Abstract
Background
Despite the widespread household use of cleaning and personal hygiene products containing antibacterial ingredients, their effects on the incidence of infectious disease symptoms have not been studied.
Objective
To evaluate the effect of antibacterial cleaning and handwashing products for consumers on the occurrence of infectious disease symptoms in households.
Design
Randomized, double-blind clinical trial.
Setting
Northern Manhattan inner-city neighborhood, New York.
Participants
238 primarily Hispanic households (1178 persons) that included at least one preschool-age child.
Interventions
Households were randomly assigned to use either antibacterial or nonantibacterial products for general cleaning, laundry, and handwashing. All products were commercially available, but the packaging was blinded and the products were provided free to participants.
Measurements
Hygiene practices and infectious disease symptoms were monitored by weekly telephone calls, monthly home visits, and quarterly interviews for 48 weeks.
Results
Symptoms were primarily respiratory: During 26.2% (717 of 2736) of household-months, 23.3% (640 of 2737) of household-months, and 10.2% (278 of 2737) of household-months, one or more members of the household had a runny nose, cough, or sore throat, respectively. Fever was present during 11% (301 of 2737) of household-months, vomiting was present in 2.2% (61 of 2737), diarrhea was present in 2.5% (69 of 2737), and boils or conjunctivitis were present in 0.77% (21 of 2737). Differences between intervention and control groups were not significant for any symptoms (all unadjusted and adjusted relative risks included 1.0) or for numbers of symptoms (overall incidence density ratio, 0.96 [95% CI, 0.82 to 1.12]).
Conclusions
The tested antibacterial products did not reduce the risk for symptoms of viral infectious diseases in households that included essentially healthy persons. This does not preclude the potential contribution of these products to reducing symptoms of bacterial diseases in the home.
Changing demographic and social patterns, such as more working parents, increased numbers of meals eaten in restaurants, and more child-care outside of the home, are causing concomitant changes in patterns of infectious diseases (1). For example, recent foodborne outbreaks have resulted from widespread distribution of contaminated foods, such as meat or ice cream. Media attention to such outbreaks and the resultant public concern about disease transmission may be one reason for the burgeoning of various products that are labeled “antibacterial” and that are readily available for personal hygiene and general cleaning.
These demographic and social shifts raise the question of the relative importance of home hygienic practices in the prevention of infectious diseases. The home environment has been implicated as one important source of spread of infectious diseases (2-4), and hygienic interventions have resulted in reduced incidence, particularly in less-developed countries (5). In the United States, several studies have demonstrated the effectiveness of hygienic interventions in reducing transmission of infections in child-care centers and schools (6-9). However, despite the fact that 75% of liquid and 29% of bar soaps available in the U.S. consumer market contain antibacterial ingredients (10), their benefits in terms of reducing the incidence of infectious diseases in households have not been demonstrated. In addition, concerns have been raised about the potential for long-term use of such products to increase resistance to antiseptics or cross-resistance with antibiotics (11, 12). Therefore, we sought to evaluate the effect of antibacterial cleaning and handwashing products on the occurrence of infectious disease symptoms in households.
Methods
In this double-blind clinical trial, we randomly assigned households to one of 2 intervention groups: those who used handwashing and household cleaning products with antibacterial ingredients and those who used products without such ingredients. The interventions lasted for 48 weeks.
Sample and Setting
We conducted the study in an inner-city neighborhood in northern Manhattan, New York, with a predominantly immigrant population in multigenerational households. Almost 30% of residents spoke little or no English, and about 90% of the households had telephones (13). To qualify for the study, a household unit had to include 3 or more persons with at least one preschool-age child and had to have access to a telephone. In addition, household members had to speak English or Spanish. In a preliminary survey (14) conducted in this neighborhood, 78.5% of 398 households reported infectious disease symptoms within the previous month, and in 37.9% of these households, at least one person sought medical attention and received specific treatment or antibiotics for an infectious disease symptom or symptoms. On the basis of this pilot work, we concluded that a randomized clinical trial with sufficient statistical power was feasible.
Context
Household cleaning products containing antibacterial ingredients are widely available and popular. Although manufacturers use claims of health benefits to market these products, evidence linking the use of antibacterial products to health outcomes has been lacking.
Contribution
This innovative trial found no difference in episodes of infectious disease symptoms over one year in 228 inner-city households randomly assigned to use antibacterial household cleaning products or identically packaged products without antibacterial ingredients.
Implications
These findings highlight the need to better educate consumers about the use and limitations of household antibacterial cleaning products.
-The Editors
Recruitment was by word of mouth, referral, and English- and Spanish-language flyers (preapproved by the institutional review board) posted throughout the community. Participants were recruited by an experienced, trained interviewer who resided in the community and who was a native Spanish speaker.
We determined sample size by power analysis. With 100 households for each intervention group and a household incidence of infectious disease symptoms of about 35% per month, on the basis of the pilot study, it would be possible to detect an absolute difference between the 2 intervention groups of 20 percentage points or more (for example, from 35% to 15%) with a power of 80% and an α value of less than 0.05 (15). We recruited an additional 19% above this desired sample size to account for potential loss to follow-up and dropouts. A total of 238 households were randomly assigned, and 224 (94.1%) completed the entire 48 weeks of data collection. Fourteen households (5.9%) did not complete the entire study period, 9 (64.3%) because the household moved out of the study area, 3 (21.4%) because the household did not continue to use the products, and 2 (14.3%) because the household was inadvertently supplied with the wrong product (Figure 1).
Figure 1.
