Abstract
Objectives
The goal of this study was to characterize left ventricular diastolic function in the sickle cell disease (SCD) population and to relate echocardiographic measures of dysfunction with pulmonary hypertension and mortality.
Background
Pulmonary hypertension has been identified as a predictor of death in the adult SCD population. Although diastolic dysfunction is also observed in this population, its prevalence, association with high pulmonary artery systolic pressure, and attributable mortality remain unknown.
Methods
Diastolic function assessment using tissue Doppler imaging was performed in a group of 141 SCD patients. Conventional echocardiographic parameters of diastolic function were performed in a total of 235 SCD patients.
Results
Diastolic dysfunction was present in 18% of patients. A combination of diastolic dysfunction and pulmonary hypertension was present in 11% of patients, and diastolic dysfunction accounted for only 10% to 20% of the variability in tricuspid regurgitation (TR) jet velocity. Diastolic dysfunction, as reflected by a low E/A ratio, was associated with mortality with a risk ratio of 3.5 (95% confidence interval 1.5 to 8.4, p < 0.001), even after adjustment for tricuspid regurgitation (TR) jet velocity. The presence of both diastolic dysfunction and pulmonary hypertension conferred a risk ratio for death of 12.0 (95% confidence interval 3.8 to 38.1, p < 0.001).
Conclusions
Diastolic dysfunction and pulmonary hypertension each contribute independently to prospective mortality in patients with SCD. Patients with both risk factors have an extremely poor prognosis. These data support the implementation of echocardiographic screening of adult patients with SCD to identify high-risk individuals for further evaluation.
The hemoglobinopathy of sickle cell disease (SCD) leads to entrapment of sickle erythrocytes within the microvasculature, producing episodic vaso-occlusion and ischemia-reperfusion injury and infarction in multiple organ systems. Although the survival of patients in the U.S. and the developed world has increased significantly over the last 3 decades, the poorly controlled lifelong hemolytic anemia and repetitive cycles of organ infarction ultimately lead to a progressive systemic vasculopathy and chronic organ failure (1). Related to this vasculopathy is the development of pulmonary hypertension (PH), mirrored by increasing reports of sudden death (2–4). Pulmonary hypertension has been closely associated with mortality (2,3,5–8) and was the most common finding in a recent autopsy series (9).
We recently reported a prevalence of PH of 32% in a prospective cohort of 195 SCD patients (5). This diagnosis was associated with a risk ratio (RR) for death of 10.1 (95% confidence interval [CI] 2.2 to 47.0). Although the association between increased pulmonary pressures and mortality is impressive and has been reproduced in multiple studies (8,10,11), the degree of PH is modest (5,12,13), making it unclear whether this is a cause or a correlate of the increased mortality. A central controversy in the field involves the contribution of left ventricular (LV) dysfunction to increased pulmonary pressures in patients with SCD. Invasive hemodynamic measurements have shown a mixed picture of high pulmonary artery pressures and elevated pulmonary capillary wedge pressures (5,6), suggesting that LV diastolic or systolic dysfunction may contribute to the high pulmonary artery systolic pressures and increased risk of death. Therefore, our goal was to characterize LV structure and function in the SCD population and to relate echocardiographic measures of dysfunction with PH and mortality.
Methods
Patient population
This study was approved by the National Heart, Lung, and Blood Institute Institutional Review Board, and all patients signed informed consent. The study population consisted of 235 adult patients with documented SCD (mean age 35 ± 11 years, range 18 to 70 years) and 41 age- and gender-balanced control patients (mean age 37 ± 11 years, range 21 to 69) who were consecutively enrolled. Consistent with the distribution of SCD in the U.S., nearly all of the study patients were African American or African, with a very small percentage of Hispanic patients. Consequently, healthy African American patients were chosen as the most appropriate ethnically comparable control group. This study included 191 patients from a subgroup of 195 patients who have been previously described in detail (5). Nine patients with moderate or more mitral or aortic regurgitation were excluded from the study population. The study population included 22 patients with mild aortic regurgitation. At the time of their initial evaluations, 37% of patients were on hydroxyurea therapy and no patients were receiving treatment for PH.
Echocardiography
Transthoracic echocardiography was performed with the use of the Acuson Sequoia (Siemens, Malvern, Pennsylvania) and Sonos 5500 (Philips, Andover, Massachusetts) systems. Echocardiograms were performed at a community hospital and at a tertiary referral center starting in February 2001 and were read in a blinded manner. Cardiac measurements were performed according to American Society of Echocardiography guidelines (14). The LV ejection fraction was assessed using the modified Quinones formula and the biplanar Simpson method (14). Measurement of stroke volume and cardiac output was performed in a standard manner (15). Left atrial volumes were measured using the biplanar method of discs incorporating both apical 4- and 2-chamber views (16). Tricuspid regurgitation (TR) and pulmonary artery systolic pressure were assessed as previously described (5). The mean right atrial pressure was calculated according to the degree of collapse of the inferior vena cava with inspiration: 5 mm Hg for a collapse of at least 50% and 15 mm Hg for a collapse of <50% (17–19). Pulmonary hypertension was prospectively defined as a peak TR jet velocity of at least 2.5 m/s.
Left ventricular mass (LVM) and LV mass index (LVMI) were calculated using the formula described by Devereux et al. (20): LVM (g) = 0.8 (1.04 [septum + posterior wall + LV internal diastolic dimension]³ − [LV diastolic dimension]³) + 0.6. Left ventricular hypertrophy (LVH) was defined as an LVMI higher than the 95th percentile for children and adults with a gender-independent partition value of 51 g/m2.7 (21). Relative wall thickness (RWT) was defined as: (PW × 2)/ LVIDD (22). Patients with LVH had concentric hypertrophy if their RWT was elevated (>0.41) and eccentric LVH if their RWT was normal (≤0.41). Patients with a normal LVMI and increased RWT were classified as having concentric remodeling.
