cells) from 3 pigtail macaques showed comparable compositions in terms of the cell types present (Supplemental Table 1). The mPBMCs from each individual animal were infected with HIV-1 or SIV at a multiplicity of 1 TCID50/cell or mock-infected with a volume of culture supernatant from uninfected cells equal to the volume of virus stocks. After a 120 minute incubation period at 37 °C, unattached virus was removed by 3 washes with phosphate-buffered saline. Mock-infected cells were washed in the same manner. The cells were then divided in 24-well tissue culture plates at 0.5 × 106 cells/well in 2 ml of complete medium per well per animal. For each animal, 2 wells of cells infected with each virus or mock were harvested on days 2, 4, 6 and 8 p.i.. Cells were counted at harvesting and the percentage of viable cells was determined by trypan blue dye exclusion. The cells were then collected by centrifugation and lysed in buffer RLT from RNeasy RNA isolation kit (Qiagen, CA). Culture supernatants were monitored for p27 or p24 levels by antigen-capture ELISA (ZeptoMetrix Corp. NY). The percentage of infected cells was estimated by indirect immunofluorescence assay (IFA) using pooled sera from SIV-infected macaques or HIV-1-infected humans as described previously (Agy et al., 1991).
Microarray analyses
For oligonucleotide array analyses, total RNA was isolated by RNeasy RNA isolation kit (Qiagen, CA). Equal amount of RNA isolated from 2 duplicate wells was combined before the complimentary RNA (cRNA) amplification. The cRNA was then amplified using the Agilent low RNA input linear amplification kit (Agilent Technologies, CA). The quantity and quality of cRNA were evaluated by capillary electrophoresis using an Agilent Technologies 2100 Bioanalyzer. Probe labeling and microarray hybridizations were performed as described in the Agilent 60-mer oligo microarray processing protocol (Agilent Technologies, CA). Labeled probes derived from SIV-infected cells, HIV-1-infected cells and their time matched mock-infected controls were co-hybridized against the probes derived from a common reference RNA sample on the Agilent macaque oligonucleotide Microarrays (Version 2) (Agilent Technologies, CA). The common reference RNA was pooling RNA isolated from mPBMCs of 10 uninfected pigtail macaques excluding the animals used in this study and the common reference probes were produced in the same fashion as the other probes. Slides were scanned with an Agilent microarray scanner and image analysis was performed using Agilent Feature Extractor Software. Each microarray experiment was done using a dye-swapping technique, thereby providing 2 technical replicates for each measurement (Kerr & Churchill, 2001).
All data were entered into a custom-designed database, Expression Array Manager, and then uploaded into Resolver 5.0 (Rosetta Biosoftware, WA) for analyses. The expression value of each individual gene was the average of 3 expression values from 3 animals calculated by the Resolver software using the Rosetta error model (Weng et al., 2006). For determining differential expression, the p-value for any given gene represents contributions from both the variance among measurements from the 3 animals, as well as the estimated errors in microarray signal measurements. Initially, genes were selected as differentially expressed based on a criterion of a greater than 99% probability of being differentially expressed (P ≤ 0.01). Biological gene sets (referred to as Biosets) were compiled for various cellular processes by selecting genes of interest that were both represented on the microarray and which had Gene Ontology (GO) annotation (Gene Ontology Consortium, 2004). Once compiled, Biosets were used to characterize and compare gene expression patterns between experiments. These Biosets were also analyzed using the Ingenuity Pathway Analysis (IPA) Software (www.ingenuity.com, Ingenuity Systems, CA) to assign functional information and biological relevance to the groups of genes being regulated. In accordance with proposed standards (Brazma et al., 2001), all data described in this report, including sample information, intensity measurements, gene lists, error analysis, microarray content, and slide hybridization conditions, are available in the public domain through Expression Array Manager at http://www.expression.washington.edu/public.