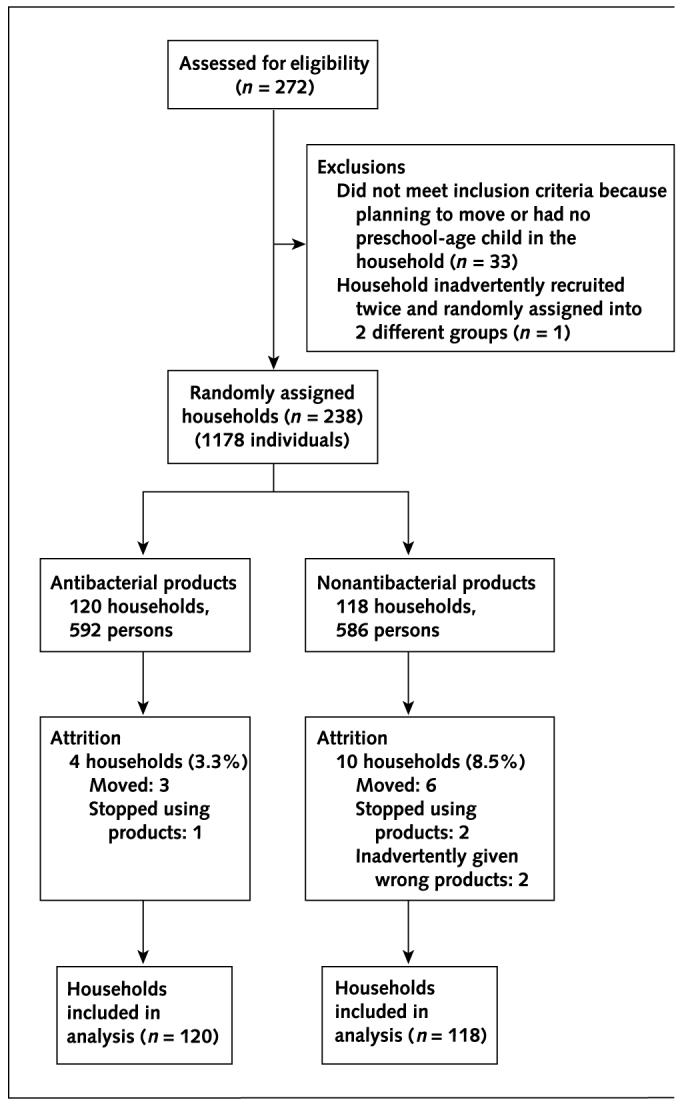
Profile of randomized, clinical trial.
Intervention
Criteria for selecting products to be tested were as follows: The products had to be readily available over the counter; have the same or similar formulation, except for the presence or absence of an antibacterial ingredient; be representative of a particular category of product so that results could be generalized to other similar products; and be developed by reputable companies known for good manufacturing practices. Antibacterial was defined as the presence of triclosan, quaternary ammonium compounds, hypochlorite, or another recognized microbicidal agent in amounts greater than preservative levels. Also, the product label had to include the term antibacterial or disinfectant.
Households randomly assigned to the antibacterial group were provided with the following: a liquid kitchen spray and “all-purpose” hard-surface cleaner containing a quaternary ammonium compound, liquid handwashing soap containing triclosan, and a laundry detergent containing oxygenated bleach. The nonantibacterial group received parallel products with similar compositions that did not contain antibacterial ingredients. Both intervention groups were provided with the same liquid dishwashing detergent and bar soap, neither of which contained antibacterial ingredients.
Procedures
The study was approved by the Columbia Presbyterian Medical Center Institutional Review Board. After we obtained written informed consent, households were randomly assigned to one of the intervention groups; the master key code was retained by the biostatistician. All products were provided without cost, were packaged identically with a generic label indicating their use, and were delivered to the household monthly. On the initial home visit, we collected baseline data on home hygiene practices and the presence of infectious disease symptoms within the previous month for each household member by using our Home Hygiene Assessment Form.
We made a weekly telephone call and a monthly home visit to each household. During the monthly visit, we assessed adherence to the product regimens by weighing the remainder of products with a postal scale and inspecting the home for the presence of other products. Every 3 months, we assessed symptoms in individual household members, and the Home Hygiene Assessment Form was readministered to determine whether any hygienic practices had changed. We conducted an average of 226 interviews each week. For most of the households (98.8%), at least 20 weekly interviews were completed, and for 89.0% of households, 45 or more weekly interviews were completed.
Data were collected by 3 interviewers who received extensive training using a written orientation manual, practice sessions with return demonstrations, and inter-rater reliability assessments. The interviewers and project director were native Spanish speakers; 3 were physicians, and the fourth was a trained community health worker. Initially and on a random monthly basis, each interviewer was accompanied by the project director on 10% of the home visits for ongoing quality control.
Instrument
Because cleaning and hygiene practices within the home would probably affect the dependent variable, infectious disease symptoms, we collected extensive data on cleaning and hygiene practices at baseline and at quarterly intervals. The Home Hygiene Assessment Form is a 31-page interview booklet that includes questions about demographic characteristics and illness (age, sex, ethnicity, country of birth, hours per week spent outside the home, type of work for adults, school or child-care arrangements for children, state of health, and presence of chronic diseases), home hygiene practices (54 items), and other relevant household factors (numbers and ages of household members, size of living space, presence of pets and visitors, type of building, and heating and cooling systems). We also asked participants about their attitudes and beliefs about how “germs” are spread and what they did to prevent infections in their home. The components of the instrument were originally derived from a literature search, focus groups of consumers, and a panel of environmental sanitation experts. The Home Hygiene Assessment Form was tested extensively for validity and reliability (16). Whenever possible, direct observations were made to confirm self-reports.
Measurement of Dependent Variable (Infectious Disease Symptoms)
The presence of infections was assessed symptomatically. We instructed participants to call their interviewer if any member of the household had vomiting, diarrhea, fever, sore throat, cough, runny nose, skin infection, or conjunctivitis (“pinkeye”). We provided each household with a supply of single-use thermometers (Tempa-DOT, 3M Health Care, St. Paul, Minnesota) and gave instructions for their use.