The LV and right ventricular (RV) areas were measured in the apical 4-chamber view (23). The RV-to-LV area ratio at end-diastole was calculated from these measurements. Right ventricular function was assessed using the RV percent change in area (14).
Diastolic function was assessed in all patients using pulsed Doppler peak E and A velocities, E/A ratio, and deceleration time (15,24). Isovolumic relaxation time was measured as the time from aortic valve closure to the start of mitral inflow. After initial reports of elevated pulmonary capillary wedge pressure in SCD patients undergoing cardiac catheterization (5,6), we added tissue Doppler imaging to the evaluation of patients undergoing echocardiogram starting in January 2003. The tissue Doppler imaging of the septal and lateral mitral annulus was performed in the subsequent 141 consecutive SCD echocardiograms. The peak Em velocity was used to calculate a septal and lateral E/Em ratio. Diastolic dysfunction was graded as normal or as mild, moderate, or severe dysfunction according to the criteria described below. Because the SCD patients that we studied were young (average age 35 ± 11 years) but had a wide range in age from 18 to 70 years, we defined a low E/A ratio as <1.0 (representing approximately the 5th percentile of our population). Mild diastolic dysfunction was defined as an E/A ratio of <1.0 and/or a deceleration time of >240 ms. Moderate diastolic dysfunction was defined as an E/A ratio of ≥1.0 and an E/Em ratio of >10 (25). Severe diastolic dysfunction was defined as an E/A ratio higher than the 95th percentile for age (26) or deceleration time (DT) <140 ms (27) and E/Em >10.
Statistical analysis
Comparisons of distributions of continuous variables in SCD patients and control patients, as well as in patients with TR jet velocity <2.5 and ≥2.5 m/s, were made using the t test. Associations between continuous variables were assessed using the Spearman rank correlation coefficient. Adjustment for hemoglobin in bivariate associations between TR jet velocity and echocardiographic or laboratory variables was done by linear regression using ranks, with TR jet velocity the dependent variable and hemoglobin and the other variable of interest the independent variable. Associations between a continuous and a dichotomous variable were evaluated using the Wilcoxon rank-sum test. Multiple linear regression modeling was used to identify sets of variables that were independently associated with TR jet velocity. This modeling used a stepwise procedure in which a potential independent variable was considered if it had a p value <0.15 for bivariate association with TR jet velocity; the final model included variables with p ≤ 0.05 in multiple regression. Multiple regression using ranks for all variables was used to confirm the most important correlates. Associations of echocardiographic variables with mortality were assessed using proportional hazards (Cox) regression modeling. All p values <0.05 were considered statistically significant, and p values shown in the tables were not adjusted for multiple comparisons; marked values represent associations that remain significant after a Bonferroni correction, in which the p value is multiplied by the number of comparisons in the table.
Because this was a registry study, prospective power analysis was not performed. Mortality data were collected by periodic telephone call or clinic follow-up and confirmatory review of the Social Security Death Index. Patients were followed up until death or last contact with clinic staff, at which time they were censored.
Results
Cardiac chamber morphology and systolic function
The characteristics of the 235 patients and 41 control patients are shown in Table 1. Left and right heart chamber volumes and mass indexes were increased in the SCD patients compared with normal volunteers, as seen in Table 2. We found no significant difference in heart rate between SCD patients and control patients, despite the fact that hemoglobin levels were significantly decreased in SCD patients. There were 9 patients with concentric LVH, 7 patients with eccentric LVH, and 35 patients with concentric remodeling. In patients with a TR jet velocity of 2.5 m/s or more, LV chamber size and mass increased (LV end-diastolic distention 52.9 ± 5.9 vs. 50.4 ± 4.6, p = 0.001; LVMI 41.4 ± 12.9 vs. 33.5 ± 8.3, p < 0.001), although LV systolic function did not change (p = 0.2). The average LVEF was 65 ± 8% (range 42% to 83%), and only 9% of patients had evidence of systolic dysfunction with an ejection fraction <55%. Although there was a trend toward RV enlargement with increasing pulmonary pressures, RV systolic function was preserved at higher pressures (RV percent area change 53 ± 11 vs. 54 ± 11, p = 0.5), suggesting no evidence of resting RV functional impairment. These echocardiographic parameters were not significantly different in patients on hydroxyurea treatment.
Table 1.
Characteristics of Sickle Cell Patients Versus Normal Controls
| SCD |
Controls |
||||
|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | p Value* | |
| Age (yrs) | 235 | 35 ± 11 | 41 | 37 ± 11 | 0.50 |
| Female gender (%) | 235 | 60 | 41 | 59 | 0.93 |
| Heart rate (beats/min) | 235 | 73 ± 11 | 41 | 70 ± 12 | 0.10 |
| Systolic BP (mm Hg) | 210 | 121 ± 18 | 36 | 133 ± 19 | <0.001 |
| Diastolic BP (mm Hg) | 211 | 67 ± 11 | 36 | 77 ±12 | <0.001 |
| Mean arterial pressure (mm Hg) | 211 | 85 ± 12 | 36 | 95 ± 14 | <0.001 |
| Creatinine (mg/dl)† | 224 | 0.9 ± 1.3 | 41 | 0.9 ± 0.2 | 0.909 |
| Hemoglobin (g/dl) | 226 | 9.6 ± 1.8 | 41 | 13.5 ± 1.6 | <0.001 |
| Ferritin (mg/l)† | 219 | 880 ± 1,325 | 38 | 75 ± 61 | <0.001 |
| Transferrin (mg/dl)† | 224 | 205 ± 53 | 37 | 271 ± 51 | <0.001 |
For continuous variables, from 2-sided 2-sample t test; for gender, from chi-square without continuity correction
logarithms (base 10) used.
BP = blood pressure; SCD = sickle cell disease.