qRT-PCR
Quantitative real-time PCR (rtPCR) was used to verify the gene expression changes. Total RNA samples were treated with DNase using DNA-free DNase Treatment and Removal Reagents (Ambion Inc. TX). Reverse Transcription was performed using TaqMan Reverse Transcription Reagents (Applied Biosystems, CA). Primer and probe sets for each of the target sequences were designed using macaque EST sequences available from Macaque.org (Supplemental Table 3) and mapping them to the M. mulatta genome available from Baylor College of Medicine. rtPCR was performed on the ABI 7500 Real-time PCR System using TaqMan chemistry (Applied Biosystems, CA). Each target was run in quadruplicate, with 20 µL reaction volumes of TaqMan 2× PCR Universal Master Mix (Applied Biosystems, CA). 18S RNA was chosen as endogenous control to normalize quantification of the targets. The relative quantification of the viral genome RNA was performed by using the SIV-3′LTR primer/probe set and the HIV-1-3′LTR primer/probe set (Supplemental Table 3). The relative quantification of SIV or HIV-1 genome RNA of each time point was calculated by using the quantity of the corresponding virus genome RNA from day 2 p.i. as the base line.
Supplementary Material
Supplemental data are available at: http://viromics.washington.edu/publications/liyu
Acknowledgments
The authors gratefully thank Drs. Satya Dandekar and Michael B. Agy for their helpful comments. This work was supported by the NIH National Center for Research Resources (grants RR016354 and RR00166 to M.G.K.) and the National Institute on Drug Abuse (grant 1P30DA01562501 to M.G.K.). Y.L. and E.Y.C. are supported by the Rosetta Inpharmatics Fellowship in Molecular Profiling.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agy MB, Frumkin LR, Corey L, Coombs RW, Wolinsky SM, Koehler J, Morton WR, Katze MG. Infection of Macaca nemestrina by human immunodeficiency virus type-1. Science. 1992;257:103–106. doi: 10.1126/science.1621083. [DOI] [PubMed] [Google Scholar]
- 2.Agy MB, Foy K, Gale MJ, Benveniste RE, Clark EA, Katze MG. Viral and cellular gene expression in CD4+ human lymphoid cell lines infected by the simian immunodeficiency virus, SIV/Mne. Virology. 1991;183:170–180. doi: 10.1016/0042-6822(91)90130-4. [DOI] [PubMed] [Google Scholar]
- 3.Agy MB, Schmidt A, Florey MJ, Kennedy BJ, Schaefer G, Katze MG, Corey L, Morton WR, Bosch ML. Serialin VivoPassage of HIV-1 Infection inMacaca nemestrina. Virology. 1997;238:336–343. doi: 10.1006/viro.1997.8832. [DOI] [PubMed] [Google Scholar]
- 4.Arase N, Arase H, Hirano S, Yokosuka T, Sakurai D, Saito T. IgE-Mediated Activation of NK Cells Through Fc{gamma}RIII. The Journal of Immunology. 2003;170:3054–3058. doi: 10.4049/jimmunol.170.6.3054. [DOI] [PubMed] [Google Scholar]
- 5.Bahbouhi B, Landay A, Al-Harthi L. Dynamics of cytokine expression in HIV productively infected primary CD4+ T cells. Blood. 2004;103:4581–4587. doi: 10.1182/blood-2003-12-4172. [DOI] [PubMed] [Google Scholar]
- 6.Batten CJ, De RR, Wilson KM, Agy MB, Chea S, Stratov I, Montefiori DC, Kent SJ. Comparative evaluation of simian, simian-human, and human immunodeficiency virus infections in the pigtail macaque (Macaca nemestrina) model. AIDS Res Hum Retroviruses. 2006;22:580–588. doi: 10.1089/aid.2006.22.580. [DOI] [PubMed] [Google Scholar]