In the weekly telephone call from the interviewer, we also solicited information on symptoms. If participants reported a cough, they were queried by a physician about whether it could be due to allergies or asthma. The cough was recorded only when other noninfectious causes were ruled out. Sore throat was not recorded for children younger than 3 years of age. If one or more symptoms were present, the informant was asked whether medical attention was sought, whether any treatment and antibiotics were administered, and whether the symptom or symptoms resulted in missed work or school.
We assessed the reliability and validity of the self-reports of symptoms as follows. For the first 100 reports of illness, an on-call physician verified the presence of the symptom or symptoms by direct observation in a home visit. In 93 of 100 reports, the physician was able to directly confirm the presence of the reported symptom. In addition, in 3 of these 100 home visits, a symptom that had not been reported by a participant was identified by the interviewer. Thus, the sensitivity and specificity of the first 100 self-reports of symptoms were 0.93 and 0.97, respectively. We considered this an acceptable level of reliability, and for the remainder of the study, we did not continue to have on-call staff verify every symptom report; however, symptoms present at the monthly home visits were confirmed by direct observation. No treatment was provided during the study; if symptoms were judged to warrant attention, participants were referred to their primary care provider or to a local urgent-care clinic.
Statistical Analysis
Data were entered by an independent data entry firm (CDPS, Inc., Milford, Ohio). Members of the research team remained blinded to the household study group until after analyses were completed. The primary outcome of the study was the presence of at least one infectious disease symptom within the household for each one-month period. For the design of intention-to-treat analysis, all households randomly assigned to the 2 groups were included.
We performed chi-square or Student t-tests to compare the characteristics of the antibacterial and nonantibacterial groups for each hygienic practice and demographic variable, as well as the health status of household members at baseline. Unadjusted relative risks and 95% CIs were calculated for each symptom. We then performed logistic regression analysis by using the SUDAAN software program (Research Triangle Institute, Research Triangle Park, North Carolina) (17). The generalized estimating equations approach was used because it accounts for intracorrelated binary data at each time point (18-20). The estimated relative risk was generated by adjustment for potential confounders, including the number of children younger than 6 years of age, the number of people who rated their health as poor or fair or who had chronic conditions, the number of people who spent 40 or more hours outside the household per week, the size of the household, and any factors that differed substantially between the intervention groups in the univariate analyses.
To determine whether the use of antibacterial products had a cumulative effect, we also tested in separate regression models the interaction of treatment with the number of months that each group used the assigned products. In addition, we used Poisson regression models to examine the number of different symptoms reported by each household, and the incidence density ratio comparing the number of symptoms in the antibacterial and nonantibacterial groups was estimated. Finally, using chisquare analyses, we examined the effect of the intervention among persons (not at the household level) who might be at particular risk for infection—those with poor health or chronic disease, children who were 5 years of age or younger or were attending daycare, and adults working outside the household for 40 or more hours per week.
Role of the Funding Source
The funding source had no role in the design, conduct, or reporting of the study or in the decision to submit the manuscript for publication.
Results
Participant Characteristics
Initially, 238 households with 1178 members were enrolled. Over the 48 weeks, 22 households had some minor variation in members as persons entered or left; a total of 2737 household-months were studied. Most household members (51.9%) were 19 years of age or younger, 98.3% were Hispanic, and 53.2% were born outside of the United States. Most (83%) considered themselves in good health, and 12.1% had one or more chronic conditions, such as diabetes or asthma (Table 1). Most households lived in large, multiple-unit buildings (90.8% and 94.1% in the antibacterial and nonantibacterial groups, respectively). The mean number of household members was 4.97 for both groups (range, 3 to 13 members). Differences in any demographic variable between the 2 randomly assigned groups were not significant.
Table 1.
Characteristics of Household Members
| Characteristic | Antibacterial Group | Nonantibacterial Group | Total | P Value* |
|---|---|---|---|---|
| Age, n (%) | ||||
| 0-5 y | 180 (30.4) | 176 (30.0) | 356 (30.2) | >0.2 |
| 6-10 y | 72 (12.2) | 62 (10.6) | 134 (11.4) | |
| 11-19 y | 60 (10.1) | 61 (10.4) | 121 (10.3) | |
| 20-35 y | 163 (27.5) | 174 (29.7) | 337 (28.6) | |
| 36-45 y | 73 (12.3) | 66 (11.3) | 139 (11.8) | |
| 46-60 y | 32 (5.4) | 39 (6.7) | 71 (6.0) | |
| >60 y | 12 (2.0) | 8 (1.4) | 20 (1.7) | |
| Sex, n (%) | ||||
| Male | 276 (46.6) | 266 (45.5) | 542 (46.0) | >0.2 |
| Female | 316 (53.4) | 320 (54.6) | 636 (54.0) | |
| Ethnicity, n (%) | ||||
| Hispanic | 585 (98.8) | 573 (97.8) | 1158 (98.3) | 0.16 |
| African American | 5 (0.8) | 6 (1.0) | 11 (0.9) | |
| White, non-Hispanic | 0 | 5 (0.9) | 5 (0.4) | |
| Other | 2 (0.3) | 2 (0.3) | 4 (0.3) | |
| Location of birth, n (%) | ||||
| United States | 267 (45.5) | 281 (48.0) | 548 (46.8) | >0.2 |
| Outside United States | 320 (54.5) | 304 (52.0) | 624 (53.2) | |
| State of health, n (%) | ||||
| Excellent or good | 490 (84.1) | 478 (82.0) | 968 (83.0) | >0.2 |
| Fair or poor | 93 (15.9) | 105 (18.0) | 198 (17.0) | |
| Chronic condition, n (%) | ||||
| Yes | 69 (12.1) | 69 (12.1) | 138 (12.1) | >0.2 |
| No | 499 (87.9) | 501 (87.9) | 1000 (87.9) | |
| Occupations of adults†, n (%) | ||||
| Child care | 62 (20.5) | 60 (21.0) | 122 (20.7) | >0.2 |
| Homemaker | 54 (17.9) | 62 (21.7) | 116 (19.7) | |
| Food services | 29 (9.6) | 24 (8.4) | 53 (9.0) | |
| Health care | 20 (6.6) | 9 (3.1) | 29 (4.9) | |
| Other | 137 (45.4) | 131 (45.8) | 268 (45.6) |
Chi-square test comparing antibacterial and nonantibacterial groups.