Table 2.
Echocardiographic and Doppler Features of Sickle Cell Patients Versus Normal Controls
| SCD |
Controls |
||||
|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | p Value* | |
| LV structure and function | |||||
| LVED (mm) | 233 | 51.3 ± 5.1 | 41 | 47.6 ± 3.8 | <0.001* |
| LVES (mm) | 233 | 32.7 ± 5.5 | 41 | 27.4 ± 4.3 | <0.001* |
| Septal thickness (mm) | 235 | 9.4 ± 1.5 | 41 | 8.9 ± 1.1 | 0.043 |
| PW thickness (mm) | 235 | 9.4 ± 1.4 | 41 | 9.0 ± 1.1 | 0.038 |
| LVMI (g/m2.7) | 214 | 36.2 ± 10.8 | 31 | 30.4 ± 6.2 | 0.002* |
| LVEF (%)† | 233 | 65.2 ± 7.9 | 41 | 72.2 ± 7.1 | <0.001* |
| LV diastolic function | |||||
| Max LA volume/BSA (ml/m²) | 235 | 40.5 ± 13.8 | 41 | 22.8 ± 5.9 | <0.001* |
| Peak E velocity (m/s) | 225 | 0.9 ± 0.2 | 37 | 0.8 ± 0.1 | 0.007 |
| Peak A velocity (m/s) | 225 | 0.6 ± 0.2 | 37 | 0.5 ± 0.1 | 0.007 |
| E/A ratio | 225 | 1.6 ± 0.6 | 37 | 1.6 ± 0.4 | 0.788 |
| Deceleration time (ms) | 223 | 189 ± 38 | 37 | 184 ± 31 | 0.427 |
| Isovolumic relaxation time (ms) | 215 | 73 ± 21 | 33 | 79 ± 14 | 0.102 |
| Right heart size and function | |||||
| Right atrial area (cm²) | 205 | 17.9 ± 4.5 | 34 | 14.6 ± 3.3 | <0.001* |
| RV end-diastolic area (cm²) | 182 | 19.5 ± 4.7 | 34 | 17.4 ± 4.3 | 0.017 |
| RV end-systolic area (cm²) | 182 | 9.0 ± 3.0 | 34 | 8.0 ± 1.8 | 0.056 |
| RV area change (%) | 179 | 53.9 ± 11.0 | 32 | 53.9 ± 9.8 | 0.998 |
| RV:LV diastolic area ratio | 182 | 0.5 ± 0.1 | 34 | 0.5 ± 0.1 | 0.430 |
From t test; marked values represent associations with p < 0.05 after Bonferroni adjustment for number of associations in table.
Quinones ejection fraction.
BSA = body surface area; LA = left atrium; LV = left ventricle; LVED = left ventricle end-diastolic dimension; LVEF = left ventricular ejection fraction; LVES = left ventricle end-systolic dimension; LVMI = left ventricular mass index; PW = posterior wall; RV = right ventricle; other abbreviations as in Table 1.
Prevalence of diastolic dysfunction in patients with SCD
Because the most comprehensive diastolic assessment using tissue Doppler was available in 141 patients, we evaluated the prevalence of diastolic dysfunction in this group and found that 25 patients (18%) had evidence of mild, moderate, or severe diastolic dysfunction. Figure 1A shows the distribution of patients with PH and diastolic dysfunction. There were 15 patients with mild diastolic dysfunction and 9 patients with moderate diastolic dysfunction. We found that in patients with mild diastolic dysfunction (E/A <1), Em was significantly reduced (9.4 ± 3.4 vs. 11.3 ± 3.4, p = 0.04). Interestingly, patients with moderate diastolic dysfunction had an E/A ratio of only 1.2 ± 0.2. In this group of 141 patients, there was only 1 patient with criteria for severe diastolic dysfunction.
Figure 1. Prevalence of Diastolic Dysfunction in Sickle Cell Disease.
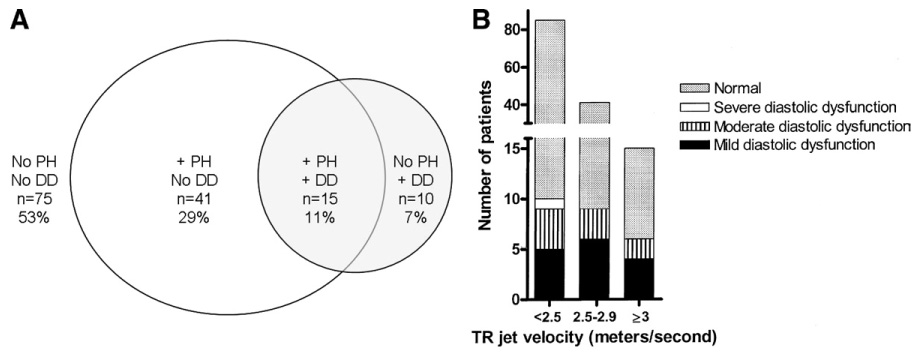
(A) Distribution of patients with pulmonary arterial hypertension (PH) and diastolic dysfunction (DD). Venn diagram indicating the number of patients without PH (tricuspid regurgitation [TR] <2.5 m/s), with PH (TR ≥2.5) and tissue Doppler results indicating DD. (B) Detailed distribution by degree of PH and DD. The number of patients without or with mild, moderate, or severe DD are shown in groups based on their TR jet velocity (TR <2.5 m/s, TR 2.5 to 2.9 m/s, and TR ≥3 m/s). In the 2 high TR velocity groups, there were no patients with severe DD.
