Abstract
Background
In patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary PCI, few data exist on the magnitude of platelet activation, aggregation and dosing of glycoprotein (GP) IIb/IIIa receptor inhibitors.
Methods
Sixty STEMI patients were randomised to abciximab, to high-dose tirofiban or to no additional GP IIb/IIIa inhibitor treatment. Platelet activation (P-selectin expression) was measured using flow cytometry and the level of inhibition of platelet aggregation was assessed using the Plateletworks assay. Additionally, the PFA-100 with the collagen/adenosine-diphosphate cartridge (CADP) was used to compare the levels of platelet inhibition. All measurements were performed at baseline (T0), immediately after (T1), 30 minutes (T2), 60 minutes (T3) and 120 minutes (T4) after primary PCI.
Results
The level of platelet activation in both GP IIb/IIIa receptor inhibitor treated groups was significantly lower compared with the control group at all time points after primary PCI (p=0.04). Also the administration of the currently recommended dose of abciximab resulted in significantly lower levels of inhibition of aggregation compared with high-dose tirofiban (p<0.0001). In addition, the CADP closure times were significantly prolonged in both GP IIb/IIIa inhibitor treated groups compared with the control group at time points T1 (p=0.006) and T4 (p<0.0001).
Conclusion
The administration of high-dose tirofiban resulted in a significantly higher inhibition of platelet aggregation compared with the currently recommended dose of abciximab. Large clinical trials are needed to assess whether this laboratory superiority of high-dose tirofiban translates into higher clinical efficacy. (Neth Heart J 2007;15:375-81.)
Keywords: myocardial infarction, platelets, IIb/IIIa receptor inhibitors, thrombosis
Platelet activation is pivotal in the pathogenesis of acute myocardial infarction (AMI) and in the occurrence of complications after percutaneous coronary interventions (PCI).1 In the Netherlands, primary PCI is the preferred reperfusion strategy in the treatment of ST-segment elevation myocardial infarction (STEMI) and recent studies in advancing the treatment of STEMI include the use of GP IIb/IIIa receptor inhibitors.2-5 Most data relate to abciximab, which has been registered for this indication. Specifically, the ADMIRAL (Abciximab before Direct angioplasty and stenting in Myocardial Infarction Regarding Acute and Long-term followup) trial showed that early administration of abciximab in patients with STEMI improves coronary patency, left ventricular function and clinical outcomes.4 However, data about the use of tirofiban in the setting of STEMI are limited. In the On-Time (ONgoing Tirofiban In Myocardial infarction Evaluation) study, tirofiban started before hospitalisation did not improve TIMI III flow compared with tirofiban started after angiography.5
After the completion of the TARGET (Tirofiban And ReoPro Give similar Efficacy outcomes Trial), several laboratory studies using optical light aggregometry have suggested that the currently used dosing regimen of tirofiban is probably too low.6-9 The TARGET dosing regimen of tirofiban inhibited 20 μM ADPinduced aggregation by only 60 to 66%.6,7 This in contrast to abciximab, as it was dosed in the TARGET trial, which inhibited 90 to 95% of aggregation.6,7,9 This difference in the extent of inhibition of platelet aggregation has been proposed as the reason why more procedure-related ischaemic events occurred among subjects receiving tirofiban in the TARGET. Higher dosing may improve outcome and this hypothesis needs to be tested in a clinical trial.
In the present study, the extent of platelet activation and aggregation were examined in 60 STEMI patients undergoing PCI who were randomised to either abciximab (currently recommended dose), tirofiban (high dose) or no additional GP IIb/IIIa receptor inhibitor treatment.
Methods
Patient population
The inclusion criteria were the presence of chest pain for more than 30 minutes, ST deviation detected on a 12-lead ECG of 0.1 mV in two or more limb leads, or 0.2 mV in two or more contiguous precordial leads and the ability to perform primary angioplasty within six hours after the start of symptoms. Patients over 80 years of age, patients who had been treated with thrombolytic therapy, warfarin or clopidogrel within the previous seven days, patients with a contraindication to GP IIb/IIIa receptor antagonists, patients with severe heart failure (Killip class III or IV) and patients with a creatinine level of >120 μmol/l were excluded. Oral informed consent was obtained in the emergency room. After PCI, written informed consent was requested. The study was conducted according to the principles of the Declaration of Helsinki and with the laws and regulations applicable in the Netherlands. The local institutional review board approved the protocol.