Only occupations likely to increase the risk for exposure to infections were specifically reported; “other” category includes, for example, construction or office work.
Home Hygiene Practices
In most households (58.8%), one person prepared 11 or more meals per week at home. Few (2.2%) had automatic dishwashers, and 34.5% used a commercial or shared laundry facility (the remainder owned their own washing machines). The treatment groups were homogeneous; differences in any cleaning or personal hygiene practices observed or reported during the study were not significant.
Rates of Infectious Disease Symptoms
A mean of 32.7% of households (range, 25.3% to 42.2%) had one or more members with infectious disease symptoms monthly. The most common symptoms were respiratory: During 26.2% (717 of 2736) and 23.3% (640 of 2737) of household-months, household members had a runny nose or cough, respectively, and during 10.2% of household-months (278 of 2737) household members had a sore throat. Fever was present during 11% (301 of 2737) of household-months, vomiting was present in 2.2% (61 of 2737), and diarrhea was present in 2.5% (69 of 2737). Skin symptoms (boils or conjunctivitis) occurred in 0.77% (21 of 2737) of household-months.
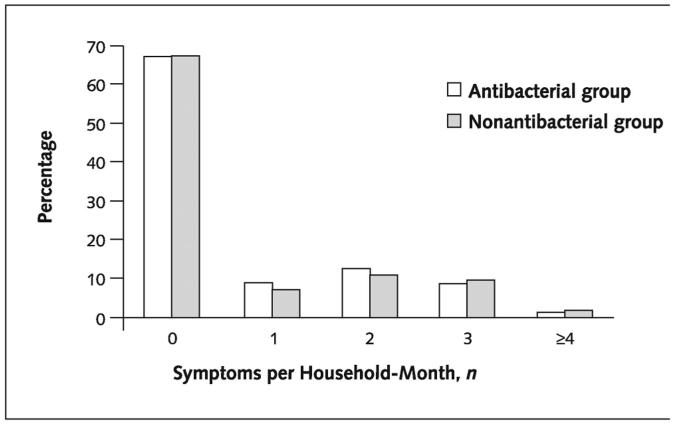
Differences between treatment groups in rates of any symptom by household-month were not statistically significant (33.1% and 32.3% in the antibacterial and nonantibacterial groups, respectively). Over the 48 weeks, both unadjusted and adjusted relative risks for each symptom showed no significant effect of antibacterial product use on infectious disease symptoms (Table 2). No interaction of treatment effect with number of months using the products was found in logistic regression analyses for any of the symptoms. Furthermore, differences in number of symptoms reported over the study period (Figure 2) were not significant between the treatment groups.
Table 2.
Rates of at Least One Infectious Disease Symptom for Each Household-Month by Group
| Symptom | Rate | Unadjusted Relative Risk (95% CI) | P Value | Adjusted Relative Risk (95% CI)* | P Value | |
|---|---|---|---|---|---|---|
| Antibacterial Group | Nonantibacterial Group | |||||
| % (n/n) | ||||||
| Vomiting | 2.2 (31/1396) | 3.0 (30/1341) | 0.75 (0.47-1.19) | >0.2 | 0.77 (0.47-1.27) | >0.2 |
| Diarrhea | 2.4 (33/1396) | 2.9 (36/1341) | 0.88 (0.55-1.41) | >0.2 | 0.90 (0.54-1.50) | >0.2 |
| Fever | 10.2 (142/1396) | 11.9 (159/1341) | 0.87 (0.70-1.08) | >0.2 | 0.84 (0.63-1.12) | >0.2 |
| Sore throat | 10.0 (140/1396) | 10.3 (138/1341) | 0.98 (0.78-1.22) | >0.2 | 0.95 (0.71-1.26) | >0.2 |
| Runny nose | 26.8 (374/1395) | 25.6 (343/1341) | 1.04 (0.91-1.18) | >0.2 | 1.03 (0.81-1.32) | >0.2 |
| Cough | 23.2 (324/1396) | 23.6 (316/1341) | 0.99 (0.86-1.14) | >0.2 | 0.97 (0.79-1.18) | >0.2 |
| Skin/conjunctivitis | 0.01 (7/1396) | 0.01 (14/1341) | 0.48 (0.20-1.19) | 0.11 | 0.46 (0.18-1.21) | 0.11 |
Generalized estimating equations for logistic regression analysis, adjusted for number of children younger than 6 years of age, number of people who rated health as poor or fair or who had chronic conditions, size of the household, and number of people spending 40 or more hours outside of the household per week.
Figure 2. Percentage of households with infectious disease symptoms for each household-month.
Poisson regression is adjusted for the number of children younger than 6 years of age, the number of people who rated their health as poor or fair or who had chronic conditions, the size of the household, and the number of people who spent 40 hours or more outside of the house per week. The incidence density ratio comparing the number of infectious disease symptoms in the 2 treatment groups was 0.96 (CI, 0.82 to 1.12).
The antibacterial and nonantibacterial groups did not differ significantly for any symptom among individuals or among the following subgroups: children 5 years of age or younger (P > 0.2 for all comparisons), children attending daycare (P > 0.2 for all comparisons), and persons who worked outside the home for 40 or more hours per week (P > 0.10 for all comparisons). However, persons with chronic disease or poor health in the antibacterial group were significantly more likely than those in the nonantibacterial group to have fever (11.5% and 4.4%, respectively; P = 0.01), runny nose (21.2% and 9.3%, respectively; P = 0.007), and cough (21.6% and 6.6%, respectively; P < 0.001). Among all persons, the cumulative incidence of infectious disease symptoms for the antibacterial and nonantibacterial groups was 38.0% and 32.1%, respectively (P = 0.19).