We also examined the prevalence of PH in this group of 141 patients. We found 56 patients (40%) with a TR jet velocity of ≥2.5 m/s and 15 patients (11%) with a TR jet velocity of ≥3 m/s. As seen in Figure 1B, patients with diastolic dysfunction were found at all levels of TR jet velocity. Patients with moderate and severe diastolic dysfunction, which may contribute to high filling pressures and subsequent high pulmonary pressures, were uncommon and represented only 9% of those with a TR jet velocity ≥2.5 m/s (5 of 56 patients) and 13% of those with a TR jet velocity ≥3 m/s (2 of 15 patients).
Associations between PH and diastolic dysfunction
Because the elevation of pulmonary pressures in SCD patients is mild, the contribution of LV systolic or diastolic dysfunction to PH remains controversial. We explored correlations between all echocardiographic and Doppler parameters and TR jet velocity. Table 3 shows that after adjustment for the effect of hemoglobin level and multiple comparisons, the relationships with LV size, LVM, and left atrial area remain significant, but only could explain approximately 10% to 15% of the variability in TR jet velocities (ρ² = 0.06 for LVMI, 0.12 for left atrial area, 0.02 for E/A, and 0.03 for deceleration time). The LV systolic function did not correlate significantly with TR. In multiple regression modeling, right atrial area, LV wall thickness, lactate dehydrogenase (LDH), and transferrin, a reflection of iron overload, were the most important correlates of TR velocity, all with p < 0.05. A model using ranks for all of these variables gave similar results, except that the significance of LDH was slightly decreased (p = 0.08). The significant, but weak, associations between measures of diastolic dysfunction and TR velocity confirm the results of the tissue Doppler analysis: only a small subgroup of patients with PH has concomitant diastolic dysfunction that potentially contributes to elevated pulmonary pressures.
Table 3.
Associations With TR Velocity, Spearman Rank Correlation Coefficient ρ
| Adjusted for Hemoglobin |
|||||||
|---|---|---|---|---|---|---|---|
| Category | Variable | ρ | n | p | ρ | n | p* |
| LV structure | LVED (mm) | 0.19 | 233 | 0.005 | 0.15 | 227 | 0.023 |
| LVES (mm) | 0.13 | 233 | 0.047 | 0.10 | 227 | 0.12 | |
| Septal thickness (mm) | 0.29 | 235 | <0.0001* | 0.28 | 228 | <0.0001* | |
| PW thickness (mm) | 0.27 | 235 | <0.0001* | 0.25 | 228 | 0.0002* | |
| LVMI (g/m2.7) | 0.25 | 213 | 0.0002* | 0.22 | 207 | 0.002* | |
| LV diastolic function | Left atrial area (cm²) | 0.35 | 235 | <0.0001* | 0.29 | 228 | <0.0001* |
| MV E/A ratio | −0.15 | 225 | 0.022 | −0.22 | 218 | 0.001* | |
| Deceleration time (ms) | 0.18 | 223 | 0.008 | 0.19 | 216 | 0.006 | |
| IVRT (ms) | 0.09 | 215 | 0.21 | 0.15 | 209 | 0.031 | |
| Septal Em/Am (cm/s) | −0.05 | 140 | 0.57 | −0.13 | 140 | 0.13 | |
| Septal E/Em ratio | 0.08 | 140 | 0.37 | 0.06 | 140 | 0.46 | |
| Right heart size and function | Right atrial area (cm²) | 0.33 | 229 | <0.0001* | 0.30 | 222 | <0.0001* |
| RV end-diastolic area (cm²) | 0.10 | 206 | 0.16 | 0.06 | 200 | 0.43 | |
| RV end-systolic area (cm²) | 0.08 | 206 | 0.27 | 0.02 | 200 | 0.79 | |
| RV area change (%) | −0.02 | 203 | 0.78 | 0.03 | 197 | 0.72 | |
| RV:LV diastolic area ratio | −0.05 | 206 | 0.48 | −0.04 | 200 | 0.54 | |
| Laboratory | Hemoglobin (g/dl) | −0.28 | 228 | <0.0001* | n/a | n/a | n/a |
| LDH (U/l) | 0.23 | 205 | 0.0009* | 0.07 | 204 | 0.32 | |
| Creatinine (mg/dl) | 0.18 | 227 | 0.005 | 0.24 | 226 | 0.0003* | |
| Transferrin (mg/dl) | −0.27 | 224 | <0.0001* | −0.20 | 223 | 0.002* | |
Marked values represent associations with p < 0.05 after Bonferroni adjustment for number of associations in table.
From regression on ranks.
IVRT = isovolumic relaxation time; MV = mitral valve; TR = tricuspid regurgitation; other abbreviations as in Table 2.
LV diastolic dysfunction and PH are independent and additive risk factors for death
Of 235 patients, 3 had no follow-up for mortality, so the survival analysis included 232 patients. There were 20 deaths, and these patients were followed up for 0.1 to 44.6 months (median 18.5 months). The 212 survivors were followed up for 1.3 to 48.6 months (median 30.8 months).
Proportional hazards regression analysis showed that several diastolic parameters, including E/A (p < 0.001), peak E velocity (p = 0.002), deceleration time (p = 0.002), and tissue Doppler septal Em/Am ratio (p = 0.026), were independent predictors of mortality. Patients with a low mitral E/A and a low tissue Doppler Em/Am ratio had a significantly increased risk of mortality, with an RR (25th relative to 75th percentile) of 4.8 (95% CI 1.9 to 12.1 for E/A, p < 0.001 and 1.0 to 22.9 for Em/Am) (Table 4). The TR velocity remained a significant predictor of mortality, with an RR of 5.1. After adjustment for E/A ratio, the RR for TR jet velocity ≥2.5 m/s was 3.4 (95% CI 1.2 to 9.4, p = 0.014). In separate analyses of other echocardiographic and Doppler variables, low EF (p = 0.04) was also found to be a significant independent risk factor for death. The associations of echocardiographic parameters with mortality were not explained by hemoglobin, LDH, creatinine, direct bilirubin, or transferrin. The E/A ratio remained significantly associated with mortality (< 0.001) when adjusted for age, log10 creatinine, or mean arterial pressure, either separately or simultaneously. For every 0.1-U decrease in the E/A ratio, the mortality risk increased by 25% (95% CI 10% to 43%).