- 7.Beer BE, Brown CR, Whitted S, Goldstein S, Goeken R, Plishka R, Buckler-White A, Hirsch VM. Immunodeficiency in the Absence of High Viral Load in Pig-Tailed Macaques Infected with Simian Immunodeficiency Virus SIVsun or SIVlhoest. The Journal of Virology. 2005;79:14044–14056. doi: 10.1128/JVI.79.22.14044-14056.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.berola-Ila J, Hernandez-Hoyos G. The Ras/MAPK cascade and the control of positive selection. Immunological Reviews. 2003;191:79–96. doi: 10.1034/j.1600-065x.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 9.Boussiotis VA, Freeman GJ, Berezovskaya A, Barber DL, Nadler LM. Maintenance of Human T Cell Anergy: Blocking of IL-2 Gene Transcription by Activated Rap1. Science. 1997;278:124–128. doi: 10.1126/science.278.5335.124. [DOI] [PubMed] [Google Scholar]
- 10.Bowick GC, Fennewald SM, Elsom BL, Aronson JF, Luxon BA, Gorenstein DG, Herzog NK. Differential Signaling Networks Induced by Mild and Lethal Hemorrhagic Fever Virus Infections. The Journal of Virology. 2006;80:10248–10252. doi: 10.1128/JVI.01384-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, Aach J, Ansorge W, Ball CA, Causton, et al. Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nature Genetics. 2001;29:365–371. doi: 10.1038/ng1201-365. [DOI] [PubMed] [Google Scholar]
- 12.Broussard SR, Staprans SI, White R, Whitehead EM, Feinberg MB, Allan JS. Simian Immunodeficiency Virus Replicates to High Levels in Naturally Infected African Green Monkeys without Inducing Immunologic or Neurologic Disease. The Journal of Virology. 2001;75:2262–2275. doi: 10.1128/JVI.75.5.2262-2275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carey KD, Dillon TJ, Schmitt JM, Baird AM, Holdorf AD, Straus DB, Shaw AS, Stork PJS. CD28 and the Tyrosine Kinase Lck Stimulate Mitogen-Activated Protein Kinase Activity in T Cells via Inhibition of the Small G Protein Rap1. Molecular and Cellular Biology. 2000;20:8409–8419. doi: 10.1128/mcb.20.22.8409-8419.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carreno LJ, Gonzalez PA, Kalergis AM. Modulation of T cell function by TCR/pMHC binding kinetics. Immunobiology. 2006;211:47–64. doi: 10.1016/j.imbio.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Chiu D, Ma K, Scott A, Duronio V. Acute activation of Erk1/Erk2 and protein kinase B/akt proceed by independent pathways in multiple cell types. FEBS Journal. 2005;272:4372–4384. doi: 10.1111/j.1742-4658.2005.04850.x. [DOI] [PubMed] [Google Scholar]
- 16.Cooper MA, Fehniger TA, Fuchs A, Colonna M, Caligiuri MA. NK cell and DC interactions. Trends in Immunology. 2004;25:47–52. doi: 10.1016/j.it.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Dorner BG, Smith HRC, French AR, Kim S, Poursine-Laurent J, Beckman DL, Pingel JT, Kroczek RA, Yokoyama WM. Coordinate Expression of Cytokines and Chemokines by NK Cells during Murine Cytomegalovirus Infection. The Journal of Immunology. 2004;172:3119–3131. doi: 10.4049/jimmunol.172.5.3119. [DOI] [PubMed] [Google Scholar]
- 18.Evans DT, Desrosiers RC. Immune evasion strategies of the primate lentiviruses. Immunological Reviews. 2001;183:141–158. doi: 10.1034/j.1600-065x.2001.1830112.x. [DOI] [PubMed] [Google Scholar]
- 19.Fackler OT, Baur AS. Live and Let Die: Nef Functions beyond HIV Replication. Immunity. 2002;16:493–497. doi: 10.1016/s1074-7613(02)00307-2. [DOI] [PubMed] [Google Scholar]
- 20.Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat Rev Immunol. 2005;5:835–843. doi: 10.1038/nri1711. [DOI] [PubMed] [Google Scholar]
- 21.Fehniger TA, Herbein G, Yu H, Para MI, Bernstein ZP, O'Brien WA, Caligiuri MA. Natural Killer Cells from HIV-1+ Patients Produce C-C Chemokines and Inhibit HIV-1 Infection. The Journal of Immunology. 1998;161:6433–6438. [PubMed] [Google Scholar]
- 22.Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. The Role of Erk1 and Erk2 in Multiple Stages of T Cell Development. Immunity. 2005;23:431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Fling SP, Arp B, Pious D. HLA-DMA and -DMB genes are both required for MHC class II/peptide complex formation in antigen-presenting cells. Nature. 1994;368:554–558. doi: 10.1038/368554a0. [DOI] [PubMed] [Google Scholar]