Discussion
Approximately one fourth of visits to primary care providers are associated with infections; the estimated cost is more than $120 billion yearly (21). Most of these infections are not “serious” in that they do not result in an increased mortality rate, but even minor infections create a large burden of illness. Rhinoviruses, for example, cause more illness than any other virus in all age groups (22), and upper respiratory infections account for the largest proportion of outpatient visits for infections (23). Half of the children in the United States develop infection with respiratory syncytial virus by the age of one year, and nearly 90 000 children are hospitalized yearly for this infection (24). Approximately 1 of 78 children is hospitalized with rotavirus infection by 5 years of age (25, 26), and rotavirus accounts for more than 30 000 outpatient visits yearly among young children in the United States (27). In addition, respiratory infections (32%) and gastroenteritis (38%) were the principal causes for admission of 1599 children in one British hospital (28).
Because the home environment harbors high levels of microbial contamination (29, 30) and evidence shows that microorganisms can be readily transmitted between the ambient environment and humans (31-34), one might speculate that antibacterial products could result in a microbiologically cleaner environment, which could translate to less human infection. On the other hand, the evidence to date has been primarily indirect or circumstantial.
Relationship between Personal Hygienic Practices and Risk for Infection
A large body of evidence shows a causal link between handwashing and risk for infection (5, 35), but most of the evidence is from developing countries, multifactorial intervention studies, or hospitals. Hands are the primary source of the spread of various viruses that cause upper respiratory infections (36-38). Although several studies in group settings showed a reduced risk for infectious disease transmission when hand hygiene interventions were implemented (6, 8, 9, 39), data on the relationship between personal hygiene practices in the home and the transmission of disease are lacking (40).
In 11 studies reviewed by Keswick and colleagues (41), antibacterial soaps were associated with a significant reduction in rates of superficial cutaneous infections. In one double-blind trial of 50 patients with atopic dermatitis, those who bathed with an antibacterial soap had greater improvement in skin lesions and reduced colonization with Staphylococcus aureus compared with those using a plain soap (42).
Studies conducted several decades ago demonstrated that showering and bathing with nonantibacterial soap increased dispersal of skin bacteria into the air and ambient environment (43, 44). Skin microflora vary among persons but are remarkably consistent for each person over time. Even when a person does not bathe for many days, the flora reach a stable equilibrium (45, 46). In a recent study, 140 newborns were randomly assigned to bathing with water only or with mild soap. Differences in the types or numbers of flora before bathing, 1 hour after bathing, and 24 hours after birth were not significant, and soap had minimal effect on subsequent colonization (47). The authors concluded that there was little justification for using soap to wash newborns. Thus, while the role of personal hygiene in reducing infections has been demonstrated in specific groups (for example, persons with dermatologic problems, hospitalized patients, or persons in developing countries with suboptimal hygiene or public services), there is a paucity of data to demonstrate an advantage of antibacterial soaps for the general, healthy public.
Relationship between Antibacterial Environmental Cleaning and Risk for Infection
Although disinfectants, such as phenolic compounds or bleach, are effective (48-50), the resulting protection is relatively brief (51). In one study comparing ammonia, baking soda, borax, vinegar, a liquid dishwashing detergent, and bleach, only bleach was effective against S. aureus, Salmonella typhi, and Escherichia coli (52). Absenteeism and respiratory infections were reduced with a comprehensive infection control program that included environmental disinfection in a specialized preschool (6), and upper respiratory infections were reduced in 8 extended-care facilities with a similar intervention (53). However, we could find no definitive evidence that use of antibacterial products for environmental cleaning reduced the risk for infections in the home or hospital setting, despite the fact that some investigators have found that use of detergent alone may actually seed the environment with more microorganisms (54-56).
Any potential benefit of using antibacterial products for home hygiene must be weighed against the theoretical risk for antiseptic or antibiotic resistance. While there is no evidence that this has or will occur with the use of products containing small amounts of triclosan, in vitro tests have confirmed that some biological mechanisms allow such cross-resistance to occur in specific organisms (57).
Additional Considerations
One of the advantages of community-based studies is the opportunity to track patterns of infection. To our knowledge, even though antibacterial cleaning and handwashing products are used extensively in U.S. homes (10, 58), this is the first randomized, double-blind clinical trial to examine the effects of these products in the home. Although microbiological cultures were not obtained during the study, cultures would probably have not provided sufficient confirmatory evidence because confirmation of viral infection would have required serial serologic tests to follow antibody levels. In addition, the multiple serotypes of some common viruses, such as rhinovirus, would have made interpretation difficult. Our primary concern was measuring the public health effect rather than determining causative agents, and we therefore chose to assess infections symptomatically because symptoms measured the burden of illness on the household.
The occurrence of infectious disease symptoms in our study was slightly lower than that in the pilot study, probably because of a Hawthorne effect (that is, households knew that their cleaning practices were being studied) or because the products were provided at no charge. These factors would probably increase the frequency and thoroughness of cleaning. Although this would reduce the overall incidence of infections, it would probably have no effect on the relative differences between the antibacterial and nonantibacterial groups. The statistical power of this study was essentially the same as that estimated in the original power calculation because the a priori calculation was based on an anticipated sample size of 100 and the final sample in this study was 18% larger than anticipated. On the basis of an a posteriori calculation, we could detect a 15% or greater difference in rates of symptoms between the treatment groups with a power of 80% and a 2-sided P value of 0.05 or less (PASS, version 2002, NCSS, Kaysville, Utah). The maximum observed difference in our study was less than 1% between treatment groups, which is probably clinically insignificant.
