Abstract
Dynamin I is dephosphorylated at Ser-774 and Ser-778 during synaptic vesicle endocytosis (SVE) in nerve terminals. Phosphorylation was proposed to regulate assembly of an endocytic protein complex with amphiphysin or endophilin. Instead, we found it recruits syndapin I for SVE and does not control amphiphysin or endophilin binding in rat synaptosomes. After depolarisation, syndapin exhibited a calcineurin-mediated interaction with dynamin. A phosphorylation site-mimicking peptide disrupted the dynamin-syndapin complex, not the dynamin-endophilin complex, arrested SVE and produced glutamate release fatigue after repetitive stimulation. Pseudo-phosphorylation of Ser-774 or Ser-778 inhibited syndapin binding without affecting amphiphysin recruitment. Site mutagenesis to alanine arrested SVE in cultured neurons. The effects of the sites were additive for syndapin I binding and SVE. Thus syndapin I is a central component of the endocytic protein complex for SVE via stimulus-dependent recruitment to dynamin I and plays a key role in synaptic transmission.
Keywords: Dynamin, syndapin, protein phosphorylation, endocytosis, synaptosomes, neurons
Neurons communicate via the release of neurotransmitter by exocytosis from nerve terminals. After exocytosis, synaptic vesicles (SV) are retrieved by endocytosis to accommodate multiple cycles of synaptic transmission. Synaptic vesicle endocytosis (SVE) is triggered by a coordinated calcineurin-dependent dephosphorylation of a group of at least eight proteins called the dephosphins. They are dynamin I, amphiphysin I/II, synaptojanin, epsin, eps15, AP180 and PIP kinase Iγ 1. The dephosphins are constitutively phosphorylated in nerve terminals and their collective rephosphorylation after SVE is necessary for maintaining the continuity of SV recycling and thus maintenance of synaptic transmission. To date only one dephosphin kinase has been identified, cyclin-dependent kinase 5 (Cdk5) 2. It phosphorylates dynamin I, synaptojanin I and PIP kinase Iγ in vivo 2-4 and other dephosphins such as amphiphysin I in vitro 5. Cdk5 activity is required for SVE 2, yet it remains unknown whether each phosphorylation site in these substrates is functionally important for the basic mechanism of SVE and what functional role they serve in the process.
Dynamin I is a large GTPase enzyme, the activity of which is required for vesicle fission in SVE 6. The proline-rich domain (PRD) at the C-terminus contains numerous binding motifs for src-3-homology (SH3) domains, through which it interacts with proteins such as amphiphysin I 7, endophilin I 8, and syndapin I 9. The SH3-mediated dynamin I interactions of amphiphysin and endophilin are involved in SVE 10, 11. An emerging idea is that different synaptic proteins like endophilin and amphiphysin are involved in mechanistically different modes of SVE, such as fast and slow modes 12, 13. Amphiphysin and endophilin are able to sense membrane curvature and tubulate lipid through their Bin/Amphiphysin/RVS (BAR) domain 14. Syndapin I has a related F-BAR domain that can tubulate lipids 15. Such proteins may sense the formation of endocytic vesicles, participate in vesicle formation through membrane tubulation and localise dynamin I for vesicle scission.
The dynamin I PRD is also the site for endogenous dynamin I phosphorylation at the synapse 16. Cdk5 phosphorylates Ser-774 and Ser-778 in the PRD of dynamin I in vivo 2, 17. The functional role of dynamin I phosphorylation in SVE is obscure, but is likely to regulate its interaction with SH3-domain-containing protein partners. It is widely thought that dynamin I dephosphorylation stimulates formation of protein complexes for endocytosis 10, but this has not been directly demonstrated in vivo. Two potential candidates for phosphorylation-dependent dynamin I binding partners are amphiphysin I or endophilin I 10, 18, 19. However, phosphorylation-dependent interactions have been demonstrated only with protein domains and/or with in vitro experiments and never with endogenous proteins in intact cells. Here, we show that stimulus-dependent dynamin I dephosphorylation in neurons recruits syndapin I for SVE and we have excluded both amphiphysin I 10 and endophilin I 18.
MATERIALS AND METHODS
DNA constructs
Dynamin I-GFP (rat sequence for Iaa isoform) in pEGFP-N1 was provided by Mark A. McNiven (Mayo Clinic, Minnesota) 20. The sequence encoding the dynamin Iaa-PRD (rat, amino acids 746 - 864) was amplified from this GFP-tagged dynamin Iaa with the oligonucleotides 5′-CGGCGAATTCAACACGACCACCGTCAGCACGCCC-3′ and 5′-CTGCAGAATTGCGGCCGCTTAGAGGTCGAAGGGG-3′ and then subcloned into pGEX4T-1 vector (Amersham Biosciences). Underlining indicates unique restriction sites used for subcloning the amplified cDNA. Dynamin I point mutants were generated using the QuickChange site-directed mutagenesis kit (Stratagene) and were confirmed by DNA sequencing. All GST-fusion proteins were expressed in Escherichia coli and purified using glutathione (GSH)-sepharose beads (Amersham Biosciences) according to the manufacturer's instructions.
Pull-down experiments
Total rat brain extract was prepared by homogenising brain tissue in ice-cold lysis buffer (1% Triton X-100, 150 mM NaCl, 25 mM Tris pH 7.4, 1 mM EDTA, 1 mM EGTA, 20 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride and EDTA-free Complete protease inhibitor (Roche)). The homogenate was centrifuged twice at 75,600g for 30 min at 4°C. The supernatant was pre-cleared by addition of GSH-sepharose beads for 1 h, pelleted at 50g for 5 min at 4°C, and the supernatant collected. Various GST-DynI-PRD recombinant proteins were then incubated with an equal amount of tissue lysate at 4°C for 1 h. Beads were washed extensively with ice-cold 20 mM Tris pH 7.4 containing 1 mM EGTA, eluted in 2X SDS-PAGE sample buffer, resolved on 7.5-15% gradient SDS gels and stained with colloidal Coomassie Blue. Identification of proteins was by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) 21. Some peptides were sequenced by tandem MS/MS 22.
Synaptosomes and 32Pi labelling
Crude (P2) synaptosomes were prepared from rat brain and labelled with 32Pi 16. Synaptosomes were lysed in ice-cold lysis buffer and centrifuged at 20,442g for 20 min at 4°C. Most pull-down experiments using synaptosomes were performed sequentially. First, dynamin I was isolated from the supernatant for 1 h at 4°C using GST-syndapin I, GST-endophilin I or GST-amphiphysin I, either full-length recombinant proteins or their SH3 domains alone, bound to GSH-sepharose. Secondly, GST-AmphI-SH3 domain was used in a subsequent pull-down experiment to recover any dynamin I not captured in the first pull-down. The washed beads were heated in SDS-PAGE sample buffer and proteins were resolved on SDS gels and subjected to autoradiography.
Glutamate release assay
In all SV recycling experiments synaptosomes were prepared from rat cerebral cortex by centrifugation on discontinuous percoll gradients 23. The glutamate release assay was performed as described before 24. Data is presented as Ca2+-dependent glutamate release, calculated as the difference between release in plus and minus Ca2+ solutions. Penetratin peptides (Genemed Synthesis) were preincubated with the synaptosomes for 30 min before stimulation and had no effect on Ca2+-independent release of glutamate.