Primary endpoints
The primary endpoints were the level of platelet activation, the inhibition of ADP-induced platelet aggregation and the PFA-100 CADP closure times.
Study protocol
Primary PCI included coronary stent implantation and was performed according to institutional standards. Immediately before coronary angiography, all patients received a bolus of 70 IU/kg of unfractionated heparin intravenously (IV) together with oral aspirin (300 loading dose followed by 100 mg daily or Aspegic (900 mg IV)) in those who were unable to swallow and a 300 mg loading dose of clopidogrel. An 0.25 mg/kg IV bolus of abciximab (ReoPro, Centocor B.V., Leiden, the Netherlands) followed by an infusion of 0.125 μg kg-1 min-1 for 12 hours or a 25 μg kg-1 iv bolus of tirofiban (Aggrastat, Merck Sharp & Dohme B.V., Haarlem, the Netherlands) were given just after angiography, but before PCI, followed by an infusion dose of 0.15 μg kg-1 min-1 for 18 hours. A third group of STEMI patients (control group) did not receive an additional GP IIb/IIIa receptor inhibitor. The use of GP IIb/III receptor inhibitors as a ‘bail out’ modality after unsuccessful PCI was permitted. Patients were randomised using sealed envelopes that were generated at the central data-coordinating centre.
Specimen sampling
Blood samples were carefully taken from the antecubital vein through a 21-gauge needle at five different time points: T0) just before angiography (baseline), T1) immediately after, T2) 30 minutes, T3) 60 minutes and T4) 120 minutes after the PCI procedure. A total of 2.7 ml of blood was collected in a lithium heparin tube for measurement of potassium, sodium, creatinine and creatine kinase; 2.7 ml in ethylene diamine tetra acetic acid (EDTA) for haematocrit and platelet count; 2.7 ml in sodium citrate (0.106 M), placed on ice for prothrombin time (PT), international normalised ratio (INR) and thrombin-antithrombin (TAT) complex by enzyme immunoassay (Enzygnost, Dade Behring, the Netherlands); 4.5 ml into a Monovette tube (Sarsted, Germany) containing 0.5 ml of 0.129 M buffered sodium citrate (pH5.5) and kept at room temperature for immediate determination of platelet function and immunolabelling.
Whole blood flow cytometry to measure the level of platelet activation
Non-washed direct double-label immunofluorescence staining in whole blood was used. Three μl whole blood was diluted with 30 μl Isoton II (Beckman Coulter, the Netherlands) and incubated with 2 μl of appropriate concentrations of antibodies for 25 minutes in darkness. Then the mixture was fixated with 1% paraformaldehyde in Isoton II and analysed on the EPICS II flow cytometer (Beckman Coulter). The platelet population was identified from its light scatter characteristics and identity was confirmed with a fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody (MAb) to the membrane glycoprotein-Ib (CD42a, clone:SZ1, isotype: IgG2a, Beckman Coulter). A bitmap was set around the platelet population and adjusted for each sample in order to ensure that >98% of the particles analysed were positive for CD42a. Activated platelets were identified with phycoerythrin (PE)-conjugated MAbs to CD62p and CD63 (clone: CLB-Thromb/6 and CLB gran/12, both IgG1-mouse, Beckman Coulter). CD62p (P-selectin) becomes exposed on the platelet surface coincidently with α-granule secretion and CD63 during lysosomal secretion. The IgG1 mouse control MAb (clone: X40, isotype: IgG1, Becton Dickinson, the Netherlands) was used to correct for nonspecific binding of MAbs to resting and activated platelets. Ten thousand platelets were counted and activation state was expressed as the absolute number of platelets positive for a specific epitope using XL2 software after the subtraction of nonspecific binding of MAbs.
Measurement of platelet aggregation
Inhibition of ADP-induced aggregation (IPA) was assessed using the Plateletworks assay (Helena Laboratories, Beaumont, Texas). In brief, blood samples were collected in plastic tubes containing EDTA and in tubes containing PPACK with 20 μM/l adenosine diphosphate (ADP, Plateletworks tubes). A routine platelet count was performed on each sample using a routine blood cell counter (LH 750, Beckman Coulter, Krefeld, Germany). The platelet count in the EDTA tube was used as a reference. In the presence of the agonist ADP, platelets aggregate and associate. As the aggregated platelets exceed the threshold limitations for platelet size, they are no longer counted as individual platelets. The ratio between the nonaggregated platelets in the agonist sample and the platelet count in the reference tube was calculated as inhibition of aggregation (IPA).