The types of infections most likely to be influenced by environmental cleaning (for example, gastrointestinal disease) may be bacterial in origin (59). Because gastrointestinal symptoms were not as common as respiratory symptoms in our study, it is possible that the interventions chosen for our study may be better suited for limiting nonrespiratory symptoms. It is likely that the respiratory symptoms most common in our study were of viral origin. Gubareva and colleagues (60) recently demonstrated that influenza transmission in families is generally the result of secondary transmission and is not from other community sources. Although the active ingredients in the products that we selected for the study may have some antiviral efficacy, none were designed or claimed to deliver antiviral efficacy under expected patterns and concentrations of use. We cannot explain why some infectious disease symptoms were significantly more common in persons with poor health or chronic disease who used antibacterial products than in persons not using such products, but this observation warrants additional research.
Study Limitations
Our study had several limitations. Because it was conducted in a crowded urban setting with primarily Hispanic households, the results may not be generalizable to suburban households with smaller family sizes. A decrease in household size could reduce the bioburden below a threshold at which use of antibacterial-containing consumer products could become effective. One would expect, therefore, that if an effect of antibacterial products were present, it would be more readily detected in the larger households participating in this study. In addition, the proportion of respondents who reported that they were in fair or poor health in this study (17%) was slightly higher than the national self-assessment data for Hispanic persons from 1997 to 2000 (61).
Although verbal and written instructions were provided, we cannot guarantee that participants actually used the products as directed. The weekly telephone calls and monthly visits to households as well as the provision of free products probably increased product use, potentially biasing the study toward having fewer infectious disease symptoms in both groups because of generally increased levels of cleanliness.
Conclusions
Our findings do not support the conclusion that use of antibacterial products reduces the risk for primarily viral infections in households of healthy persons. However, this does not preclude their potential contribution in reducing bacterial symptoms or their potential usefulness in specific instances, such as when household members are immuno-suppressed or have skin or gastrointestinal infections. This suggests that manufacturers and care providers need to educate consumers about the appropriate use and limitations of household antibacterial products. Additional research is indicated to better understand potential health benefits associated with increased use of cleaning products, regardless of whether the products contain antibacterial ingredients.
Acknowledgments
The authors thank Ms. Delmy Miranda and Dr. Angela Lopez, who served as interviewers in the research team.
Grant Support: By the National Institute for Nursing Research, National Institutes of Health (grant 1 RO1 NR05251). Procter & Gamble, Cincinnati, Ohio, provided products packaged in a blinded fashion.
Potential Financial Conflicts of Interest: None disclosed.
References
- 1.Collins JE. Impact of changing consumer lifestyles on the emergence/reemergence of foodborne pathogens. Emerge Infect Dis. 1997;3:471–9. doi: 10.3201/eid0304.970409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dennehy PH. Transmission of rotavirus and other enteric pathogens in the home. Pediatr Infect Dis J. 2000;19:S103–5. doi: 10.1097/00006454-200010001-00003. [DOI] [PubMed] [Google Scholar]
- 3.Scott E. Relationship between cross-contamination and the transmission of foodborne pathogens in the home. Pediatr Infect Dis J. 2000;19:S111–3. doi: 10.1097/00006454-200010001-00005. [DOI] [PubMed] [Google Scholar]
- 4.Goldmann DA. Transmission of viral respiratory infections in the home. Pediatr Infect Dis J. 2000;19:S97–102. doi: 10.1097/00006454-200010001-00002. [DOI] [PubMed] [Google Scholar]
- 5.Aiello AE, Larson EL. What is the evidence for a causal link between hygiene and infections? Lancet Infect Dis. 2002;2:103–10. doi: 10.1016/s1473-3099(02)00184-6. [DOI] [PubMed] [Google Scholar]
- 6.Krilov LR, Barone SR, Mandel FS, Cusack TM, Gaber DJ, Rubino JR. Impact of an infection control program in a specialized preschool. Am J Infect Control. 1996;24:167–73. doi: 10.1016/s0196-6553(96)90008-5. [DOI] [PubMed] [Google Scholar]
- 7.Butz AM, Larson E, Fosarelli P, Yolken R. Occurrence of infectious symptoms in children in day care homes. Am J Infect Control. 1990;18:347–53. doi: 10.1016/0196-6553(90)90248-q. [DOI] [PubMed] [Google Scholar]
- 8.Guinan M, McGuckin M, Ali Y. The effect of a comprehensive handwashing program on absenteeism in elementary schools. Am J Infect Control. 2002;30:217–20. doi: 10.1067/mic.2002.120366. [DOI] [PubMed] [Google Scholar]
- 9.Fendler EJ, Ali Y, Hammond BS, Lyons MK, Kelley MB, Vowell NA. The impact of alcohol hand sanitizer use on infection rates in an extended care facility. Am J Infect Control. 2002;30:226–33. doi: 10.1067/mic.2002.120129. [DOI] [PubMed] [Google Scholar]
- 10.Perencevich EN, Wong MT, Harris AD. National and regional assessment of the antibacterial soap market: a step toward determining the impact of prevalent antibacterial soaps. Am J Infect Control. 2001;29:281–3. doi: 10.1067/mic.2001.115469. [DOI] [PubMed] [Google Scholar]