SV turnover and internalisation assays
Loading of FM2-10 (Molecular Probes) into recycling synaptosomal SVs was measured as previously described 24. Data is presented as Ca2+-dependent FM2-10 unloading, which is the difference between release after loading in plus and minus Ca2+ solutions. Where indicated, synaptosomes were preincubated with penetratin peptides for 30 min prior to stimulation of FM2-10 loading. Neither peptide affected the ability of the standard pulse of KCl to evoke release of FM2-10-labelled vesicles, since parallel studies on S2 Ca2+-dependent glutamate release showed no effect. The same assay was used to monitor the effect of penetratin peptides on SV exocytosis. However, now the synaptosomes were loaded with FM2-10 in plus Ca2+ Krebs-like solution and, where indicated, were preincubated with penetratin peptides for 30 min prior to stimulation of FM2-10 unloading. Data is presented as Ca2+-dependent FM2-10 unloading. The SV internalisation assay was performed as described 25. See the Supplementary Materials and Methods for details.
Cell culture
Primary cerebellar granule neuron (CGN) cultures were prepared from 7-day old Sprague-Dawley rat pups as previously described 2. Transfections were carried out using an established calcium phosphate precipitation protocol 2. CGNs were transiently transfected with 1.5 μg of DNA between 7 - 10 days in vitro and were used 48 h later.
Fluorescence imaging and image analysis
The effect of overexpression of dynamin I-GFP constructs on SV recycling in CGNs was monitored using the styryl dye FM4-64 (Molecular Probes) as described previously 2. The extent of FM4-64 loading was quantified by normalising the fluorescence value at the start of stimulation to an arbitrary value and monitoring the total decrease on unloading with a dual KCl stimulus. The rate of FM4-64 unloading (as a measure of SV exocytosis) was estimated by determining the time taken for individual nerve terminals to lose 50% of their dye content on stimulation of unloading. These values were normalised to untransfected nerve terminals within the same field of view. SV exocytosis and recycling was also monitored using the pH-sensitive VAMP-GFP (synaptopHluorin) 26. Dynamin constructs linked to mCerulean rather than to GFP were used in this part of the study. CGNs were co-transfected with synaptopHluorin and either DynIWT-mCerulean, DynIdmAA-mCerulean or DynIdmEE-mCerulean and stimulated with 50 mM KCl for 20 sec. The evoked synaptopHluorin response was recorded at 500 nm excitation and 510 - 550 nm emission (mCerulean fluorescence was not detectable at this wavelength). Co-expression of dynamin I was confirmed by recording at 430 nm excitation. Responses were analysed by calculating the increased fluorescence of synaptopHluorin over baseline for individual nerve terminals and then normalising the maxima and minima to 1 and 0.
RESULTS
Dynamin I phosphorylation-dependent protein partners
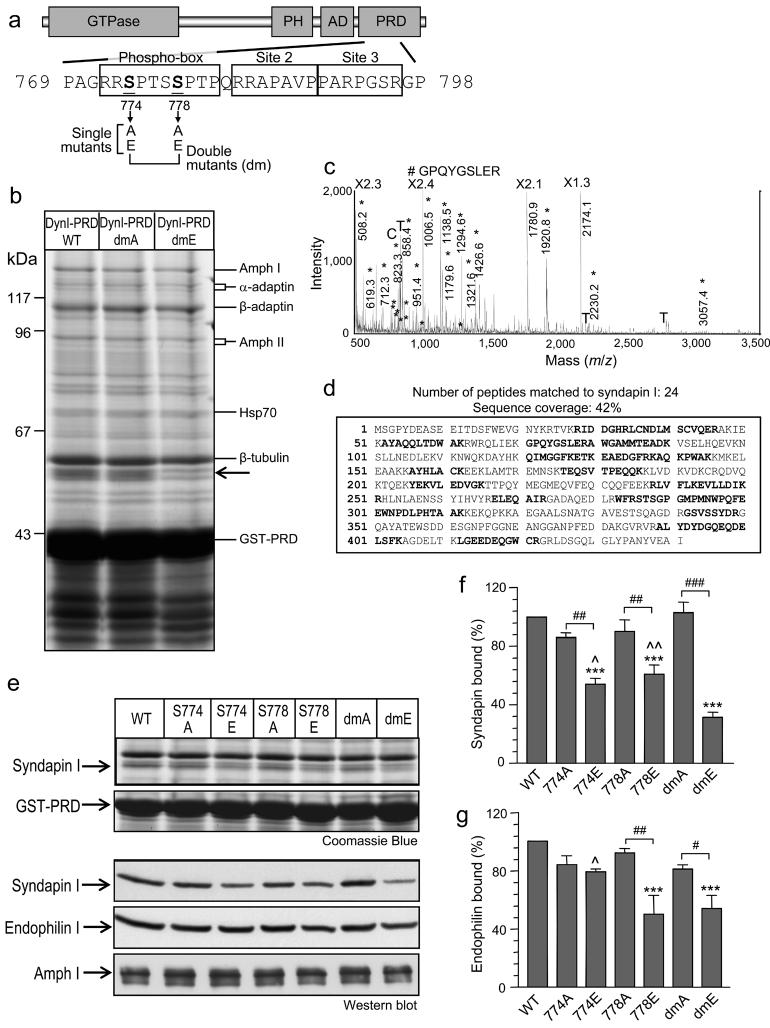
Dynamin I is phosphorylated in synaptosomes on Ser-774 and Ser-778 2, 17, and a previous report of phosphorylation at Thr-780 19 is an in vitro artefact 17 (see below). To identify proteins whose interaction with dynamin I might be regulated by these sites we generated a series of single or double point mutations (Fig. 1a). Ser to Ala mutation prevents phosphorylation while Ser to Glu mutation should mimic phosphorylation. Dynamin I PRD, either WT, dmA (non-phosphorylatable) or dmE (pseudo-phosphorylation) were expressed as GST-fusion proteins. A GST pull-down screen for binding partners revealed several known partners such as amphiphysin I / II and their binding partners, α-adaptin and β-adaptin (Fig. 1b); each being identified using MALDI-TOF MS (not shown). Pull-downs with WT and dmA or dmE DynI-PRDs were essentially the same, except the binding of one protein of 52 kDa was specifically inhibited in the DynI-PRD-dmE pull-down (Fig. 1b, arrow). It was identified as syndapin I (also known as PACSIN I) and a representative MALDI-TOF MS peptide mass map is shown (Fig. 1c). It revealed 24 matching peptides with 42% sequence coverage of syndapin I (Fig. 1d). The tryptic peptide detected at m/z of 1,006.50 was sequenced by tandem MS/MS and matched 100% to the GPQYGSLER sequence of syndapin I. The interaction of the other dynamin I binding proteins was not significantly regulated by pseudo-phosphorylation and therefore their dynamin binding was independent of syndapin. In particular, the binding of amphiphysin I or II was barely affected (Fig. 1b). This suggests Ser-774 and Ser-778 phosphorylation may inhibit the interaction of dynamin I with syndapin I, and not with amphiphysin I/II.
Fig. 1. Phosphorylation-dependent interaction of syndapin I and endophilin I with dynamin I in vitro.