Table 4.
Proportional Hazards (Cox) Regression Analysis of Mortality
| Risk Factor | Total* | No. of Deaths | p† | Risk Ratio (RR)‡ | 95% CI for RR |
|---|---|---|---|---|---|
| MV E/A ratio (negative§) | 222 | 18 | <0.001 | 4.8 | (1.9–12.1) |
| TR jet velocity ≥2.5 m/s | 232 | 20 | <0.001 | 5.1 | (2.0–13.3) |
| MV peak E (negative§) | 222 | 18 | 0.002 | 3.1 | (1.5–6.8) |
| Deceleration time | 220 | 18 | 0.002 | 2.4 | (1.4–4.1) |
| Septal Em/Am (negative§) | 141 | 7 | 0.026 | 4.8 | (1.0–22.9) |
| Ejection fraction (negative§) | 230 | 20 | 0.036 | 2.0 | (1.0–3.8) |
| Septal peak Am | 141 | 7 | 0.049 | 2.8 | (1.1–7.5) |
| MV E/A ratio (negative§), adjusted for TR jet velocity (<2.5 or ≥2.5 m/s) | 222 | 18 | <0.001 | 3.5 | (1.5–8.4) |
| TR jet velocity ≥2.5 m/s, adjusted for MV E/A ratio | 222 | 18 | 0.014 | 3.4 | (1.2–9.4) |
| MV E/A ratio (negative§) and TR jet velocity ≥2.5 m/s simultaneously | 222 | 18 | <0.001 | 12.0 | (3.8–38.1) |
Totals differ because of missing values for some variables. Not included are three subjects with no mortality follow-up.
Likelihood ratio test. For pairs of variables shown, p < 0.05 for both variables.
For TR jet velocity, RR is given for values values ≥2.5 (coded 1) relative to values <2.5 (coded 0). For all other variables, RR is given for 75th relative to 25th percentile, calculated as ecoefficient × (75th percentile − 25th percentile). For negative associations, 25th and 75th percentiles are reversed.
“Negative” indicates a continuous variable for which lower values are associated with higher risk of death.
CI = confidence interval; other abbreviations as in Table 3.
Importantly, when a high TR jet velocity and a low E/A ratio were present simultaneously, mortality increased significantly to an observed RR of 12.0 (95% CI 3.8 to 38.1). Consistent with the proportional hazard regression analysis, Kaplan-Meier survival curves show a significantly increased mortality risk in patients having one or both of these risk factors, compared with patients classified as lower risk based on a TR jet velocity of <2.5 m/s or an E/A ratio of ≥1.0 (Fig. 2).
Figure 2. Kaplan-Meier Survival Curve According to Both TR Jet Velocity and E/A Ratio.
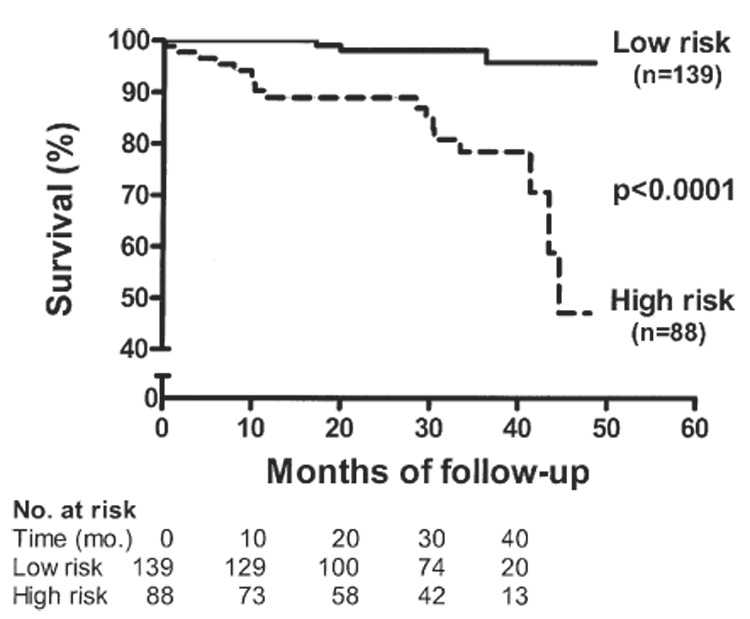
Patients were classified as low risk if they had a tricuspid regurgitation (TR) jet velocity of <2.5 m/s and an E/A ratio of ≥1.0. The high-risk group of patients had either a TR velocity of ≥2.5 m/s or an E/A ratio of <1.0 or both. Mortality was significantly increased in the group having one or both risk factors (p < 0.0001).
Clinical correlates of diastolic parameters
There was a significant correlation between the E/A ratio and blood pressure (BP) (Spearman ρ = −0.29, p < 0.0001 for systolic BP and ρ = −0.33, p < 0.001 for diastolic BP). Patients with an E/A ratio of <1 had significantly higher systolic and diastolic BP values compared with patients with an E/A ratio ≥1 (systolic BP 137 ± 22 mm Hg vs. 119 ± 16 mm Hg, p < 0.0001; diastolic BP 74 ± 12 mm Hg vs. 67 ± 11 mm Hg, p = 0.007). These patients were older, had increased wall thickness and mass, and by tissue Doppler had lower septal and lateral Em velocities and lower Em/Am ratios. There was a good correlation between the E/A ratio and creatinine level (ρ = −0.46, p < 0.0001). Although markers of hemolysis correlated significantly with chamber sizes and LVM (LDH vs. LVM/body surface area: ρ = 0.34, p < 0.0001), the association with diastolic parameters was weak (LDH vs. E/A: ρ = 0.15, p = 0.04, LDH vs. DT: ρ = 0.14, p = NS), suggesting that cardiac adaptations to anemia have more of an effect on diastolic function than direct effects of hemolysis.