- 11.Levy SB. Antibiotic and antiseptic resistance: impact on public health. Pediatr Infect Dis J. 2000;19:S120–2. doi: 10.1097/00006454-200010001-00008. [DOI] [PubMed] [Google Scholar]
- 12.Hooton TM, Levy SB. Antimicrobial resistance: a plan of action for community practice. Am Fam Physician. 2001;63:1087–98. [PubMed] [Google Scholar]
- 13.Garfield R, Broe D, Albano B. The role of academic medical centers in delivery of primary care: an urban study. Acad Med. 1995;70:405–9. doi: 10.1097/00001888-199505000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Larson E, Gomez Duarte C. Home hygiene practices and infectious disease symptoms among household members. Public Health Nurs. 2001;18:116–27. doi: 10.1046/j.1525-1446.2001.00116.x. [DOI] [PubMed] [Google Scholar]
- 15.Lachin JM. Introduction to sample size determination and power analysis for clinical trials. Control Clin Trials. 1981;2:93–113. doi: 10.1016/0197-2456(81)90001-5. [DOI] [PubMed] [Google Scholar]
- 16.Larson EL, Gomez-Duarte C, Qureshi K, Miranda D, Kain DJ, Cablish KL. How clean is the home environment?: a tool to assess home hygiene. J Community Health Nurs. 2001;18:139–50. doi: 10.1207/S15327655JCHN1803_01. [DOI] [PubMed] [Google Scholar]
- 17.Shah B, Barnwell G, Bieler G. SUDAAN User’s Manual, Release 7.5. Research Triangle Institute; Research Triangle Park, NC: 1997. [Google Scholar]
- 18.Liang KY, Zeger SL. Regression analysis for correlated data. Annu Rev Public Health. 1993;14:43–68. doi: 10.1146/annurev.pu.14.050193.000355. [DOI] [PubMed] [Google Scholar]
- 19.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–60. [PubMed] [Google Scholar]
- 20.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 21.Satcher D. Emerging infections: getting ahead of the curve. Emerg Infect Dis. 1995;1:1–6. doi: 10.3201/eid0101.950101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16:351–73. doi: 10.1093/oxfordjournals.epirev.a036158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armstrong GL, Pinner RW. Outpatient visits for infectious diseases in the United States, 1980 through 1996. Arch Intern Med. 1999;159:2531–6. doi: 10.1001/archinte.159.21.2531. [DOI] [PubMed] [Google Scholar]
- 24.Darville T, Yamauchi T. Respiratory syncytial virus. Pediatr Rev. 1998;19:55–61. doi: 10.1542/pir.19-2-55. [DOI] [PubMed] [Google Scholar]
- 25.Cunliffe NA, Kilgore PE, Bresee JS, Steele AD, Luo N, Hart CA, et al. Epidemiology of rotavirus diarrhoea in Africa: a review to assess the need for rotavirus immunization. Bull World Health Organ. 1998;76:525–37. [PMC free article] [PubMed] [Google Scholar]
- 26.Parashar UD, Holman RC, Clarke MJ, Bresee JS, Glass RI. Hospitalizations associated with rotavirus diarrhea in the United States, 1993 through 1995: surveillance based on the new ICD-9-CM rotavirus-specific diagnostic code. J Infect Dis. 1998;177:13–7. doi: 10.1086/513808. [DOI] [PubMed] [Google Scholar]
- 27.Zimmerman CM, Bresee JS, Parashar UD, Riggs TL, Holman RC, Glass RI. Cost of diarrhea-associated hospitalizations and outpatient visits in an insured population of young children in the United States. Pediatr Infect Dis J. 2001;20:14–9. doi: 10.1097/00006454-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Shears P, Wright A. Community-acquired infections among children in an urban environment: a 2-year prospective study in Liverpool, U.K. J Infect. 1995;30:173–7. doi: 10.1016/s0163-4453(95)80016-6. [DOI] [PubMed] [Google Scholar]
- 29.Bloomfield S, Scott E. A risk-assessment approach to the use of disinfectant procedures in the community. Research and Clinical Forums. 1997;19:37–47. [Google Scholar]
- 30.Bloomfield SF, Scott EA. Developing an effective policy for home hygiene: a risk-based approach. Int J Environ Health Res. 2003;13(Suppl 1):S57–66. doi: 10.1080/0960312031000102804. [DOI] [PubMed] [Google Scholar]
- 31.Rheinbaben F, Schunemann S, Gross T, Wolff MH. Transmission of viruses via contact in a household setting: experiments using bacteriophage straight phiX174 as a model virus. J Hosp Infect. 2000;46:61–6. doi: 10.1053/jhin.2000.0794. [DOI] [PubMed] [Google Scholar]
- 32.Schutze GE, Sikes JD, Stefanova R, Cave MD. The home environment and salmonellosis in children. Pediatrics. 1999;103:E1. doi: 10.1542/peds.103.1.e1. [DOI] [PubMed] [Google Scholar]
- 33.Parry SM, Salmon RL. Sporadic STEC O157 infection: secondary household transmission in Wales. Emerg Infect Dis. 1998;4:657–61. doi: 10.3201/eid0404.980419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denton M, Todd NJ, Kerr KG, Hawkey PM, Littlewood JM. Molecular epidemiology of Stenotrophomonas maltophilia isolated from clinical specimens from patients with cystic fibrosis and associated environmental samples. J Clin Microbiol. 1998;36:1953–8. doi: 10.1128/jcm.36.7.1953-1958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bryan JL, Cohran J, Larson EL. Hand washing: a ritual revisited. Crit Care Nurs Clin North Am. 1995;7:617–25. [PubMed] [Google Scholar]
- 36.Hall CB, Douglas RG., Jr. Modes of transmission of respiratory syncytial virus. J Pediatr. 1981;99:100–3. doi: 10.1016/s0022-3476(81)80969-9. [DOI] [PubMed] [Google Scholar]
- 37.Gwaltney JM, Jr, Moskalski PB, Hendley JO. Hand-to-hand transmission of rhinovirus colds. Ann Intern Med. 1978;88:463–7. doi: 10.7326/0003-4819-88-4-463. [DOI] [PubMed] [Google Scholar]