(a) Dynamin I consists of four distinct domains: the GTP hydrolysis domain (GTPase), a pleckstrin homology (PH) domain, an assembly domain (AD) and a proline-rich domain (PRD) and is phosphorylated by Cdk5 at Ser-774 and Ser-778 in vivo in the “phospho-box”. Point mutations were made in phospho-box residues at the indicated positions (arrows). (b) GST-DynI-PRD either WT, dmA or dmE coupled to GSH-sepharose were used in pull-down experiments from rat brain lysates. Bound proteins were separated by SDS-PAGE and stained with Coomassie Blue. Each labelled band was identified by MALDI-TOF mass spectrometry. The major band at 52 kDa (arrow) lost binding to DynI-PRD-dmE, revealing two underlying proteins identified as non-specific binding proteins (not shown). (c) MALDI-MS peptide mass spectrum of the 52 kDa band identified it as syndapin I. The intensity of the 4 largest peaks were reduced the indicated amounts for display purposes (X2.3 etc). * = Match to syndapin I by peptide mass fingerprinting; # = The peptide at m/z 1,006.50 was sequenced by tandem MS/MS and also matched syndapin I; C = Coomassie Blue peak; T = Trypsin autolysis product. (d) MALDI-MS revealed 42% sequence coverage of syndapin I, including many peptides not found in syndapins II or III. Figures (b-d) are representative of three independent experiments. (e) Effect of individual DynI-PRD mutants on binding of syndapin I in pull-down experiments (upper panels). The same pull-down samples were also blotted with anti-syndapin I, anti-endophilin I and anti-amphiphysin I antibodies (lower panels). The amount of syndapin I (f) or endophilin I (g) bound to GST-DynI-PRD mutants was quantified by densitometry analysis of Western blots (n = 5). Data was expressed as a percent of DynI-PRD-WT ± s.e.m. One-way ANOVA was applied (***, P<0.001 against DynI-WT; #, P<0.05; ##, P<0.01; ###, P<0.001 against DynI Ala mutants; ^, P<0.05; ^ ^, P<0.01 against DynI-dmE).
To determine individual roles for the two phospho-sites, we performed DynI-PRD pull-downs with Ser-774 or Ser-778 individually mutated to Ala or Glu. Syndapin I binding was decreased with either of the pseudo-phosphorylated point mutants, but not the Ala mutations (Fig. 1e, top panel). The effect of the two sites was almost additive. These results were confirmed by Western blot analysis (Fig. 1e, bottom panel) and by quantitative densitometry analysis (Fig. 1f). Therefore both sites are involved in syndapin I binding, while the two sites appear to act coordinately for maximal effect.
Another major dynamin interacting protein is endophilin I, the SH3 domain of which shows reduced binding to Cdk5-phosphorylated dynamin I PRD in vitro 18. Since endophilin I was not detected by Coomassie Blue stain, migrating close to the GST-PRD, the pull-downs were probed with endophilin I antibodies. Endophilin I binding was significantly reduced by the S778E mutation, but not by the S774E mutation (Fig. 1e, bottom panel, and Fig. 1g). It also showed reduced binding to DynI-PRD-dmE to the same extent as S778E alone. Amphiphysin I binding was unaffected by any of the mutations (Fig. 1e). Therefore syndapin I and endophilin I are potential phosphorylation-dependent binding partners for dynamin I in vitro. However, syndapin I binding is regulated by both sites, while endophilin I is regulated by only a single site, Ser-778.
A phosphorylation-regulated dynamin I-syndapin I complex
The preceding experiments utilised a recombinant dynamin I domain to identify full-length native binding partners in tissues. The in vitro behaviour of an isolated domain with pseudo-phosphorylation instead of “real” phosphorylation might not reflect the biological function of the full-length protein. Thus we performed reverse pull-down experiments, using recombinant syndapin I or endophilin I to capture native dynamin I with its in vivo phosphorylation status preserved. The method employed sequential pull-downs, based on observations that GST-AmphI-SH3 quantitatively binds synaptosomal dynamin independently of its phosphorylation status (Fig. 2a-c, ref 2). A pull-down with AmphI-SH3 recovered considerable dynamin I protein, while a second pull-down with AmphI-SH3 recovered less than 1% residual dynamin (provided the molar ratio of GST-AmphI-SH3:dynamin exceeded 2:1; Fig. 2a). A third sequential pull-down with two different SH3 domains recovered no residual dynamin protein (Fig. 2a), nor was anything detectable by Western blots with dynamin I or phosphorylation site-specific antibodies (not shown). Therefore AmphI-SH3 domain binds essentially all dynamin I regardless of its in vivo phosphorylation status and can be used to capture residual dynamin in the following experiments.
Fig. 2. Phosphorylation-dependent interaction of syndapin I with dynamin I in vivo.
(a) Amphiphysin I SH3 domain binds all synaptosomal dynamin I. GST-AmphI-SH3 bound to GSH-sepharose was used in pull-down experiments from P2 synaptosomes lysed in Triton X-100 in the presence of 150 mM NaCl. Dynamin I (arrow, top panel) was extracted in quadruplicate. GST-AmphI-SH3 was used in the 2nd sequential pull-down (middle panel) to recover any remaining unbound dynamin I. In the 3rd pull-down GST-SdpnI-SH3 or GST-Endo1-SH3 were used to recover any remaining unbound dynamin I (bottom panel, each was in duplicate). The band migrating above the position of dynamin was a bacterial contaminant of the recombinant protein expression. All panels were from samples loaded onto the same gel. (b) Effect of in vivo dynamin I phosphorylation on binding to the SH3 domains of syndapin I or endophilin I. GST-SdpnI-SH3 and GST-EndoI-SH3 were used in pull-down experiments from P2 synaptosomes lysed in Triton X-100 in the absence or presence of 150 mM NaCl. GST-AmphI-SH3 was used sequentially in the 2nd pull-down to recover any remaining unbound dynamin I. Dynamin I was detected by Coomassie Blue staining of gels (top two panels). Lysates from the same experiment were probed with antibodies to phospho-Ser-774 and phospho-Ser-778 (bottom four panels). (c) Effect of in vivo dynamin I phosphorylation on binding to full length syndapin I or endophilin I. Rat brain P2 synaptosomes were labelled with 32Pi and lysed with Triton X-100 in the presence of 150 mM NaCl. GST-AmphI-SH3, GST-SdpnI-FL and GST-EndoI-FL (1st pull-down) and GST-AmphI-SH3 (2nd pull-down) were used in sequential pull-down experiments (in duplicate). Bound proteins were analysed by SDS-PAGE (top two panels) and phospho-dynamin I was visualised by autoradiography (lower two panels). All samples were run on the same gel. (d) Phospho-dynamin I from synaptosomes shows reduced binding to full length syndapin I but not to full length endophilin I. Quantitation of data such as in panel c from total protein levels (densitometry of the 1st pull-downs) and phospho-dynamin I levels (phosphorimager from 1st pull-down) are presented as a ratio and were normalised to the amphiphysin value. Results are the mean ± s.e.m. for n = 6. One-way ANOVA was applied (***, P<0.001 compared to amphiphysin I; ###, P<0.001 compared to endophilin I).
We examined the ability of isolated SH3 domains of syndapin I or endophilin I to bind endogenous dynamin I from lysed synaptosomes, which contain a large pool of phosphorylated dynamin I after 45 min incubation in a Krebs-like buffer at 37°C. The remaining lysate was used for a second pull-down with GST-AmphI-SH3. Surprisingly, recombinant GST-SH3 domains gave different results to DynI-PRD. GST-SdpnI-SH3 bound all detectable dynamin I, regardless of its in vivo phosphorylation status (Fig. 2b, upper panels). The presence of the endogenously phosphorylated form of dynamin I was confirmed by blots with the two phosphorylation site-specific antibodies (Fig. 2b, lower four panels). EndoI-SH3 extracted at least 95% of the synaptosomal dynamin I in the absence of salt and the residual unbound dynamin I was captured in the second pull-down by AmphI-SH3. The unbound dynamin I was highly phosphorylated, suggesting that the interaction of endophilin I with dynamin I may have a phosphorylation-regulated component. However, when pull-downs were performed in the absence of salt (conditions not reflecting the ionic strength inside cells) there may be considerable non-specific binding. Indeed, the presence of 150 mM salt strengthened EndoI-SH3 binding to phospho-dynamin I, and virtually no phosphorylation-dependent interaction was evident (Fig. 2b). Thus full-length syndapin I and endophilin I have significant differences in their binding properties to dynamin I in comparison to their SH3 domains alone and should not be used to report phosphorylation-regulated dynamin interactions.