- 24.Folks T, Rowe T, Villinger F, Parekh B, Mayne A, Anderson D, McClure H, Ansari AA. Immune stimulation may contribute to enhanced progression of SIV induced disease in rhesus macaques. J Med Primatol. 1997;26:181–189. doi: 10.1111/j.1600-0684.1997.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 25.Franchini G, Bosch ML. Genetic relatedness of the human immunodeficiency viruses type 1 and 2 (HIV-1, HIV-2) and the simian immunodeficiency virus (SIV) Ann N Y Acad Sci. 1989;554:81–87. doi: 10.1111/j.1749-6632.1989.tb22412.x. [DOI] [PubMed] [Google Scholar]
- 26.Gartner S, Liu Y, Lewis MG, Polonis V, Elkins WR, Zack PM, Miao J, Hunter EA, Greenhouse J, Eddy GA. HIV-1 infection in pigtailed macaques. AIDS Res Hum Retroviruses. 1994b;10 2:S129–S133. [PubMed] [Google Scholar]
- 27.Gartner S, Liu Y, Lewis MG, Polonis V, Elkins WR, Zack PM, Miao J, Hunter EA, Greenhouse J, Eddy GA. HIV-1 infection in pigtailed macaques. AIDS Res Hum Retroviruses. 1994a;10 2:S129–S133. [PubMed] [Google Scholar]
- 28.Gene Ontology Consortium. Nucleic Acids Research. Vol. 32. 2004. The Gene Ontology (GO) database and informatics resource; pp. D258–D261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.George MD, Verhoeven D, McBride Z, Dandekar S. Gene expression profiling of gut mucosa and mesenteric lymph nodes in simian immunodeficiency virus-infected macaques with divergent disease course. J Med Primatol. 2006;35:261–269. doi: 10.1111/j.1600-0684.2006.00180.x. [DOI] [PubMed] [Google Scholar]
- 30.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal Activating Interaction between Natural Killer Cells and Dendritic Cells. The Journal of Experimental Medicine. 2002;195:327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibbs JS, Regier DA, Desrosiers RC. Construction and in vitro properties of SIVmac mutants with deletions in “nonessential” genes. AIDS Res Hum Retroviruses. 1994;10:607–616. doi: 10.1089/aid.1994.10.607. [DOI] [PubMed] [Google Scholar]
- 32.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, Shih R, Lewis J, Wiley DJ, Phair JP, Wolinsky SM, Detels R. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. The Journal of Infectious Diseases. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 33.gli-Esposti MA, Smyth MJ. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol. 2005;5:112–124. doi: 10.1038/nri1549. [DOI] [PubMed] [Google Scholar]
- 34.Goux D, Coudert JD, Maurice D, Scarpellino L, Jeannet G, Piccolo S, Weston K, Huelsken J, Held W. Cooperating pre-T-cell receptor and TCF-1-dependent signals ensure thymocyte survival. Blood. 2005;106:1726–1733. doi: 10.1182/blood-2005-01-0337. [DOI] [PubMed] [Google Scholar]
- 35.Gowda SD, Stein BS, Mohagheghpour N, Benike CJ, Engleman EG. Evidence that T cell activation is required for HIV-1 entry in CD4+ lymphocytes. J Immunol. 1989;142:773–780. [PubMed] [Google Scholar]
- 36.Groot F, van Capel TMM, Kapsenberg ML, Berkhout B, de Jong EC. Opposing roles of blood myeloid and plasmacytoid dendritic cells in HIV-1 infection of T cells: transmission facilitation versus replication inhibition. Blood. 2006;108:1957–1964. doi: 10.1182/blood-2006-03-010918. [DOI] [PubMed] [Google Scholar]
- 37.Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat Med. 2006;12:289–295. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- 38.Guo B, Rothstein TL. B Cell Receptor (BCR) Cross-Talk: IL-4 Creates an Alternate Pathway for BCR-Induced ERK Activation That Is Phosphatidylinositol 3-Kinase Independent. The Journal of Immunology. 2005;174:5375–5381. doi: 10.4049/jimmunol.174.9.5375. [DOI] [PubMed] [Google Scholar]