- 38.Ansari SA, Springthorpe VS, Sattar SA, Rivard S, Rahman M. Potential role of hands in the spread of respiratory viral infections: studies with human para-influenza virus 3 and rhinovirus 14. J Clin Microbiol. 1991;29:2115–9. doi: 10.1128/jcm.29.10.2115-2119.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammond B, Ali Y, Fendler E, Dolan M, Donovan S. Effect of hand sanitizer use on elementary school absenteeism. Am J Infect Control. 2000;28:340–6. doi: 10.1067/mic.2000.107276. [DOI] [PubMed] [Google Scholar]
- 40.Larson E. Skin hygiene and infection prevention: more of the same or different approaches? Clin Infect Dis. 1999;29:1287–94. doi: 10.1086/313468. [DOI] [PubMed] [Google Scholar]
- 41.Keswick B, Berge C, Bartolo R, Watson D. Antimicrobial soaps: their role in personal hygiene. In: Aly R, Beutner K, Maibach H, editors. Cutaneous Infection and Therapy. Marcel Dekker; New York: 1997. pp. 49–82. [Google Scholar]
- 42.Breneman DL, Hanifin JM, Berge CA, Keswick BH, Neumann PB. The effect of antibacterial soap with 1.5% triclocarban on Staphylococcus aureus in patients with atopic dermatitis. Cutis. 2000;66:296–300. [PubMed] [Google Scholar]
- 43.Speers R, Jr, O’Grady FW, Shooter RA, Bernard HR, Cole WR. Increased dispersal of skin bacteria into the air after shower baths: the effect of hexachlorophene. Lancet. 1966;1:1298–9. doi: 10.1016/s0140-6736(66)91203-7. [DOI] [PubMed] [Google Scholar]
- 44.Hall GS, Mackintosh CA, Hoffman PN. The dispersal of bacteria and skin scales from the body after showering and after application of a skin lotion. J Hyg (Lond) 1986;97:289–98. doi: 10.1017/s0022172400065384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Evans CA. Persistent individual differences in the bacterial flora of the skin of the forehead: numbers of propionibacteria. J Invest Dermatol. 1975;64:42–6. doi: 10.1111/1523-1747.ep12540897. [DOI] [PubMed] [Google Scholar]
- 46.Leyden JJ, McGinley KJ, Nordstrom KM, Webster GF. Skin microflora. J Invest Dermatol. 1987;88:65s–72s. doi: 10.1111/1523-1747.ep12468965. [DOI] [PubMed] [Google Scholar]
- 47.Medves JM, O’Brien B. Does bathing newborns remove potentially harmful pathogens from the skin? Birth. 2001;28:161–5. doi: 10.1046/j.1523-536x.2001.00161.x. [DOI] [PubMed] [Google Scholar]
- 48.Sattar SA, Jacobsen H, Rahman H, Cusack TM, Rubino JR. Interruption of rotavirus spread through chemical disinfection. Infect Control Hosp Epidemiol. 1994;15:751–6. doi: 10.1086/646852. [DOI] [PubMed] [Google Scholar]
- 49.Rutala WA, Barbee SL, Aguiar NC, Sobsey MD, Weber DJ. Antimicrobial activity of home disinfectants and natural products against potential human pathogens. Infect Control Hosp Epidemiol. 2000;21:33–8. doi: 10.1086/501694. [DOI] [PubMed] [Google Scholar]
- 50.Sattar SA, Jacobsen H, Springthorpe VS, Cusack TM, Rubino JR. Chemical disinfection to interrupt transfer of rhinovirus type 14 from environmental surfaces to hands. Appl Environ Microbiol. 1993;59:1579–85. doi: 10.1128/aem.59.5.1579-1585.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scott E, Bloomfield SF, Barlow CG. Evaluation of disinfectants in the domestic environment under ‘in use’ conditions. J Hyg (Lond) 1984;92:193–203. doi: 10.1017/s0022172400064214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parnes C. Efficacy of sodium hypochlorite bleach and “alternative” products in preventing transfer of bacteria to and from inanimate surfaces. Environmental Health. 1997 Jan/Feb;:14–20. [Google Scholar]
- 53.Makris AT, Morgan L, Gaber DJ, Richter A, Rubino JR. Effect of a comprehensive infection control program on the incidence of infections in long-term care facilities. Am J Infect Control. 2000;28:3–7. doi: 10.1016/s0196-6553(00)90004-x. [DOI] [PubMed] [Google Scholar]
- 54.Maki DG, Alvarado CJ, Hassemer CA, Zilz MA. Relation of the inanimate hospital environment to endemic nosocomial infection. N Engl J Med. 1982;307:1562–6. doi: 10.1056/NEJM198212163072507. [DOI] [PubMed] [Google Scholar]
- 55.Dharan S, Mourouga P, Copin P, Bessmer G, Tschanz B, Pittet D. Routine disinfection of patients’ environmental surfaces. Myth or reality? J Hosp Infect. 1999;42:113–7. doi: 10.1053/jhin.1999.0567. [DOI] [PubMed] [Google Scholar]
- 56.Josephson KL, Rubino JR, Pepper IL. Characterization and quantification of bacterial pathogens and indicator organisms in household kitchens with and without the use of a disinfectant cleaner. J Appl Microbiol. 1997;83:737–50. doi: 10.1046/j.1365-2672.1997.00308.x. [DOI] [PubMed] [Google Scholar]
- 57.Aiello AE, Larson E. Antibacterial cleaning and hygiene products as an emerging risk factor for antibiotic resistance in the community. Lancet Infect Dis. 2003;3:501–6. doi: 10.1016/s1473-3099(03)00723-0. [DOI] [PubMed] [Google Scholar]
- 58.Rosenberg S. Consumer and market use of antibacterials at home. Pediatr Infect Dis J. 2000;19(10 Suppl):114–6. doi: 10.1097/00006454-200010001-00006. [DOI] [PubMed] [Google Scholar]
- 59.Bloomfield S. Gastrointestinal disease in the domestic setting: what are the issues? J Infect. 2001;43:23–9. doi: 10.1053/jinf.2001.0848. [DOI] [PubMed] [Google Scholar]
- 60.Gubareva LV, Novikov DV, Hayden FG. Assessment of hemagglutinin sequence heterogeneity during influenza virus transmission in families. J Infect Dis. 2002;186:1575–81. doi: 10.1086/345372. [DOI] [PubMed] [Google Scholar]
- 61. A demographic and health snapshot of the U.S. Hispanic/Latino population. 2002 National Hispanic Health Leadership Summit. National Centers for Health Statistics, Centers for Disease Control and Prevention. Accessed at cdc.gov/nchs/data/hpdata2010/chcsummit.pdf on 9 January 2003.