Since the interaction of the isolated SH3 domains did not match those observed using pull-downs with dynamin I PRD, we asked whether the association of dynamin I with full-length syndapin I or endophilin I might be phosphorylation-regulated. Experiments were performed in 32Pi-labelled synaptosomes to reveal the endogenous phosphorylation status of dynamin I by autoradiography. 32Pi-labelled synaptosomal lysates were incubated with the full-length GST-fusion proteins of syndapin I (SdpnI-FL) or endophilin I (EndoI-FL) in the presence of 150 mM NaCl, followed by a second pull-down with GST-AmphI-SH3. While AmphI-SH3 domain bound almost all dynamin I and all the phosphorylated form in the first pull-down (Fig. 2c), SdpnI-FL showed considerably reduced interaction with dynamin I (Fig. 2c, top panels). SdpnI-FL bound almost exclusively to unphosphorylated dynamin I (Fig. 2c, bottom panels). In contrast, EndoI-FL strongly bound almost all dynamin I, independently of its phosphorylation status. Quantitative analysis showed that full-length syndapin I had significantly reduced binding to phospho-dynamin I relative to the total dynamin I bound to the beads (56 ± 3 % of AmphI-SH3 control), while endophilin I binding to dynamin I was clearly independent of its phosphorylation status (91 ± 4% of AmphI-SH3 control, Fig. 2d). We conclude that syndapin I is the relevant dynamin I phosphorylation-dependent protein partner for dynamin I in synaptosomes.
Since syndapin I and endophilin I utilise distinct subsets of phosphorylation sites for in vitro binding, we next attempted to further discriminate between their potential in vivo roles. We surmised that if the distribution of in vivo phosphate between Ser-774 and Ser-778 favoured the former, then syndapin I binding may be more relevant. To determine if either of the two phosphorylation sites might predominate in synaptosomes, in vivo phosphorylated dynamin I was tryptically digested and subjected to 2-D peptide mapping (see Supplementary Materials and Methods). Six related tryptic phosphopeptides migrated as a group of spots (Fig. 3a) that completely account for the total number of theoretical tryptic peptides that could be produced from digestion of 772-RRSPTSSPTPQRR-784 (which includes the phospho-box). MALDI-TOF MS and tandem MS/MS were used to identify and directly sequence the phosphopeptide within each spot (Fig. 3b). No phosphorylation on Thr was found, again suggesting that a previous report on Thr-780 was an artefact of in vitro phosphorylation studies 19. Spots A-C were derived from dynamin phosphorylated on a single site, at least 99% of which was Ser-774, while spots D-F were from the doubly phosphorylated form. Summing the total 32Pi incorporation revealed a ratio of 3:1 for doubly (774 + 778) versus singly (774 alone) phosphorylated dynamin I. Phospho-Ser-774 predominates 2:1 over Ser-778 (Fig. 3c). Therefore phospho-Ser-774 is the major phosphate present on synaptosomal dynamin I and phospho-Ser-778 virtually never exists in isolation, supporting the conclusion that the phosphorylation-regulated dynamin I binding partner is syndapin I rather than endophilin I.
Fig. 3. Phosphopeptide mapping of dynamin I from synaptosomes.

(a) Dynamin I from 32Pi-labelled synaptosomes was isolated by a pull-down with GST-AmphI-SH3, digested with trypsin and the isolated phosphopeptides were separated by 2-D tryptic maps. An autoradiograph is shown. (b) The amount of radiation associated with each spot from panel a was quantified by Storm phosphorimager (n = 2, error bars indicate range). The specific phosphopeptides that accounted for each spot were identified by MALDI-TOF MS (shown above each bar in b; peptides phosphorylated on both Ser-774 + Ser-778 are in brackets). Tandem MS/MS was used to sequence the phosphorylation site in peptides A and D 17 and B and E (not shown). Note that due to the presence of two Arg residues at each end of the phospho-box, peptides B and E have the Arg at either end (shown with a dashed line) and each presents in two forms which are chemically identical. The phosphopeptides were identified as the following dynamin I sequences with phosphorylation at Ser-774 (A-C) or Ser-774 + Ser-778 (D-F): A 774-783 + 80, m/z = 1,137.6; B 773-783 + 80 and/or 774-784 +80, m/z = 1,293.6; C 773-784 + 80, m/z = 1,449.8; D 774-783 + 160, m/z = 1,217.5; E 773-783 + 160 and/or 774-784 +160, m/z = 1,373.7; E 773-784 + 160, m/z = 1,529.6. (c) Phospho-Ser-774 predominates 2:1. The in vivo radiation distributed between Ser-774 and Ser-778 was expressed as a percent of the total 32Pi incorporated into all these phosphopeptides (63% Ser-774 and 37% Ser-778; n = 2, error bars indicating range are too small to be seen).
We next asked whether the dynamin I-syndapin I interaction is regulated by nerve terminal stimulation which activates calcineurin-dependent dynamin I dephosphorylation 27. Dynamin I and syndapin I were immunoprecipitated from either resting or KCl depolarised synaptosomes. The two proteins co-immunoprecipitated, thereby demonstrating that their interaction occurs in vivo (Fig. 4a-b). Furthermore, the interaction of syndapin I with dynamin I was increased by brief depolarization, which caused dephosphorylation of dynamin I. This suggests that a specific endocytic protein complex is formed during SVE. To confirm the role of calcineurin, synaptosomes were treated with the antagonist cyclosporin A and the increased association of syndapin I with dynamin I was abolished. To determine whether the phospho-box sequence might account for syndapin I recruitment to dynamin I we tested the ability of a DynI769-784AA peptide (Fig. 5a) to disrupt their interaction. Syndapin I or dynamin I were immunoprecipitated from nerve terminals and the individual protein complexes were incubated with DynI769-784AA peptide. Their interaction in both cases was reduced by the peptide, as indicated by the specific release of either binding partner from the bead-bound complex (Fig. 4c). No elution of endophilin I (Fig. 4c) or amphiphysin I (data not shown) was detected, highlighting the specificity of the interaction. Thus the dynamin I phospho-box sequence can compete for syndapin I binding within the endocytic complex, but not endophilin I, demonstrating the central role for the phospho-box region in mediating complex formation.
Fig. 4. Depolarization-regulated interaction of syndapin I with dynamin I in synaptosomes.