- 39.Haks MC, Lefebvre JM, Lauritsen JP, Carleton M, Rhodes M, Miyazaki T, Kappes DJ, Wiest DL. Attenuation of [gamma][delta]TCR Signaling Efficiently Diverts Thymocytes to the [alpha][beta] Lineage. Immunity. 2005;22:595–606. doi: 10.1016/j.immuni.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Hatziioannou T, Princiotta M, Piatak M, Jr, Yuan F, Zhang F, Lifson JD, Bieniasz PD. Generation of Simian-Tropic HIV-1 by Restriction Factor Evasion. Science. 2006;314:95. doi: 10.1126/science.1130994. [DOI] [PubMed] [Google Scholar]
- 41.Heisel O, Keown P. Alterations in transcription factor binding at the IL-2 promoter region in anergized human CD4+ T lymphocytes. Transplantation. 2001;72:1416–1422. doi: 10.1097/00007890-200110270-00015. [DOI] [PubMed] [Google Scholar]
- 42.Hovanes K, Li TW, Munguia JE, Truong T, Milovanovic T, Lawrence MJ, Holcombe RF, Waterman ML. Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet. 2001;28:53–57. doi: 10.1038/ng0501-53. [DOI] [PubMed] [Google Scholar]
- 43.Ioannidis V, Beermann F, Clevers H, Held W. The beta-catenin--TCF-1 pathway ensures CD4(+)CD8(+) thymocyte survival. Nat Immunol. 2001;2:691–697. doi: 10.1038/90623. [DOI] [PubMed] [Google Scholar]
- 44.Jiang K, Zhong B, Gilvary DL, Corliss BC, Vivier E, Hong-Geller E, Wei S, Djeu JY. Syk Regulation of Phosphoinositide 3-Kinase-Dependent NK Cell Function. The Journal of Immunology. 2002;168:3155–3164. doi: 10.4049/jimmunol.168.7.3155. [DOI] [PubMed] [Google Scholar]
- 45.Kaizu M, Weiler AM, Weisgrau KL, Vielhuber KA, May G, Piaskowski SM, Furlott J, Maness NJ, Friedrich TC, Loffredo JT, Usborne A, Rakasz EG. Repeated Intravaginal Inoculation with Cell-Associated Simian Immunodeficiency Virus Results in Persistent Infection of Nonhuman Primates. The Journal of Infectious Diseases. 2006;194:912–916. doi: 10.1086/507308. [DOI] [PubMed] [Google Scholar]
- 46.Kamada K, Igarashi T, Martin MA, Khamsri B, Hatcho K, Yamashita T, Fujita M, Uchiyama T, Adachi A. Generation of HIV-1 derivatives that productively infect macaque monkey lymphoid cells. Proceedings of the National Academy of Sciences. 2006;103:16959–16964. doi: 10.1073/pnas.0608289103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kane LP, Lin J, Weiss A. Signal transduction by the TCR for antigen. Current Opinion in Immunology. 2000;12:242–249. doi: 10.1016/s0952-7915(00)00083-2. [DOI] [PubMed] [Google Scholar]
- 48.Kash JC, Basler CF, Garcia-Sastre A, Carter V, Billharz R, Swayne DE, Przygodzki RM, Taubenberger JK, Katze MG, Tumpey TM. Global Host Immune Response: Pathogenesis and Transcriptional Profiling of Type A Influenza Viruses Expressing the Hemagglutinin and Neuraminidase Genes from the 1918 Pandemic Virus. The Journal of Virology. 2004;78:9499–9511. doi: 10.1128/JVI.78.17.9499-9511.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kash JC, Muhlberger E, Carter V, Grosch M, Perwitasari O, Proll SC, Thomas MJ, Weber F, Klenk HD, Katze MG. Global Suppression of the Host Antiviral Response by Ebola- and Marburgviruses: Increased Antagonism of the Type I Interferon Response Is Associated with Enhanced Virulence. The Journal of Virology. 2006a;80:3009–3020. doi: 10.1128/JVI.80.6.3009-3020.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kash JC, Tumpey TM, Proll SC, Carter V, Perwitasari O, Thomas MJ, Basler CF, Palese P, Taubenberger JK, Garcia-Sastre A, Swayne DE, Katze MG. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature advanced online publication. 2006b doi: 10.1038/nature05181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kerr MK, Churchill GA. Statistical design and the analysis of gene expression microarray data. Genetical Research. 2001;77:123–128. doi: 10.1017/s0016672301005055. [DOI] [PubMed] [Google Scholar]