Recently, several studies have demonstrated the usefulness of this assay in measuring the magnitude of the inhibiting effects of tirofiban as well as an excellent correlation with macroaggregation as assessed with light transmittance aggregometry.10-12
Platelet function analyser (PFA-100)
The PFA-100 analyser (Dade Behring, Germany) was used to measure platelet function, in particularadhesion and aggregation, in whole blood under high shear conditions. The time needed to form a platelet plug occluding the aperture cut into a collagen/ADP (CADP)-coated membrane was determined and reported as closure time (CT) in seconds. If the formed clot is too weak, the CT can not be measured and the results are presented as >300 seconds. The PFA-100 is a high shear system generating shear rates in the order of 4000 to 5000 sec-1. A constant vacuum of 40 mBar is maintained in the system that mimics the pressure in a microcapillary in the human body.13
Statistical analysis
To detect a 10% difference in the level of inhibition of platelet aggregation, platelet activation and in GP IIb/IIIa receptor occupancy between the control group and the GP IIb/IIIa receptor antagonist treated groups with a power of 80% and a significance (p value) of <0.05, a sample size of 20 patients in each group was calculated. Analysis was performed by repeated measures using ANOVA. The Mann-Whitney U test was used for comparison of continuous variables. Discrete variables, expressed as counts, were tested by using Fisher’s exact test. The results of continuous variables are reported as the mean value ± SD.
Results
Patients
A total of 63 consecutive STEMI patients were enrolled. Baseline characteristics are shown in table 1. No significant differences were seen between the three groups.
Table 1.
Baseline demographic, clinical and angiographic characteristics of the study groups.
| Control group (n=20) | Abciximab group (n=20) | Tirofiban group (n=20) | P value | |
|---|---|---|---|---|
| Age (years) | 61±10 | 61±10 | 59±10 | 0.23 |
| Women | 4 | 4 | 3 | 0.90 |
| Height (cm) | 175±9 | 176±9 | 177±9 | 0.59 |
| Weight (kg) | 76±12 | 83±16 | 83±12 | 0.49 |
| Active smoker | 9 | 7 | 10 | 0.62 |
| Hypercholesterolaemia | 8 | 6 | 7 | 0.86 |
| Hypertension | 7 | 8 | 7 | 0.93 |
| Diabetes | 1 | 3 | 2 | 0.57 |
| Family history of AMI | 10 | 6 | 10 | 0.34 |
| Previous AMI | 5 | 2 | 4 | 0.46 |
| Multivessel disease | 5 | 7 | 6 | 0.79 |
| Anterior infarction | 14 | 13 | 11 | 0.60 |
| ST shift (mm) | 14±5 | 15±7 | 12.7±3 | 0.47 |
| Number of stents | 1.35±0.5 | 1.1±0.3 | 1.25±0.5 | 0.18 |
| TIMI 0 before PTCA | 15 | 14 | 17 | 0.60 |
| TIMI 3 after PTCA | 19 | 19 | 20 | 0.60 |
| Present thrombus | 13 | 15 | 16 | 0.34 |
| Complex lesions | 15 | 12 | 13 | 0.73 |
| Time to PCI | 191.3±55.75 | 199.1±55.6 | 192.5±49.3 | 0.44 |
Data are presented as the absolute number of patients or mean value ± SD. AMI=acute myocardial infarction, TIMI=thrombolysis in myocardial infarction, PTCA=percutaneous transluminal coronary intervention.
Three patients dropped out; in one patient the study medication was erroneously discontinued, one patient refused to participate after two blood samples had been obtained despite giving written informed consent and one patient died during the PCI procedure due to heart failure. Consequently, the final study consisted of 60 patients. Two femoral access site bleeding complications occurred during the infusion of the study medication: one in the high-dose tirofiban group and one in the abciximab group. No other major bleeding complications were reported.
Laboratory testing
There were no significant differences between the three groups in markers of coagulation (thrombinantithrombin III (TAT), international normalised ratio (INR) and prothrombin time (PT)), cholesterol, haemoglobin and platelet count at the different time points (table 2)
Table 2.