(a) A dynamin I-syndapin I complex in synaptosomes. Synaptosomes were preincubated in the presence or absence of 40 μM cyclosporin A (CysA), stimulated for 5 s (30 mM KCl), lysed, co-immunoprecipitated (Co-IP) then were blotted for dynamin I and syndapin I as indicated. Blots are representative of at least 3 independent experiments. (b) Depolarisation stimulates the formation of a calcineurin-sensitive dynamin I-syndapin I complex. The amount of dynamin I immunoprecipitated by the syndapin I antibody (as in panel a) was quantified using densitometry (n = 3, data are mean ± s.e.m.). (c) The phospho-box peptide releases syndapin I from the complex. Co-IPs were performed in synaptosome lysates using antisera against either dynamin I or syndapin I from either resting (Ctrl) or stimulated (30 mM KCl) synaptosomes. After immunoprecipitation, DynI769-784AA phospho-box peptide (2 mM) was added to protein complexes retained on the beads. Samples released from the beads by this treatment were blotted for either syndapin I (upper panel), endophilin I (middle panel) or dynamin I (lower panel). The endophilin blot included a positive control (not shown). Blots are representative of two independent experiments.
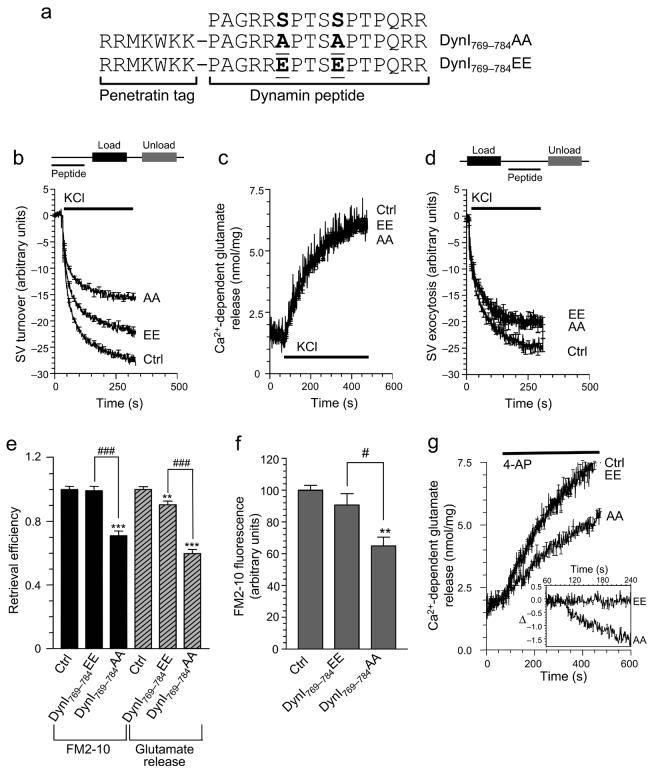
Fig. 5. Dynamin I phospho-box peptide inhibits SVE in nerve terminals.
(a) Sequences of two penetratin-linked synthetic peptides made from the sequences in the phospho-box. (b) The AA mutant blocks SV turnover. Synaptosomes were preincubated in the presence or absence of either penetratin peptide (250 μM) for 30 minutes prior to FM2-10 loading stimulated by 30 mM KCl. Accumulated FM2-10 was unloaded by stimulation with another pulse of 30 mM KCl (indicated by the solid bar). Ca2+-dependent unloading (SV turnover) is displayed for control (Ctrl) or peptide treated synaptosomes. (c) Ca2+-dependent glutamate release from control (Ctrl) or peptide-treated synaptosomes stimulated with 30 mM KCl (solid bar) is unaffected by either peptide. (d) Both peptides have small background effects on SV exocytosis. Synaptosomes were loaded with FM2-10 using a 30 mM KCl stimulus for 2 min and were then incubated for 30 min in the presence or absence of either peptide (250 μM). Unloading of dye was measured by the Ca2+-dependent fluorescence released upon subsequent stimulation with 30 mM KCl (solid bar). (b-d) n ≥ 3. (e) SVE is specifically inhibited by the AA peptide. Retrieval efficiency (SV turnover / exocytosis) is displayed using exocytosis data from either panels c or d. (f) Synaptosomes were loaded with FM2-10 as in panel b before being hypotonically lysed. SVs were purified and their fluorescence values were corrected for dye uptake in the absence of Ca2+ (Ca2+-dependent) and normalised to control (n = 3, mean ± s.e.m.). In e & f one-way ANOVA was applied (***, P<0.001; **, P<0.01 peptides compared to control: ###, P<0.001; #, P<0.05 DynI769-784AA compared to DynI769-784EE.). (g) Ca2+-dependent glutamate release from control (Ctrl) or peptide-treated synaptosomes stimulated with 4-AP (1 mM, solid bar), n = 3, using methods as described in panel e. The inset shows the same data for glutamate release for the AA or EE peptides subtracted from the control exocytosis (Δ) to emphasise the activity-dependent nature of the rundown in glutamate release. The inset is also zoomed on the first 3 minutes from 4-AP addition (which was at 60 sec) to highlight the lack of effect during the first 45 s.
SVE is inhibited by dynamin I phospho-box peptides
To determine whether the phosphorylation sites in dynamin I play a direct role in SVE we measured SVE in synaptosomes. Since isolated nerve terminals cannot be transfected with DNA, a membrane-permeable peptide strategy was employed 28. Phospho-mimetic peptides were designed corresponding to the amino acid sequence of the dynamin I phospho-box (Fig. 5a) and were tagged with a penetratin heptapeptide to facilitate their delivery into synaptosomes 28. The DynI769-784AA peptide specifically reduced the dynamin I-syndapin I interaction, but not the dynamin I-endophilin I interaction (Fig. 4c). If the phosphorylation-regulated syndapin I interaction itself is essential for SVE, the penetratin-linked peptides may inhibit the process. Both peptides significantly reduced SV turnover to differing extents (Fig. 5b). The DynI769-784AA peptide (250 μM) inhibited turnover by more than 40% (57.1 ± 2.1 % of control), compared to less than 20% with the DynI769-784EE peptide (250 μM, 81.1 ± 2.4 % of control).
A block in SV turnover cannot be ascribed to an inhibition of SVE until an effect on exocytosis has been excluded. Therefore we used two independent exocytosis assays: endogenous glutamate release and FM2-10 unloading 24. Neither DynI769-784AA nor DynI769-784EE peptide had any significant effect on KCl-evoked Ca2+-dependent glutamate release (DynI769-784AA - 92.5 ± 8.4 % of control; DynI769-784EE - 90.2 ± 9.5 % of control, Fig. 5c). Both peptides had small effects on FM2-10 unloading evoked by 30 mM KCl (DynI769-784AA - 81.1 ± 0.8 % of control; DynI769-784EE - 82.3 ± 0.6 % of control, Fig. 5d). Finally, the extent of SVE inhibition by the peptides was quantified by calculating “retrieval efficiency” which takes into account prior exocytosis; a value of less than one indicating a selective SVE inhibition 24. DynI769-784EE had little effect on retrieval efficiency using data from either exocytosis assay, whereas there was a robust inhibition with DynI769-784AA (Fig. 5e). Thus DynI769-784AA exerts a selective inhibition of SVE in synaptosomes (Fig. 5e) by disrupting the dynamin I-syndapin I interaction (Fig. 4c).
To directly demonstrate that the block of SVE by DynI769-784AA was due to an inhibition of SV retrieval from the plasma membrane, rather than a post-endocytic recycling defect, we performed an FM2-10 SV internalisation assay, based on purification of SVs after their loading and directly quantifying their dye content. If SVE was arrested before SV fission occurred, a reduction in FM2-10 content of the SVs should be apparent, while a block of recycling should produce no such reduction. DynI769-784AA inhibited FM2-10 accumulation by approximately 35% (64.9 ± 5.5 % of control), whereas DynI769-784EE had no significant effect (90.7 ± 7.1 % of control, Fig. 5f).