- 52.Kestler H, Kodama T, Ringler D, Marthas M, Pedersen N, Lackner A, Regier D, Sehgal P, Daniel M, King N. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990;248:1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- 53.Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, Hatta Y, Kim JH, Halfmann P, Hatta M, Feldmann F, Alimonti JB, Fernando L, Li Y, Katze MG, Feldmann H, Kawaoka Y. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- 54.Levy JA. The search for the CD8+ cell anti-HIV factor (CAF) Trends in Immunology. 2003;24:628–632. doi: 10.1016/j.it.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 55.Li X, Yost HJ, Virshup DM, Seeling JM. Protein phosphatase 2A and its B56 regulatory subunit inhibit Wnt signaling in Xenopus. EMBO J. 2001;20:4122–4131. doi: 10.1093/emboj/20.15.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li X, Hansen PA, Xi L, Chandraratna RAS, Burant CF. Distinct Mechanisms of Glucose Lowering by Specific Agonists for Peroxisomal Proliferator Activated Receptor {gamma} and Retinoic Acid X Receptors. Journal of Biological Chemistry. 2005;280:38317–38327. doi: 10.1074/jbc.M505853200. [DOI] [PubMed] [Google Scholar]
- 57.Lichterfeld M, Yu XG, Le Gall S, Altfeld M. Immunodominance of HIV-1-specific CD8+ T-cell responses in acute HIV-1 infection: at the crossroads of viral and host genetics. Trends in Immunology. 2005;26:166–171. doi: 10.1016/j.it.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 58.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 59.Mavilio D, Benjamin J, Daucher M, Lombardo G, Kottilil S, Planta MA, Marcenaro E, Bottino C, Moretta L, Moretta A, Fauci AS. Natural killer cells in HIV-1 infection: Dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proceedings of the National Academy of Sciences. 2003;100:15011–15016. doi: 10.1073/pnas.2336091100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Messmer B, Eissmann P, Stark S, Watzl C. CD48 Stimulation by 2B4 (CD244)-Expressing Targets Activates Human NK Cells. The Journal of Immunology. 2006;176:4646–4650. doi: 10.4049/jimmunol.176.8.4646. [DOI] [PubMed] [Google Scholar]
- 61.Michie AM, Trop S, Wiest DL, Zuniga-Pflucker JC. Extracellular Signal-regulated Kinase (ERK) Activation by the Pre-T Cell Receptor in Developing Thymocytes In Vivo. The Journal of Experimental Medicine. 1999;190:1647–1656. doi: 10.1084/jem.190.11.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller JR, Moon RT. Signal transduction through beta-catenin and specification of cell fate during embryogenesis. Genes Dev. 1996;10:2527–2539. doi: 10.1101/gad.10.20.2527. [DOI] [PubMed] [Google Scholar]
- 63.Molina TJ, Kishihara K, Siderovskid DP, van Ewijk W, Narendran A, Timms E, Wakeham A, Paige CJ, Hartmann KU, Veillette A, Davidson D, Mak TW. Profound block in thymocyte development in mice lacking p56lck. Nature. 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 64.Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol. 2002;2:957–964. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- 65.Nurieva RI, Duong J, Kishikawa H, Dianzani U, Rojo JM, Ho Ic, Flavell RA, Dong C. Transcriptional Regulation of Th2 Differentiation by Inducible Costimulator. Immunity. 2003;18:801–811. doi: 10.1016/s1074-7613(03)00144-4. [DOI] [PubMed] [Google Scholar]
- 66.Okamura RM, Sigvardsson M, Galceran J, Verbeek S, Clevers H, Grosschedl R. Redundant Regulation of T Cell Differentiation and TCR[alpha] Gene Expression by the Transcription Factors LEF-1 and TCF-1. Immunity. 1998;8:11–20. doi: 10.1016/s1074-7613(00)80454-9. [DOI] [PubMed] [Google Scholar]