Although prospective studies will be necessary to define optimal BP levels in SCD patients, our study found the 75th and 90th percentiles for systolic BP to be 131 mm Hg and 144 mm Hg, respectively. Using either of these levels as cut points, the SCD patients with lower BP values had significantly decreased wall thickness and LVM and higher E/A values, suggesting that diastolic dysfunction had not yet developed. In patients with suspected LV diastolic dysfunction (E/A <1), the pulse pressure was significantly higher than in patients with an E/A ≥1 (63 ± 19 vs. 52 ± 13, p = 0.001).
Discussion
To our knowledge, this study represents the largest echocardiographic study of cardiac function ever undertaken in patients with SCD. Our data suggest that 18% of all adults with SCD have diastolic dysfunction and 11% have both PH and LV diastolic dysfunction. We found some associations between measures of diastolic dysfunction and TR jet velocities; however, the maximum ρ² value of 0.12 suggests that <20% of the observed variability in pulmonary artery systolic pressure is secondary to diastolic dysfunction. Importantly, mild increases in pulmonary pressures and diastolic LV filling abnormalities, when present simultaneously, can predict high rates of mortality. Adjustment of TR velocity for E/A ratio in the Cox proportional hazards analysis reduces the RR from 5.1 to 3.5, and adjustment of E/A for TR reduces the RR from 4.8 to 3.4. Thus, these data strongly support the thesis that PH, measured by echocardiographic Doppler, and diastolic dysfunction develop largely independently, but can have an important additive impact on mortality.
Cardiac alterations in SCD
The hemodynamic consequences of SCD are reflective of a volume overload state and have been well described in earlier studies (12,28–32). Persistent anemia results in a need for increased cardiac output, which is achieved by dilation of all cardiac chambers with very little change in heart rate. Dilation of the LV results in increased systolic wall stress and compensatory LVH. Our study confirms findings from an earlier series of 191 SCD patients (30) and shows no significant impairment of LV or RV systolic function in the majority of patients. However, those with LV systolic dysfunction do have an increased risk for mortality. Although RV disease has been proposed as one etiology for high mortality rates in other diseases, such as idiopathic pulmonary arterial hypertension (33), resting steady-state RV parameters were included in our survival analysis of SCD patients and were not found to be useful in the risk assessment of these patients.
Prevalence and prognosis of diastolic dysfunction in SCD
We found that diastolic dysfunction is common in SCD, and our findings agree with earlier reports of Doppler filling abnormalities in SCD (28,31). Although a high E/A ratio has been associated with a worse outcome in heart failure (34) and acute myocardial infarction (35), the importance of a low E/A ratio has been recognized only recently (36). Our study extends these findings to the SCD population and confirms prior associations among age, blood pressure, and abnormal diastolic filling (37). In our study, SCD patients with an E/A ratio of <1.0 were older than the remaining patients and had significantly higher systolic and diastolic blood pressures and creatinine levels. Because E/A ratios are known to decrease with age (38), we adjusted for age as well as for mean arterial pressure and found that the association of a low E/A with mortality remains significant. Tissue Doppler indexes support the finding of mild or moderate relaxation abnormalities in the patients at highest risk based on the E/A ratio, and suggest that the majority of these patients are distinct from the group with elevated pulmonary pressures.
Mechanisms of diastolic dysfunction
Diastolic dysfunction in SCD patients may be a consequence of relative systemic hypertension or direct myocardial damage from either microvascular vaso-occlusive disease or iron overload. Pegelow et al. (39) have shown that the normal range of blood pressure is lower in healthy SCD patients compared with the general population. They and others (39–41) found that those SCD patients with blood pressure values above the expected range for SCD patients, “relative systemic hypertension,” had an increased risk of stroke and death. Our study findings suggest that patients with a low E/A ratio have higher blood pressures than the general SCD population, and the E/A ratio may be a marker for end-organ effects of relative systemic hypertension. This is supported by the finding of increased wall thickness and mass in patients with low E/A ratios. Blood pressure values that would be considered normal for the general population may be significantly elevated above the normal range for SCD patients and may contribute to increased risk in these patients through end-organ effects in the heart and kidneys.
Clinical implications
There is a growing body of results associating relative systemic hypertension with stroke, diastolic dysfunction, and increased mortality in SCD patients, suggesting the need for clinical trials to determine whether controlling blood pressure to lower SCD norms would reduce morbidity and mortality. Not surprisingly, our study found an increased prevalence of diastolic dysfunction alone in older SCD patients and PH alone in younger patients. Redfield et al. (42) have shown in a community population that even mild diastolic dysfunction carries more than a 5-fold increase in mortality risk compared with patients with normal diastolic function. Our results, therefore, support the use of echocardiographic screening with supplemental cardiac catheterization confirmation in adult SCD patients to identify high-risk individuals that may benefit from additional treatment. Further studies are warranted to determine underlying etiologies for both the diastolic dysfunction and PH.
Study limitations
The results of this study are based on mortality events in a relatively small group of patients with SCD. The ability to obtain mechanistic insights into the deaths is, therefore, limited. Although an E/A ratio of <1 identifies a high-risk group in our study, this parameter is known to be dependent on intravascular volume status and results in SCD patients may be confounded by chronic anemia and fluid overload. Although we did not perform Valsalva maneuvers or assess pulmonary venous profiles, our results are supported by the tissue Doppler finding of septal Em/Am having an RR of 4.8 for mortality. It is possible that as follow-up time increases, we may find more prognostic information from other tissue Doppler parameters. Because information regarding cause of death is not yet available in this registry, it cannot be determined whether diastolic dysfunction is a direct contributor to early death, or only a marker of overall health status in SCD. Finally, because echocardiography has known limitations in the assessment of pulmonary pressures and LV filling pressures, it is imperative that suspected PH and diastolic dysfunction by echocardiography be further evaluated by invasive hemodynamic measurements.