A selective inhibition of SVE by the phospho-box peptide should produce a rundown or fatigue of glutamate release after repeated stimuli, since the supply of SVs will progressively decline. To produce repetitive stimulation in synaptosomes we used the K+ channel blocker 4-aminopyridine (4-AP) 29, 30. This produces a tetrodotoxin-sensitive repetitive firing that mimics in vivo stimulation. We previously showed that 4-AP evokes continual SV recycling for at least 15 minutes in synaptosomes 30. The DynI769-784AA peptide produced an activity-dependent depression of release that increased with continued stimulation (Fig. 5g). Significantly, it was without effect on the first 45 sec of 4-AP evoked exocytosis (see inset in Fig. 5g), emphasising the activity-dependent nature of the rundown. Therefore, blocking syndapin I binding to dynamin I produces a functional block in SVE that induces synaptic fatigue.
Dynamin I phosphorylation sites are required for SVE
To show the key role of the phosphorylation sites by independent methods in cultured neurons we examined their role in SV turnover in nerve terminals of CGNs transfected with dynamin I-GFP. SV turnover in neurons transfected with DynIWT-GFP was indistinguishable from that in untransfected neurons (Fig. 6a-c). However the double mutants DynIdmA-GFP (Fig. 6d-f) or DynIdmE-GFP (Fig. 6g-i) produced greatly reduced loading of FM4-64. The extent of SV turnover was quantified by unloading the terminals with two sequential KCl stimuli (Fig. 6j). The total SV recycling pool was reduced by approximately 70% in CGNs transfected with DynIdmA-GFP (28 ± 3.5 % of control) and 40% with DynIdmE-GFP (64.4 ± 6.2 % of control) (Fig. 6k). However, transfection of DynIWT-GFP had no detectable effect (100.1 ± 5.2 % of control, Fig. 6k). We next examined the phosphorylation sites separately. Both Ala and Glu mutation on Ser-774 and Ser-778 reduced SV turnover by approximately 35% each (Fig. 6k). However, the inhibitory effect of DynIdmA-GFP was even greater, being additive with that of the two single mutants. This shows that both sites contribute to SV turnover. The reduction in the total SV recycling pool could be the result of a decrease in either SVE or exocytosis. To discriminate, we examined the kinetics of FM4-64 unloading after stimulation with KCl, since this is independent of the extent of dye accumulation. The unloading kinetics were not significantly different with any construct (Fig. 6l). Thus, the reduction in the total SV recycling pool was mediated by an inhibition of SVE and not by an effect on exocytosis.
Fig. 6. DynIdmA and DynIdmE inhibit SVE in cerebellar granule neurons.
(a) Primary cultures of CGNs were transfected with DynIWT-GFP (green) and stimulated uptake of FM4-64 (red) was visualized by fluorescence microscopy. Note that axons from both transfected and untransfected neurons inter-twine. (b) Monochrome FM4-64 image shows puncta representing sites of SV turnover. (c) CGNs were depolarized to unload the accumulated FM4-64. The fluorescence of all puncta was reduced. After transfection of DynIdmA-GFP (d-f) or DynIdmE-GFP (g-i) into CGNs there was considerably less accumulation of FM4-64 (e and h). Scale bar is 5 μm. (j) The extent of FM4-64 accumulation and thus of SV turnover, was quantified from the change in fluorescence (ΔF) by unloading the CGN nerve terminals with two sequential KCl stimuli (S1 and S2). Solid bars indicate the period of stimulation. (k) Both Ser-774 and Ser-778 contribute to SV turnover in an additive fashion. Quantitative analysis shows the effects of Ser-774, Ser-778 and double mutations on SV turnover (ΔS1 + ΔS2). (l) SV exocytosis is unaffected. Exocytosis was determined by examining the kinetics of FM4-64 unloading. Data (k-l) was from 3-5 independent experiments for each mutant (n ≥ 30 for each transfected and n ≥ 1,744 for untransfected synaptic puncta) and was expressed as a percent of untransfected neurons ± s.e.m. One-way ANOVA was applied (***, P<0.001 against DynIWT; ###, P<0.001 against DynIdmE).
To address whether the inhibition of FM4-64 uptake was due to a SVE arrest or to an impaired rate of SVE, we applied the FM4-64 to transfected sparse cultures of CGNs after stimulation. Sparse cultures avoided potential artefacts from intertwining of transfected and non-transfected neurons. There was no increase in dye accumulation after transfection with DynIdmA-GFP or DynIdmE-GFP relative to DynIWT-GFP (supplementary Fig. S1). Although this points towards an SVE arrest, an effect on even slower SVE rates cannot yet be ruled out.
Finally, we used an SVE assay that was independent of FM dyes to determine the significance of the dynamin I phosphorylation sites. SynaptopHluorin is a fusion construct of a pH-sensitive GFP with the integral synaptic vesicle protein synaptobrevin/VAMP, that detects vesicle fusion by an increased fluorescence and vesicle retrieval by a subsequent decrease 26. When neurons were cotransfected with synaptopHluorin and DynIWT-mCerulean, KCl evoked robust exocytosis, followed by SVE on stimulus removal (Fig. 7a). DynIdmA-mCerulean and DynIdmE-mCerulean did not affect exocytosis, but each selectively inhibited SVE (Fig. 7b-c). This provides further biological evidence that the phospho-sites specifically regulate neuronal SVE. The near complete inhibition of SVE by DynIdmA-mCerulean may suggest an SVE arrest, but a rate effect cannot be completely ruled out without additional experiments.
Fig. 7. Overexpression of DynIdmA and DynIdmE arrest SVE when assayed using synaptopHluorin.
CGN cultures were co-transfected with synaptopHluorin and either DynIWT-mCerulean (a), DynIdmE-mCerulean (b) or DynIdmA-mCerulean (c). Representative time courses of synaptopHluorin fluorescence responses evoked by 50 mM KCl are displayed, with fluorescence normalised to a maximum of 1 unit. The change in fluorescence (ΔF) normalized to initial fluorescence (F0), averaged over the first six data points, is plotted as a function of time. The bar indicates the 20 s period of KCl addition. (d) Collated data showing the amount of fluorescence remaining (i.e. synaptopHlourin remaining on the cell surface) 60 s after termination of KCl stimulation (n = 27 DynIWT-mCerulean; n = 34 DynIdmE-mCerulean and n = 12 DynIdmA-mCerulean ± s.e.m.).
DISCUSSION
Calcineurin stimulates SVE through dephosphorylation of the dephosphins 1. Dynamin I dephosphorylation was suggested by previous in vitro studies to regulate protein complex formation with amphiphysin I or endophilin I 10, 18, 19. However, we now demonstrate these effects do not occur either in vivo or with the full-length proteins, rather, their association with dynamin I appears to be constitutive in vitro (see Supplementary Discussion). We reveal that the biological significance and functional role of dynamin I phosphorylation on Ser-774 and Ser-778 is to control syndapin I recruitment for SVE. Dynamin I and syndapin I form a complex in nerve terminals, their interaction is increased by rapid depolarization and this can be reversed by treatment with calcineurin antagonists. This places syndapin I at the centre of the main endocytic machinery for SVE at the synapse.