Laboratory parameters.
| Control group(n=20) | Abciximab group(n=20) | Tirofiban group(n=20) | P value | |
|---|---|---|---|---|
| Haemoglobin at T0 | 8.75±0.76 | 8.66±0.69 | 8.63±0.68 | 0.68 |
| Haemoglobin at T1 | 7.99±0.73 | 7.87±0.85 | 7.99±0.74 | 0.39 |
| Haemoglobin at T2 | 8.47±0.75 | 8.46±0.82 | 8.29±0.63 | 0.65 |
| Haemoglobin at T3 | 8.49±0.68 | 8.48±0.73 | 8.42±0.76 | 0.34 |
| Haemoglobin at T4 | 8.50±0.66 | 8.41±0.72 | 8.39±0.97 | 0.49 |
| Platelet count at T0 | 235.8±45.7 | 264.3±97.9 | 268.2±42.3 | 0.46 |
| Platelet count at T1 | 209.9±40.8 | 227.1±96.4 | 248.4±30.0 | 0.51 |
| Platelet count at T2 | 219.5±45.0 | 228.7±102.0 | 258.7±34.7 | 0.32 |
| Platelet count at T3 | 219.9±44.0 | 232.7±88.5 | 261.8±38.9 | 0.27 |
| Platelet count at T4 | 222.9±46.5 | 246.7±93.6 | 256.9±42.3 | 0.62 |
| TAT at T0 | 6.23±15.12 | 4.90±9.23 | 5.14±7.55 | 0.32 |
| TAT at T1 | 11.06±24.68 | 13.68±27.63 | 8.88±7.21 | 0.31 |
| TAT at T2 | 3.35±3.37 | 3.75±3.63 | 5.12±4.13 | 0.31 |
| TAT at T3 | 5.18±13.37 | 3.22±2.73 | 4.70±4.03 | 0.39 |
| TAT at T4 | 2.20±1.99 | 5.62±8.39 | 6.70±7.79 | 0.33 |
| International normalised ratio at T0 | 1.11±0.099 | 1.11±0.091 | 1.11±0.097 | 0.54 |
| International normalised ratio at T4 | 1.14±0.122 | 1.13±0.071 | 1.17±0.178 | 0.17 |
| Prothrombin time at T0 | 17.08±3.90 | 20.14±10.33 | 16.49±2.09 | 0.40 |
| Prothrombin time at T4 | 15.70±0.89 | 17.32±5.49 | 16.97±2.63 | 0.27 |
| GP IIb/IIIa receptor genotype | ||||
| - A1/A1 | 15 | 14 | 13 | 0.79 |
| - A1/A2 | 5 | 5 | 6 | 0.92 |
| - A2/A2 | 0 | 1 | 1 | 0.60 |
| Cholesterol | 4.99±1.07 | 5.02±0.88 | 5.22±1.08 | 0.48 |
Data are presented as the absolute number of patients or mean value ± SD. TAT= thrombin-antithrombin, T0= pre-PCI, T1= directly after PCI, T2= 30 minutes after PCI, T3= 60 minutes after PCI, T4= 90 minutes after PCI.
The level of platelet activation
At baseline, surface expression of P-selectin was not significantly different between the three groups. Pselectin expression was maximal at the time of primary PCI and decreased rapidly during the following two hours. Compared with the control group, both GP IIb/IIIa receptor inhibitors resulted in significantly less P-selectin surface expression at time points T1 to T4 (p=0.04). There were no differences between abciximab and tirofiban in decreasing P-selectin surface expression (p=0.9) (figure 1).
Figure 1.
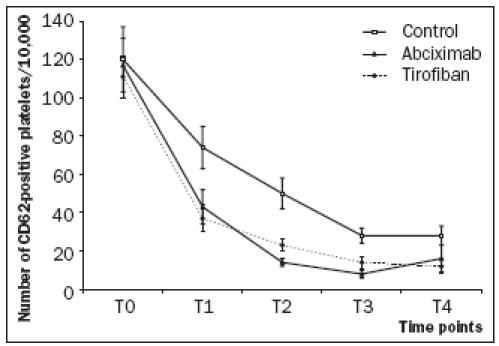
At T0 (baseline), the mean level of platelet activation was not significantly different between the three groups. Compared with the control group, both GP IIb/IIIa receptor inhibitors resulted in significantly less P-selectin surface expression at time points T1 to T4 (p=0.04). There were no differences between abciximab and tirofiban in decreasing P-selectin surface expression (p=0.9).