- 67.Onanga R, Souquiere S, Makuwa M, Mouinga-Ondeme A, Simon F, Apetrei C, Roques P. Primary Simian Immunodeficiency Virus SIVmnd-2 Infection in Mandrills (Mandrillus sphinx) The Journal of Virology. 2006;80:3301–3309. doi: 10.1128/JVI.80.7.3301-3309.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pandrea I, Onanga R, Kornfeld C, Rouquet P, Bourry O, Clifford S, Telfer PT, Abernethy K, White LT, Ngari P. High levels of SIVmnd-1 replication in chronically infected Mandrillus sphinx. Virology. 2003;317:119–127. doi: 10.1016/j.virol.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 69.Pasieka TJ, Baas T, Carter VS, Proll SC, Katze MG, Leib DA. Functional Genomic Analysis of Herpes Simplex Virus Type 1 Counteraction of the Host Innate Response. The Journal of Virology. 2006;80:7600–7612. doi: 10.1128/JVI.00333-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peden K, Emerman M, Montagnier L. Changes in growth properties on passage in tissue culture of viruses derived from infectious molecular clones of HIV-1LAI, HIV-1MAL, and HIV-1ELI. Virology. 1991;185:661–672. doi: 10.1016/0042-6822(91)90537-l. [DOI] [PubMed] [Google Scholar]
- 71.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent Stimulation and Inhibition of Dendritic Cells by Natural Killer Cells. The Journal of Experimental Medicine. 2002;195:335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ploquin M, Desoutter JF, Santos P, Pandrea I, Diop O, Hosmalin A, Butor C, Barre-Sinoussi F, Muller-Trutwin M. Distinct expression profiles of TGF-beta1 signaling mediators in pathogenic SIVmac and non-pathogenic SIVagm infections. Retrovirology. 2006;3:37. doi: 10.1186/1742-4690-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Powis SJ. CLIP-region mediated interaction of Invariant chain with MHC class I molecules. FEBS Letters. 2006;580:3112–3116. doi: 10.1016/j.febslet.2006.04.060. [DOI] [PubMed] [Google Scholar]
- 74.Rodrigo AG, Goracke PC, Rowhanian K, Mullins JI. Quantitation of target molecules from polymerase chain reaction-based limiting dilution assays. AIDS Res Hum Retroviruses. 1997;13:737–742. doi: 10.1089/aid.1997.13.737. [DOI] [PubMed] [Google Scholar]
- 75.Rudd CE, Schneider H. Unifying concepts in CD28, ICOS and CTLA4 co-receptor signalling. Nat Rev Immunol. 2003;3:544–556. doi: 10.1038/nri1131. [DOI] [PubMed] [Google Scholar]
- 76.Sancho J, Ledbetter JA, Choi MS, Kanner SB, Deans JP, Terhorst C. CD3-zeta surface expression is required for CD4-p56lck-mediated upregulation of T cell antigen receptor-CD3 signaling in T cells. Journal of Biological Chemistry. 1992;267:7871–7879. [PubMed] [Google Scholar]
- 77.Schindler M, Munch J, Kutsch O, Li H, Santiago ML, Bibollet-Ruche F, Muller-Trutwin MC, Novembre FJ, Peeters M, Courgnaud V. Nef-Mediated Suppression of T Cell Activation Was Lost in a Lentiviral Lineage that Gave Rise to HIV-1. Cell. 2006;125:1055–1067. doi: 10.1016/j.cell.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 78.Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, Wei B, Hogg N, Garside P, Rudd CE. Reversal of the TCR Stop Signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 79.Sherman MA, Weber DA, Jensen PE. DM enhances peptide binding to class II MHC by release of invariant chain-derived peptide. Immunity. 1995;3:197–205. doi: 10.1016/1074-7613(95)90089-6. [DOI] [PubMed] [Google Scholar]