Conclusions
We found that diastolic dysfunction is common in the SCD population, but only contributes directly to the elevated pulmonary pressures in a small fraction of patients with a high TR jet velocity. Of all echocardiographic and Doppler parameters, the most important information on risk assessment was obtained from the standard E/A ratio in combination with the TR jet velocity. The presence of either a TR jet velocity ≥2.5 m/s and/or an E/A ratio <1.0 identifies a high-risk group with a 4-year mortality of approximately 60%. Early identification of the diastolic dysfunction with echocardiography may allow early intervention of the underlying etiology. Diastolic dysfunction and PH can develop independently, each contributing to increased mortality alone, and patients with both risk factors have a poor prognosis. The apparent contribution of relative hypertension to the development of diastolic dysfunction in SCD suggests a compelling need for trials to determine whether correcting relative hypertension in patients with SCD will attenuate the development of diastolic dysfunction and its associated mortality rate.
Acknowledgments
The authors acknowledge the imaging contributions of Cynthia L. Brenneman, RN, and Charles W. Birdsall, RDCS, the clinical contributions of Lori Hunter, RN, and James Nichols, RN, the statistical contributions of Xiuli Xu, PhD, and Mary K. Hall for invaluable protocol support. We are grateful to Drs. W. Armstrong and M. Domanski for editorial review of the manuscript.
Dr. Gladwin is supported by intramural funds from the Department of Intramural Research of the National Heart, Lung, and Blood Institute, Bethesda, Maryland, and a Collaborative Research and Development Agreement with INO Therapeutics, Clinton, New Jersey. Dr. Gladwin is named as a co-inventor on a patent application by the National Heart, Lung, and Blood Institute for the therapeutic use of nitrite.
Abbreviations and Acronyms
- BP
blood pressure
- LV
left ventricle/ventricular
- LVH
left ventricular hypertrophy
- PH
pulmonary hypertension
- RV
right ventricle/ventricular
- RWT
relative wall thickness
- SCD
sickle cell disease
- TR
tricuspid regurgitant/regurgitation
REFERENCES
- 1.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 2.Haque AK, Gokhale S, Rampy BA, Adegboyega P, Duarte A, Saldana MJ. Pulmonary hypertension in sickle cell hemoglobinopathy: a clinicopathologic study of 20 cases. Hum Pathol. 2002;33:1037–1043. doi: 10.1053/hupa.2002.128059. [DOI] [PubMed] [Google Scholar]
- 3.Manci EA, Culberson DE, Yang YM, et al. Causes of death in sickle cell disease: an autopsy study. Br J Haematol. 2003;123:359–365. doi: 10.1046/j.1365-2141.2003.04594.x. [DOI] [PubMed] [Google Scholar]
- 4.Perronne V, Roberts-Harewood M, Bachir D, et al. Patterns of mortality in sickle cell disease in adults in France and England. Hematol J. 2002;3:56–60. doi: 10.1038/sj.thj.6200147. [DOI] [PubMed] [Google Scholar]
- 5.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 6.Castro O, Hoque M, Brown BD. Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. Blood. 2003;101:1257–1261. doi: 10.1182/blood-2002-03-0948. [DOI] [PubMed] [Google Scholar]
- 7.Vichinsky EP. Pulmonary hypertension in sickle cell disease. N Engl J Med. 2004;350:857–859. doi: 10.1056/NEJMp038250. [DOI] [PubMed] [Google Scholar]
- 8.Ataga KI, Moore CG, Jones S, et al. Pulmonary hypertension in patients with sickle cell disease: a longitudinal study. Br J Haematol. 2006;134:109–115. doi: 10.1111/j.1365-2141.2006.06110.x. [DOI] [PubMed] [Google Scholar]
- 9.Darbari DS, Kple-Faget P, Kwagyan J, Rana S, Gordeuk VR, Castro O. Circumstances of death in adult sickle cell disease patients. Am J Hematol. 2006;81:858–863. doi: 10.1002/ajh.20685. [DOI] [PubMed] [Google Scholar]
- 10.Ataga KI, Jones S, Olajide O, Strayhorn D, Lail A, Orringer EP. The relationship of pulmonary hypertension and survival in sickle cell disease (abstr) Blood. 2004;104:463a. [Google Scholar]
- 11.De Castro L, Jonassaint J, Graham F, Ashley-Koch A, Telen M. Pulmonary hypertension in SS, SC, and SB thalassemia: prevalence, associated clinical syndromes, and mortality (abstr) Blood. 2004;104:462a. [Google Scholar]
- 12.Ahmed S, Siddiqui AK, Sadiq A, Shahid RK, Patel DV, Russo LA. Echocardiographic abnormalities in sickle cell disease. Am J Hematol. 2004;76:195–198. doi: 10.1002/ajh.20118. [DOI] [PubMed] [Google Scholar]
- 13.Ataga KI, Sood N, De Gent G, et al. Pulmonary hypertension in sickle cell disease. Am J Med. 2004;117:665–669. doi: 10.1016/j.amjmed.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–184. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 16.Lester SJ, Ryan EW, Schiller NB, Foster E. Best method in clinical practice and in research studies to determine left atrial size. Am J Cardiol. 1999;84:829–832. doi: 10.1016/s0002-9149(99)00446-4. [DOI] [PubMed] [Google Scholar]
- 17.Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66:493–496. doi: 10.1016/0002-9149(90)90711-9. [DOI] [PubMed] [Google Scholar]
- 18.Ommen SR, Nishimura RA, Hurrell DG, Klarich KW. Assessment of right atrial pressure with 2-dimensional and Doppler echocardiography: a simultaneous catheterization and echocardiographic study. Mayo Clin Proc. 2000;75:24–29. doi: 10.4065/75.1.24. [DOI] [PubMed] [Google Scholar]