Inhibition of platelet aggregation
At baseline, there were no significant differences between the three groups (p=0.8). Immediately post- PCI and the time points thereafter, inhibition of aggregation was significantly higher in both GP IIb/IIIa inhibitor groups than in the control group (p<0.0001). The administration of high-dose tirofiban resulted in a mean inhibition of over 80% at all time points. With abciximab, the mean inhibition of aggregation increased over the two hours after PCI but did not reach the threshold of 80% inhibition (figure 2). Inhibition of aggregation was significantly higher in the tirofiban group as compared with the abciximab group at time points T1 to T4 (p<0.0001).
Figure 2.
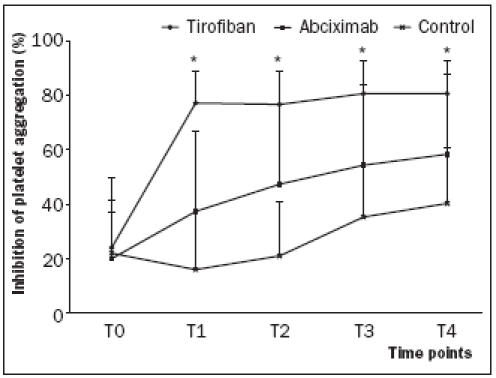
At T0 (baseline), the mean inhibition of aggregation was not significantly different between the three groups. *Immediately after administration of either abciximab or high-dose tirofiban and the time points thereafter, the inhibition of aggregation was significantly different between the three groups (p<0.0001). In each group, the inhibition of aggregation was significantly different between T0 and T4 (p<0.0001). High-dose tirofiban administration resulted in a higher inhibition compared with abciximab as measured with the Plateletworks assay.
PFA-100 COL/ADP closure times
The CADP closure times in the three different groups are presented in figure 3. The baseline closure times were not significantly different between the three groups.
Figure 3.
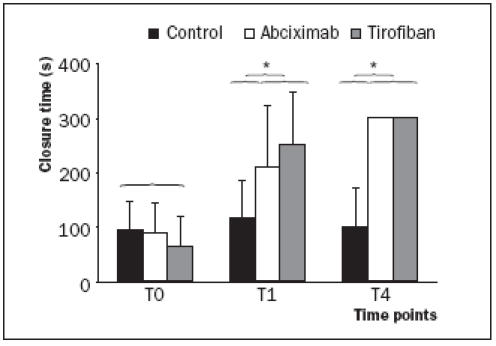
At T0 (baseline), COL/ADP induced closure times were not significantly different between the three groups. Immediately after the administration of either abciximab or high-dose tirofiban (T1), the PFA-100 CADP closure time was significantly prolonged compared with its baseline value (T0). No differences were seen in CADP closure time values between the two GP IIb/IIIa inhibitor treated groups at time points T1 and T4 (p=0.33 at T1 and p=1.0 at T4). *The CADP closure times were significantly prolonged in both GP IIb/IIIa inhibitor treated groups as compared with the control group at time points T1 (p=0.006) and T4 (p<0.0001).
The closure times significantly increased after administration of either abciximab or high-dose tirofiban. No differences were seen between the two GP IIb/IIIa inhibitor treated groups at time point T1 and T4 (p=0.333 at T1 and p=1.00 at T4). The CADP closure times were significantly prolonged in both GP IIb/IIIa inhibitor treated groups compared with the control group at time points T1 (p=0.006) and T4 (p<0.0001).
Discussion
The present study demonstrates that in STEMI patients: 1) The level of platelet activation is maximal at the time of primary PCI and decreases rapidly thereafter. 2) Both GP IIb/IIIa receptor inhibitors significantly decrease platelet activation within two hours compared with the control group. 3) ADP-induced aggregation is more effectively inhibited by high-dose tirofiban than by abciximab. 4) Immediately after the administration of both abciximab and high-dose tirofiban, COL/ADP closure times increase and exceed >300 seconds after primary PCI.