- 80.Silvestri G, Sodora DL, Koup RA, Paiardini M, O'Neil SP, McClure HM, Staprans SI, Feinberg MB. Nonpathogenic SIV Infection of Sooty Mangabeys Is Characterized by Limited Bystander Immunopathology Despite Chronic High-Level Viremia. Immunity. 2003;18:441–452. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 81.Simon V, Ho DD, bdool Karim Q. HIV/AIDS epidemiology, pathogenesis, prevention, and treatment. The Lancet. 2006;368:489–504. doi: 10.1016/S0140-6736(06)69157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signaling: Altered phospho-[zeta] and lack of zap70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 83.Sugie K, Jeon MS, Grey HM. Activation of naive CD4 T cells by anti-CD3 reveals an important role for Fyn in Lck-mediated signaling. Proceedings of the National Academy of Sciences. 2004;101:14859–14864. doi: 10.1073/pnas.0406168101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tan AHM, Wong SC, Lam KP. Regulation of Mouse Inducible Costimulator (ICOS) Expression by Fyn-NFATc2 and ERK Signaling in T Cells. Journal of Biological Chemistry. 2006;281:28666–28678. doi: 10.1074/jbc.M604081200. [DOI] [PubMed] [Google Scholar]
- 85.Thomas MJ, Agy MB, Proll SC, Paeper BW, Li Y, Jensen KL, Korth MJ, Katze MG. Functional gene analysis of individual response to challenge of SIVmac239 in M. mulatta PBMC culture. Virology. 2006;348:242–252. doi: 10.1016/j.virol.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 86.Wang ZW, Sarmento L, Wang Y, Li Xq, Dhingra V, Tseggai T, Jiang B, Fu ZF. Attenuated Rabies Virus Activates, while Pathogenic Rabies Virus Evades, the Host Innate Immune Responses in the Central Nervous System. The Journal of Virology. 2005;79:12554–12565. doi: 10.1128/JVI.79.19.12554-12565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weng L, Dai H, Zhan Y, He Y, Stepaniants SB, Bassett DE., Jr Rosetta error model for gene expression analysis. Bioinformatics. 2006 doi: 10.1093/bioinformatics/btl045. btl045. [DOI] [PubMed] [Google Scholar]
- 88.Wortman B, Darbinian N, Sawaya BE, Khalili K, Amini S. Evidence for Regulation of Long Terminal Repeat Transcription by Wnt Transcription Factor TCF-4 in Human Astrocytic Cells. The Journal of Virology. 2002;76:11159–11165. doi: 10.1128/JVI.76.21.11159-11165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu X, Anderson JL, Campbell EM, Joseph AM, Hope TJ. Proteasome inhibitors uncouple rhesus TRIM5alpha restriction of HIV-1 reverse transcription and infection. Proc Natl Acad Sci U S A. 2006;103:7465–7470. doi: 10.1073/pnas.0510483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xing H, Zhang S, Weinheimer C, Kovacs A, Muslin AJ. 14-3-3 proteins block apoptosis and differentially regulate MAPK cascades. EMBO J. 2000;19:349–358. doi: 10.1093/emboj/19.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu TK, Caudell EG, Smid C, Grimm EA. IL-2 Activation of NK Cells: Involvement of MKK1/2/ERK But Not p38 Kinase Pathway. The Journal of Immunology. 2000;164:6244–6251. doi: 10.4049/jimmunol.164.12.6244. [DOI] [PubMed] [Google Scholar]
- 92.Zennou V, Bieniasz PD. Comparative analysis of the antiretroviral activity of APOBEC3G and APOBEC3F from primates. Virology. 2006;349:31–40. doi: 10.1016/j.virol.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 93.Zheng YH, Peterlin BM. Intracellular immunity to HIV-1: newly defined retroviral battles inside infected cells. Retrovirology. 2005;2:25. doi: 10.1186/1742-4690-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zwart W, Griekspoor A, Kuijl C, Marsman M, van Rheenen J, Janssen H, Calafat J, van Ham M, Janssen L, van Lith M. Spatial Separation of HLA-DM/HLA-DR Interactions within MIIC and Phagosome-Induced Immune Escape. Immunity. 2005;22:221–233. doi: 10.1016/j.immuni.2005.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental data are available at: http://viromics.washington.edu/publications/liyu