- 19.Nagueh SF, Kopelen HA, Zoghbi WA. Relation of mean right atrial pressure to echocardiographic and Doppler parameters of right atrial and right ventricular function. Circulation. 1996;93:1160–1169. doi: 10.1161/01.cir.93.6.1160. [DOI] [PubMed] [Google Scholar]
- 20.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 21.de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH. Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol. 1995;25:1056–1062. doi: 10.1016/0735-1097(94)00540-7. [DOI] [PubMed] [Google Scholar]
- 22.Ganau A, Devereux RB, Roman MJ, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550–1558. doi: 10.1016/0735-1097(92)90617-v. [DOI] [PubMed] [Google Scholar]
- 23.Kaul S, Tei C, Hopkins JM, Shah PM. Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J. 1984;107:526–531. doi: 10.1016/0002-8703(84)90095-4. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician’s Rosetta stone. J Am Coll Cardiol. 1997;30:8–18. doi: 10.1016/s0735-1097(97)00144-7. [DOI] [PubMed] [Google Scholar]
- 25.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–1533. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 26.Benjamin EJ, Levy D, Anderson KM, et al. Determinants of Doppler indexes of left ventricular diastolic function in normal subjects (the Framingham Heart Study) Am J Cardiol. 1992;70:508–515. doi: 10.1016/0002-9149(92)91199-e. [DOI] [PubMed] [Google Scholar]
- 27.Munagala VK, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Association of newer diastolic function parameters with age in healthy subjects: a population-based study. J Am Soc Echocardiogr. 2003;16:1049–1056. doi: 10.1016/S0894-7317(03)00516-9. [DOI] [PubMed] [Google Scholar]
- 28.Balfour IC, Covitz W, Arensman FW, Eubig C, Garrido M, Jones C. Left ventricular filling in sickle cell anemia. Am J Cardiol. 1988;61:395–399. doi: 10.1016/0002-9149(88)90952-6. [DOI] [PubMed] [Google Scholar]
- 29.Braden DS, Covitz W, Milner PF. Cardiovascular function during rest and exercise in patients with sickle-cell anemia and coexisting alpha thalassemia-2. Am J Hematol. 1996;52:96–102. doi: 10.1002/(SICI)1096-8652(199606)52:2<96::AID-AJH5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 30.Covitz W, Espeland M, Gallagher D, Hellenbrand W, Leff S, Talner N. The heart in sickle cell anemia. The Cooperative Study of Sickle Cell Disease (CSSCD) Chest. 1995;108:1214–1219. doi: 10.1378/chest.108.5.1214. [DOI] [PubMed] [Google Scholar]
- 31.Lewis JF, Maron BJ, Castro O, Moosa YA. Left ventricular diastolic filling abnormalities identified by Doppler echocardiography in asymptomatic patients with sickle cell anemia. J Am Coll Cardiol. 1991;17:1473–1478. doi: 10.1016/0735-1097(91)90634-l. [DOI] [PubMed] [Google Scholar]
- 32.Lamers L, Ensing G, Pignatelli R, et al. Evaluation of left ventricular systolic function in pediatric sickle cell anemia patients using the end-systolic wall stress-velocity of circumferential fiber shortening relationship. J Am Coll Cardiol. 2006;47:2283–2288. doi: 10.1016/j.jacc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655–1665. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 34.Giannuzzi P, Temporelli PL, Bosimini E, et al. Independent and incremental prognostic value of Doppler-derived mitral deceleration time of early filling in both symptomatic and asymptomatic patients with left ventricular dysfunction. J Am Coll Cardiol. 1996;28:383–390. doi: 10.1016/0735-1097(96)00163-5. [DOI] [PubMed] [Google Scholar]
- 35.Oh JK, Ding ZP, Gersh BJ, Bailey KR, Tajik AJ. Restrictive left ventricular diastolic filling identifies patients with heart failure after acute myocardial infarction. J Am Soc Echocardiogr. 1992;5:497–503. doi: 10.1016/s0894-7317(14)80041-2. [DOI] [PubMed] [Google Scholar]
- 36.Bella JN, Palmieri V, Roman MJ, et al. Mitral ratio of peak early to late diastolic filling velocity as a predictor of mortality in middle-aged and elderly adults: the Strong Heart Study. Circulation. 2002;105:1928–1933. doi: 10.1161/01.cir.0000015076.37047.d9. [DOI] [PubMed] [Google Scholar]
- 37.Gardin JM, Arnold AM, Bild DE, et al. Left ventricular diastolic filling in the elderly: the cardiovascular health study. Am J Cardiol. 1998;82:345–351. doi: 10.1016/s0002-9149(98)00339-7. [DOI] [PubMed] [Google Scholar]
- 38.Klein AL, Burstow DJ, Tajik AJ, Zachariah PK, Bailey KR, Seward JB. Effects of age on left ventricular dimensions and filling dynamics in 117 normal persons. Mayo Clin Proc. 1994;69:212–224. doi: 10.1016/s0025-6196(12)61059-3. [DOI] [PubMed] [Google Scholar]
- 39.Pegelow CH, Colangelo L, Steinberg M, et al. Natural history of blood pressure in sickle cell disease: risks for stroke and death associated with relative hypertension in sickle cell anemia. Am J Med. 1997;102:171–177. doi: 10.1016/s0002-9343(96)00407-x. [DOI] [PubMed] [Google Scholar]
- 40.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91:288–294. [PubMed] [Google Scholar]
- 41.Rodgers GP, Walker EC, Podgor MJ. Is “relative” hypertension a risk factor for vaso-occlusive complications in sickle cell disease? Am J Med Sci. 1993;305:150–156. doi: 10.1097/00000441-199303000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]