Abciximab has demonstrated benefit in optimising the clinical outcome of STEMI patients undergoing primary PCI.4 However, data about the use of tirofiban in the setting of primary PCI for STEMI do not support its use in this setting, possibly as a result of suboptimal dosing.7-9 In the present study, we used a higher dose of tirofiban than currently recommended for several reasons: first, previous tirofiban dose-finding studies used citrate as anticoagulant which leads to calcium chelation thereby overestimating the inhibitory effects of tirofiban.14,15 Second, the TARGET dosing regimen of tirofiban inhibited 20 μM ADP macroaggregation by only 60 to 66%. This in contrast to abciximab, as it was dosed in the TARGET trial, which inhibited 90 to 95% of 20 μM ADP induced platelet aggregation.6 This difference in the extent of platelet aggregation inhibition was proposed as the reason why more procedure-related ischaemic events occurred among subjects receiving tirofiban.7-9 Third, in previous studies elective patients were analysed who have less platelet activation and submaximal GP IIb/IIIa receptor expression compared with STEMI patients. Consequently, previously used doses might have been too low for patients with STEMI.
Platelet activation
It is well known that the rupture of an atherosclerotic plaque and the consequent exposure of platelets to strong agonists such as thrombin and collagen results in the activation of circulating platelets and the consequent formation of a thrombus. The present study confirms this observation: platelet activation is maximal at the time of primary PCI and decreases rapidly thereafter. Both abciximab and high-dose tirofiban led to a rapid decrease of platelet activation compared with patients not receiving any GP IIb/IIIa inhibitor therapy.
Of note, Scarborough and colleagues demonstrated that the total number of expressed GP IIb/IIIa receptors increases by as much as 50% during the acute phase of STEMI.16 Consequently, STEMI patients undergoing primary PCI have more expressed GP IIb/IIIa receptors compared with patients with stable angina and may therefore need a higher dosage of a GP IIb/IIIa receptor inhibitor.
Platelet aggregation
Previous trials of GP IIb/IIIa inhibitors have demonstrated that it is important to achieve and maintain a sufficient level of inhibition of aggregation since the absolute level of aggregation inhibition is an independent predictor of major cardiac events after PCI.17,18 However, it has recently been demonstrated that microaggregation (which is measured with singleplatelet counting [Plateletworks]) is perhaps a more sensitive marker of platelet inhibition since it discriminates more effectively between different degrees of inhibition.11 Specifically, despite a strong relationship between the inhibition of macroaggregation (as measured by gold standard light transmittance aggregometry) and the inhibition of microaggregation (as measured with the Plateletworks assay), the inhibition of microaggregation reaches the middle range of platelet inhibition whereas macroaggregation reaches the upper limits of inhibition of macroaggregation.11
Our results support the findings of Ernst and coworkers who reported in a nonrandomised study that a high-dose tirofiban regimen leads to a mean level of >80% inhibition of microaggregation in STEMI patients after primary PCI whereas the current recommended dose of abciximab did not achieve the 80% threshold.[12] The observed superiority of high-dose tirofiban in inhibiting ADP-induced aggregation compared with abciximab can be explained in the perspective of its different pharmacological properties and dosing.19-21
Pharmacological properties and dosing
Abciximab has a higher binding affinity to the GP IIb/IIIa receptor, reflected in substantially longer platelet-bound half-life compared with tirofiban, but its plasma half-life is far shorter than that of tirofiban. Consequently, tirofiban is more likely to block the new externalised GP IIb/IIIa receptors after platelet stimulation during STEMI.22,23 In other words: the new externalised GP IIb/IIIa receptors after platelet stimulation (as in STEMI) can only be inhibited if a sufficient unbound plasma fraction of a GP IIb/IIIa receptor inhibitor is present in the plasma. A higher bolus dose and more importantly, a higher infusion dose of abciximab will probably result in a better inhibition of the newly externalised receptors after additional platelet stimulation.
Platelet aggregation under high shear stress
It is commonly known that the combination of high shear stress and the presence of platelet-stimulating agents (collagen and ADP) influences the inhibitory capacities of antiplatelet therapy. Nonetheless, the administration of either abciximab or high-dose tirofiban in our study resulted in a rapid prolongation of the PFA-100 CADP closure time beyond the upper limit of 300 seconds. The PFA-100 CADP assay is therefore of limited use to determine any differences in inhibitory capacities between abciximab (in its currently used dose) and high-dose tirofiban.