There were a number of major physiological correlations in this study supporting our conclusion that the dynamin-syndapin interaction is essential for SVE, and ultimately for synaptic transmission. Firstly, the DynI769-784AA phospho-box peptide blocked syndapin I binding, not endophilin I, reducing its availability for recruitment by endogenous dynamin I for SVE in synaptosomes. When introduced into synaptosomes, this correlated with SVE inhibition. Secondly, as expected for a specific endocytic block, the DynI769-784AA peptide also produced a progressive rundown of glutamate release after prolonged repetitive stimulation induced by 4-AP (Fig. 5g) but not KCl (Fig. 5c). This emphasises the central role of SVE in sustaining synaptic transmission. The apparent conflict between results with 4-AP and KCl is not surprising and is due to the stimulation method. Prolonged KCl stimulation does not support efficient SV recycling at or after 300 s of stimulation in synaptosomes 30, potentially due to desensitization of Ca2+-channels, an increased prevalence of bulk endocytosis or another as yet unknown mechanism. Thirdly, when transfected into neurons DynIdmA-GFP blocked SVE in two independent types of assay - uptake of FM4-64 or retrieval of membrane-inserted synaptopHluorin. An apparent conflict between experiments in synaptosomes and cultured neurons is discussed in the Supplementary Discussion. Finally, we found that both Ser-774 and Ser-778 contributed individually to syndapin I binding in vitro, their effects being additive. When DynIS774A-GFP and DynIS778A-GFP single mutants were transfected into neurons, the resultant inhibition of SVE precisely correlated with the in vitro effects of each mutant. This reveals an essential biological role for both Ser-774 and Ser-778 in SVE and suggests that the two sites operate coordinately to control both SVE and syndapin I binding. This is the first report showing that mutation of a phosphorylation site in an endocytic protein creates a dominant-negative function in SVE. We propose that both the Ala and Glu mutations interfere with the cycle of dynamin I dephosphorylation and phosphorylation by preventing appropriate binding and release (respectively) of syndapin.
What might be the role of syndapin I in SVE? Syndapin I interacts with dynamin I as well as synaptojanin I, synapsin I and N-WASP, an activator of the Arp2/3 complex 9. It has been suggested that syndapin I may connect the actin cytoskeleton with dynamin-mediated vesicle fission 31 and dynamin binds a number of proteins that regulate actin 32-34. Unfortunately, most studies have not considered a distinction between dynamins I and II. In non-neuronal cells, there is strong evidence for an active role for actin in providing force for dynamin II-mediated endocytosis in some circumstances 35, 36. Similarly, overexpression of the SH3-domain of syndapin I inhibits receptor-mediated endocytosis in non-neuronal cells 37. However, syndapin I expression is restricted to neurons and SH3 domain over-expression may deplete other PRD-containing proteins that may be required for the process. A major role for the actin cytoskeleton in the molecular machinery of SVE is somewhat unlikely. In neurons, actin is proposed to act either in the fission and transport of SVs away from sites of endocytosis 38 or as a non-propulsive scaffolding system for regulatory molecules 39. Recently, membrane sensing and lipid tubulating properties have been identified in the N-terminal F-BAR domain of syndapin I 15, as previously reported in amphiphysin I 14 and endophilin I 40. The three proteins have the same domain architecture: BAR domain at the N-terminus and a dynamin binding SH3 domain at the C-terminus. We propose a new model for syndapin I function as a component of a major endocytic protein complex important immediately before dynamin I recruitment to the recycling SV. In this model the F-BAR domain of syndapin I induces plasma membrane curvature and/or shapes the neck of the budding vesicle, but has no ability to cut the neck. For vesicle fission, dynamin I is dephosphorylated upon depolarisation, binds the SH3 domain of syndapin I, co-encircling the vesicle neck and provides energy from GTP hydrolysis for SV fission. We favour the hypothesis that syndapin I mediates SVE via a membrane tubulating function rather than via a connection with the actin cytoskeleton, since its SH3 domain is already occupied by dynamin I at this stage of endocytosis.
The discovery that the phosphorylation-regulated partner for dynamin I in nerve terminals is syndapin I provides a key new insight into the molecular mechanisms underlying synaptic transmission. The results illustrate how endocytosis regulates exocytosis under conditions of higher or longer stimulation. Syndapin I should now be considered as a protein at the heart of the endocytic machinery in the synapse, rather than as a protein peripheral to the process. The focus should now turn to determining the distinctions between syndapin I, endophilin I and amphiphysin I in order to fully understand their individual roles when in partnership with dynamin I in different modes of endocytosis.
Supplementary Material
Acknowledgements
We thank the large number of our colleagues who have generously provided materials for this study. We particularly wish to thank J. Xue, A. Quan and G. Evans for technical advice and assistance and E. van Dam, R. Duncan, M. Shipston and P. Rowe for critical reading of the manuscript. This work was supported by grants from the National Health and Medical Research Council of Australia, the Wellcome Trust (Ref: GR070569), an Australian Bicentennial Scholarship (to VA), a University of Sydney Postgraduate Award (to VA) and a University of Edinburgh Medical Faculty Scholarship (to KJS).
Abbreviations
- SVE
synaptic vesicle endocytosis
- SH3
src-3 homology
- SV
synaptic vesicle
- PRD
proline-rich domain
- CGN
cerebellar granule neuron
- Cdk5
cyclin-dependent kinase 5
- 4-AP
4-aminopyridine
Reference List
- 1.Cousin MA, Robinson PJ. The dephosphins: Dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends Neurosci. 2001;24:659–665. doi: 10.1016/s0166-2236(00)01930-5. [DOI] [PubMed] [Google Scholar]
- 2.Tan TC, et al. Cdk5 is essential for synaptic vesicle endocytosis. Nat. Cell Biol. 2003;5:701–710. doi: 10.1038/ncb1020. [DOI] [PubMed] [Google Scholar]
- 3.Lee SY, Wenk MR, Kim Y, Nairn AC, De Camilli P. Regulation of synaptojanin 1 by cyclin-dependent kinase 5 at synapses. Proc. Natl. Acad. Sci. U. S. A. 2004;101:546–551. doi: 10.1073/pnas.0307813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SY, et al. Regulation of the interaction between PIPKIgamma and talin by proline-directed protein kinases. J. Cell Biol. 2005;168:789–799. doi: 10.1083/jcb.200409028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Floyd SR, et al. Amphiphysin binds the cdk5 regulatory subunit p35 and is phosphorylated by cdk5 and cdc2. J. Biol. Chem. 2001;276:8104–8110. doi: 10.1074/jbc.M008932200. [DOI] [PubMed] [Google Scholar]
- 6.Sweitzer SM, Hinshaw JE. Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell. 1998;93:1021–1029. doi: 10.1016/s0092-8674(00)81207-6. [DOI] [PubMed] [Google Scholar]
- 7.Grabs D, et al. The SH3 domain of amphiphysin binds the proline-rich domain of dynamin at a single site that defines a new SH3 binding consensus sequence. J. Biol. Chem. 1997;272:13419–13425. doi: 10.1074/jbc.272.20.13419. [DOI] [PubMed] [Google Scholar]