Study limitations
There are two notable study limitations. First, we determined the magnitude of platelet inhibition by either high-dose tirofiban or abciximab with the relatively new Plateletworks assay. As yet, this assay has not demonstrated any inverse relationship between the absolute level of platelet inhibition and the occurrence of atherothrombotic events. Nonetheless, small studies have demonstrated a perfect correlation with turbidometric light transmittance aggregometry, the gold standard method for assessing platelet inhibition by GP IIb/IIIa inhibitors. Secondly, the small sample size of our study was insufficient to demonstrate the possible inverse relationship between the absolute amount of platelet inhibition and adverse clinical outcome.
Conclusions
In the present randomised study we have demonstrated that the administration of the currently recommended dose of abciximab or a high dose of tirofiban significantly decreases the high levels of platelet activation in patients presenting with STEMI. Furthermore, we confirm in a randomised design that the currently recommended dose of abciximab is less effective in preventing ADP-induced aggregation compared with high-dose tirofiban. The next step in assessing the usefulness of high-dose tirofiban in the treatment of STEMI patients is the development of large clinical trials to assess whether this laboratory superiority of high-dose tirofiban translates into higher clinical efficacy. The currently running On-TIME-2 trial in the Netherlands will hopefully answer this important question.
References
- 1.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2000; 20:1262-75. [DOI] [PubMed] [Google Scholar]
- 2.Zijlstra F, Hoorntje JC, de Boer MJ, Reiffers S, Miedema K, Ottervanger JP, et al. Long-term benefit of primary angioplasty as compared with thrombolytic therapy for acute myocardial infarction. N Engl J Med 1999;341:1413-9. [DOI] [PubMed] [Google Scholar]
- 3.Stone GW, Grines CL, Cox DA, Garcia E, Tcheng JE, Griffin JJ, et al. Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) Investigators. Comparison of angioplasty with stenting, with or without abciximab, in acute myocardial infarction. N Engl J Med 2002;346: 957-66.11919304 [Google Scholar]
- 4.Montalescot G, Barragan P, Wittenberg O, Ecollan P, Elhadad S, Villain P, et al., for the ADMIRAL Investigators. Platelet glycoprotein IIb/IIIa inhibition with coronary stenting for acute myocardial infarction. N Engl J Med 2002; 344:1895-903. [DOI] [PubMed] [Google Scholar]
- 5.Van ’t Hof AWJ, Ernst N, de Boer MJ, de Winter R, Boersma E, Bunt T, et al., for the On-TIME study group. Facilitation of primary coronary angioplasty by early start of glycoprotein IIb/IIIa inhibitor: results of the ongoing tirofiban in myocardial infarction evaluation (On-TIME) trial. Eur Heart J 2004;25:837-46. [DOI] [PubMed] [Google Scholar]
- 6.The TARGET Investigators. Comparison of two platelet glycoprotein IIb/IIIa inhibitors, tirofiban and abciximab, for prevention of ischemic events with percutaneous coronary revascularization. N Engl J Med 2001;344:1888-94. [DOI] [PubMed] [Google Scholar]
- 7.Schneider DJ, Herrmann HC, Lakkis N, Aguirre F, Wan Y, Aggarwal A, et al. Enhanced early inhibition of platelet aggregation with an increased bolus of tirofiban. Am J Cardiol 2002;90:1421-4. [DOI] [PubMed] [Google Scholar]
- 8.Soffer D, Moussa I, Karatepe M, Harjai KJ, Boura J, Dixon SR, et al. Suboptimal inhibition of platelet aggregation following tirofiban bolus in patients undergoing percutaneous coronary intervention for unstable angina pectoris. Am J Cardiol 2003;91:872-5. [DOI] [PubMed] [Google Scholar]
- 9.Kabbani SS, Aggarwal A, Terrien EF, DiBattiste PM, Sobel BE, Schneider DJ. Suboptimal early inhibition of platelets by treatment with tirofiban and implications for coronary interventions. Am J Cardiol 2002;89:647-50. [DOI] [PubMed] [Google Scholar]
- 10.Lakkis NM, George S, Thomas E, Ali M, Guyer K, Carville D. Use of ICHOR-platelet works to assess platelet function in patients treated with GP IIb/IIIa inhibitors. Catheter Cardiovasc Interv 2001;53:346-51. [DOI] [PubMed] [Google Scholar]
- 11.Siotia A, Buckland R, Judge HM, Sastry P, Storey RF. Utility of a whole blood single platelet counting assay to monitor the effects of tirofiban in patients with acute coronary syndromes scheduled for coronary intervention. Thromb Haemost 2006;95:997-1002. [DOI] [PubMed] [Google Scholar]
- 12.Ernst NM, Suryapranata H, Miedema K, Slingerland RJ, Ottervanger JP, Hoorntje JC, et al. Achieved platelet aggregation inhibition after different antiplatelet regimens during percutaneous coronary intervention for ST-segment elevation myocardial infarction. J Am Coll Cardiol 2004;44:1187-93. [DOI] [PubMed] [Google Scholar]
- 13.Mammen EF, Comp PC, Gosselin R C, Greenberg C, Hoots WK, Kessler CM, et al. PFA-100 system: a new method for assessment of platelet dysfunction. Semin Thromb Hemost 1998;24:195-202. [DOI] [PubMed] [Google Scholar]
- 14.Phillips DR, Teng W, Arfsten A, Nannizzi-Alaimo L, White MM, Longhurst C, et al. Effect of Ca2+ on GP IIb-IIIa interactions with integrilin: enhanced GP IIb-IIIa binding and inhibition of platelet aggregation by reductions in the concentration of ionized calcium in plasma anticoagulated with citrate. Circulation 1997;96:1488-94. [DOI] [PubMed] [Google Scholar]
- 15.Marciniak SJ Jr, Jordan RE, Mascelli MA. Effect of Ca2+ chelation on the platelet inhibitory ability of the GPIIb/IIIa antagonists abciximab, eptifibatide and tirofiban. Thromb Haemost 2001;85:539-43. [PubMed] [Google Scholar]
- 16.Scarborough RM, Kleiman NS Philips DR. Platelet Glycoprotein IIb/IIIa Antagonists. What are the relevant issues concerning their pharmacology and clinical use? Circulation 1999;100:437-44. [DOI] [PubMed] [Google Scholar]
- 17.The EPIC Investigaters. Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. N Engl J Med 1994;330:956-61. [DOI] [PubMed] [Google Scholar]
- 18.Steinhubl SR, Talley JD, Braden GA, Tcheng JE, Casterella PJ, Moliterno et al. Point-of-care measured platelet inhibition correlates with a reduced risk of an adverse cardiac event after percutaneous coronary intervention: results of the GOLD (AU-Assessing Ultegra) multicenter study. Circulation 2001;103:2572-8. [DOI] [PubMed] [Google Scholar]
- 19.Kleiman NS, Raizner AE, Jordan R, Wang AL, Norton D, Mace KF, et al. Differential inhibition of platelet aggregation induced by adenosine diphosphate or a thrombin receptor-activating peptide in patients treated with chimeric 7E3 Fab: implications for inhibition of the internal pool of GP IIb/IIIa receptors. J Am Coll Cardiol 1995;26:1665-71. [DOI] [PubMed] [Google Scholar]
- 20.Schror K, Weber AA. Comparative pharmacology of GP IIb/IIIa antagonists. J Thromb Thrombolysis 2003;15:71-80. [DOI] [PubMed] [Google Scholar]
- 21.Cook NS, Kottirsch G, Zerwes H-G. Platelet glycoprotein IIb/IIIa antagonists. Drugs Future 1994;19:135-59. [Google Scholar]
- 22.Matzdorff A, Voss R. Upregulation of GP IIb/IIIa receptors during platelet activation: Influence on efficacy of receptor blockade. Thromb Res 2006;117:307-14. [DOI] [PubMed] [Google Scholar]
- 23.Matzdorff AC, Kühnel G, Kemkes-Matthes B, Comparison of GP IIb/IIIa inhibitors and their activity as measured by aggregometry, flow cytometry, single platelet counting, and the rapid platelet function analyzer. J Thromb Thrombolysis 2001;12:129-39. [DOI] [PubMed] [Google Scholar]
- 24.Renda G, Rocca B, Crocchiolo R, De Cristofaro R, Landolfi R. Effect of fibrinogen concentration and platelet count on the inhibitory effect of abciximab and tirofiban Thromb Haemost 2003;89:348-54. [PubMed] [Google Scholar]