- 8.Ringstad N, Nemoto Y, De Camilli P. The SH3p4/Sh3p8/SH3p13 protein family: Binding partners for synaptojanin and dynamin via a Grb2-like Src homology 3 domain. Proc. Natl. Acad. Sci. U. S. A. 1997;94:8569–8574. doi: 10.1073/pnas.94.16.8569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qualmann B, Roos J, DiGregorio PJ, Kelly RB. Syndapin I, a synaptic dynamin-binding protein that associates with the neural Wiskott-Aldrich syndrome protein. Mol. Biol. Cell. 1999;10:501–513. doi: 10.1091/mbc.10.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slepnev VI, Ochoa GC, Butler MH, Grabs D, DeCamilli P. Role of phosphorylation in regulation of the assembly of endocytic coat complexes. Science. 1998;281:821–824. doi: 10.1126/science.281.5378.821. [DOI] [PubMed] [Google Scholar]
- 11.Gad H, et al. Fission and uncoating of synaptic clathrin-coated vesicles are perturbed by disruption of interactions with the SH3 domain of endophilin. Neuron. 2000;27:301–312. doi: 10.1016/s0896-6273(00)00038-6. [DOI] [PubMed] [Google Scholar]
- 12.Verstreken P, et al. Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron. 2003;40:733–748. doi: 10.1016/s0896-6273(03)00644-5. [DOI] [PubMed] [Google Scholar]
- 13.Jockusch WJ, Praefcke GJ, McMahon HT, Lagnado L. Clathrin-dependent and clathrin-independent retrieval of synaptic vesicles in retinal bipolar cells. Neuron. 2005;46:869–878. doi: 10.1016/j.neuron.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Peter BJ, et al. BAR domains as sensors of membrane curvature: The amphiphysin BAR structure. Science. 2004;303:495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- 15.Itoh T, et al. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev Cell. 2005;9:791–804. doi: 10.1016/j.devcel.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Robinson PJ, et al. Dynamin GTPase regulated by protein kinase C phosphorylation in nerve terminals. Nature. 1993;365:163–166. doi: 10.1038/365163a0. [DOI] [PubMed] [Google Scholar]
- 17.Larsen MR, Graham ME, Robinson PJ, Roepstorff P. Improved detection of hydrophilic phosphopeptides using graphite powder micro-columns and mass spectrometry: Evidence for in vivo doubly phosphorylated dynamin I and dynamin III. Mol Cell Proteomics. 2004;3:456–465. doi: 10.1074/mcp.M300105-MCP200. [DOI] [PubMed] [Google Scholar]
- 18.Solomaha E, Szeto FL, Yousef MA, Palfrey HC. Kinetics of SH3 domain association with the proline rich domain of dynamins: specificity, occlusion and the effects of phosphorylation. J. Biol. Chem. 2005;280:23147–23156. doi: 10.1074/jbc.M501745200. [DOI] [PubMed] [Google Scholar]
- 19.Tomizawa K, et al. Cophosphorylation of amphiphysin I and dynamin I by Cdk5 regulates clathrin-mediated endocytosis of synaptic vesicles. J. Cell Biol. 2003;163:813–824. doi: 10.1083/jcb.200308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao H, Garcia F, McNiven MA. Differential distribution of dynamin isoforms in mammalian cells. Mol. Biol. Cell. 1998;9:2595–2609. doi: 10.1091/mbc.9.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brymora A, Valova VA, Larsen MR, Roufogalis BD, Robinson PJ. The brain exocyst complex interacts with RalA in a GTP-dependent manner: Identification of a novel mammalian Sec3 gene and a second Sec15 gene. J. Biol. Chem. 2001;276:29792–29797. doi: 10.1074/jbc.C100320200. [DOI] [PubMed] [Google Scholar]
- 22.Xue J, et al. Phosphorylation of G-Septin on Ser-91 by cyclic GMP-dependent protein kinase-I in nerve terminals. Biochem. J. 2004;381:753–760. doi: 10.1042/BJ20040455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunkley PR, Jarvie PE, Heath JW, Kidd GJ, Rostas JA. A rapid method for isolation of synaptosomes on Percoll gradients. Brain Res. 1986;372:115–129. doi: 10.1016/0006-8993(86)91464-2. [DOI] [PubMed] [Google Scholar]
- 24.Cousin MA, Robinson PJ. Ca2+ inhibition of dynamin arrests synaptic vesicle recycling at the active zone. J. Neurosci. 2000;20:949–957. doi: 10.1523/JNEUROSCI.20-03-00949.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Paolo G, et al. Decreased synaptic vesicle recycling efficiency and cognitive deficits in amphiphysin 1 knockout mice. Neuron. 2002;33:789–804. doi: 10.1016/s0896-6273(02)00601-3. [DOI] [PubMed] [Google Scholar]
- 26.Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 27.Liu JP, Sim ATR, Robinson PJ. Calcineurin inhibition of dynamin I GTPase activity coupled to nerve terminal depolarization. Science. 1994;265:970–973. doi: 10.1126/science.8052858. [DOI] [PubMed] [Google Scholar]
- 28.Cousin MA, et al. Synapsin I-associated phosphatidylinositol 3-kinase mediates synaptic vesicle delivery to the readily releasable pool. J. Biol. Chem. 2003;278:29065–29071. doi: 10.1074/jbc.M302386200. [DOI] [PubMed] [Google Scholar]
- 29.Tibbs GR, Dolly JO, Nicholls DG. Evidence for the induction of repetitive action potentials in synaptosomes by K+-channel inhibitors: An analysis of plasma membrane ion fluxes. J. Neurochem. 1996;67:389–397. doi: 10.1046/j.1471-4159.1996.67010389.x. [DOI] [PubMed] [Google Scholar]
- 30.Cousin MA, Robinson PJ. Two mechanisms of synaptic vesicle recycling in rat brain nerve terminals. J. Neurochem. 2000;75:1645–1653. doi: 10.1046/j.1471-4159.2000.0751645.x. [DOI] [PubMed] [Google Scholar]
- 31.Kessels MM, Qualmann B. The syndapin protein family: linking membrane trafficking with the cytoskeleton. J. Cell Sci. 2004;117:3077–3086. doi: 10.1242/jcs.01290. [DOI] [PubMed] [Google Scholar]
- 32.Witke W, et al. In mouse brain profilin I and profilin II associate with regulators of the endocytic pathway and actin assembly. EMBO J. 1998;17:967–976. doi: 10.1093/emboj/17.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNiven MA, et al. Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J. Cell Biol. 2000;151:187–198. doi: 10.1083/jcb.151.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kessels MM, Engqvist-Goldstein AEY, Drubin DG, Qualmann B. Mammalian Abp1, a signal-responsive F-actin-binding protein, links the actin cytoskeleton to endocytosis via the GTPase dynamin. J. Cell Biol. 2001;153:351–366. doi: 10.1083/jcb.153.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orth JD, Krueger EW, Cao H, McNiven MA. The large GTPase dynamin regulates actin comet formation and movement in living cells. Proc. Natl. Acad. Sci. U. S. A. 2002;99:167–172. doi: 10.1073/pnas.012607899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee E, De Camilli P. Dynamin at actin tails. Proc. Natl. Acad. Sci. U. S. A. 2002;99:161–166. doi: 10.1073/pnas.012607799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kessels MM, Qualmann B. Syndapins integrate N-WASP in receptor-mediated endocytosis. EMBO J. 2002;21:6083–6094. doi: 10.1093/emboj/cdf604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shupliakov O, et al. Impaired recycling of synaptic vesicles after acute perturbation of the presynaptic actin cytoskeleton. Proc. Natl. Acad. Sci. U. S. A. 2002;99:14476–14481. doi: 10.1073/pnas.212381799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sankaranarayanan S, Atluri PP, Ryan TA. Actin has a molecular scaffolding, not propulsive, role in presynaptic function. Nat. Neurosci. 2003;6:127–135. doi: 10.1038/nn1002. [DOI] [PubMed] [Google Scholar]
- 40.Farsad K, et al. Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J. Cell Biol. 2001;155:193–200. doi: 10.1083/jcb.200107075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.