Abstract
Study Objective:
People with narcolepsy and mice lacking orexin/hypocretin have disrupted sleep/wake behavior and reduced physical activity. Our objective was to identify physiologic mechanisms through which orexin deficiency reduces locomotor activity.
Design:
We examined spontaneous wheel running activity and its relationship to sleep/wake behavior in wild type (WT) and orexin knockout (KO) mice. Additionally, given that physical activity promotes alertness, we also studied whether orexin deficiency reduces the wake-promoting effects of exercise.
Measurements and Results:
Orexin KO mice ran 42% less than WT mice. Their ability to run appeared normal as they initiated running as often as WT mice and ran at normal speeds. However, their running bouts were considerably shorter, and they often had cataplexy or quick transitions into sleep after running. Wheel running increased the total amount of wakefulness in WT and orexin KO mice similarly, however, KO mice continued to have moderately fragmented sleep/wake behavior. Wheel running also doubled the amount of cataplexy by increasing the probability of transitioning into cataplexy.
Conclusions:
Orexin KO mice run significantly less than normal, likely due to sleepiness, imminent cataplexy, or a reduced motivation to run. Orexin is not required for the wake-promoting effects of wheel running given that both WT and KO mice had similar increases in wakefulness with running wheels. In addition, the clear increase in cataplexy with wheel running suggests the possibility that positive emotions or reward can trigger murine cataplexy, similar to that seen in people and dogs with narcolepsy.
Citation:
España RA; McCormack SL; Mochizuki T; Scammell TE. Running promotes wakefulness and increases cataplexy in orexin knockout mice. SLEEP 2007;30(11):1417-1425.
Keywords: Orexin, hypocretin, cataplexy, locomotor activity, running, running wheels, obesity, motivation, positive affect
INTRODUCTION
NARCOLEPSY WITH CATAPLEXY IS ASSOCIATED WITH A LOSS OF THE OREXIN (HYPOCRETIN)-CONTAINING NEURONS IN THE HYPOTHALAMUS.1–3 MICE LACKING OREXIN are an excellent model of narcolepsy as they have sleepiness and episodes of sudden atonia resembling human cataplexy.4,5 In addition, mice lacking the orexin neurons can become mildly obese similar to that seen in human narcolepsy.6 In part, this obesity may be a consequence of reduced physical activity because orexin-deficient mice have less spontaneous movement than WT mice.5,7,8 To better understand the interaction of physical activity with sleepiness and cataplexy, we examined the influence of wheel running on the behavior of wild type (WT) and orexin knockout (KO) mice.
First, we examined whether sleepiness, cataplexy, or reduced motivation might contribute to the reduced locomotor activity (LMA) observed in orexin KO mice. One possibility is that orexin KO mice might be less motivated to run because they lack excitatory orexin signaling to reward pathways between the ventral tegmental area (VTA) and nucleus accumbens.9–13 Alternatively, given that orexin promotes arousal and suppresses cataplexy, the reduced LMA in orexin deficient animals could be a direct consequence of sleepiness or cataplexy. Therefore, we examined spontaneous wheel running and its relationship to sleep-wake behavior.
Second, we examined whether orexin is necessary for the wake-promoting effects of exercise. In healthy people, exercise reduces the likelihood of sleep and promotes alertness, whereas forced bed rest has the opposite effects.14,15 In rodents, wheel running increases wakefulness and markedly lengthens wake bouts.16–18 Although the arousing effects of exercise have long been recognized, the neural mechanisms through which physical activity promotes wakefulness remain largely unknown. The orexin neurons are well-situated to mediate this effect because they receive inputs from brain regions that control locomotion (e.g., the substantia nigra, periaqueductal gray, and midbrain locomotor region),19 and they heavily innervate and excite numerous wake-promoting brain regions.20 Furthermore, the orexin neurons are particularly active during locomotion as shown by extracellular recordings and the expression of Fos.21–25 To test whether orexin mediates the wake-promoting effects of exercise, we measured wheel running in WT and orexin KO mice.
Last, we examined whether running increases cataplexy in orexin KO mice. In people and dogs, cataplexy is often triggered by positive emotions such as mirth or surprise, but it remains unknown whether positive emotions trigger cataplexy in narcoleptic mice. Wheel running is rewarding for mice and probably elicits positive emotions.26,27 Accordingly, wheel running provides an excellent opportunity to better understand emotional triggering of murine cataplexy.
METHODS
Animals
These experiments used 12-15 week old, male, homozygous orexin KO mice (n = 11) and male WT littermates (n = 9). Mean weights were 26 grams in each group. Mice had ad lib access to water and regular mouse chow (PicoLab Rodent Diet 20, Purina). Founder lines of mice were on a C57BL/6J-129/SvEV background4 and then backcrossed with C57BL/6J mice for seven to eight generations. Mice were genotyped using PCR with a neo primer, 5'-CCGCTATCAGGACATAGCGTTGGC, or a genomic primer, 5′-GACGACGGCCTCAGACTTCTTGGG, and a genomic primer, 3′-TCACCCCCTTGGGATAGCCCTTCC, common to KO and WT mice. All experiments were approved by the Institutional Animal Care and Use Committees of Beth Israel Deaconess Medical Center and Harvard Medical School.
Surgery
Mice were anaesthetized with ketamine/xylazine (100 and 10 mg/kg, i.p.) and placed in a stereotaxic apparatus. Two stainless-steel screws for recording the EEG were placed; one at 1 mm rostral and 1.5 mm lateral to bregma and the other at 1 mm rostral and 1.5 mm lateral to lambda. EMG signals were acquired with a pair of multistranded stainless steel wires (Cooner Wire, Chatsworth, CA) inserted into the neck extensor muscles. All leads were attached to a 2 x 2 pin head plug that was secured to the skull using dental acrylic.
Experimental Protocol
One week after surgery, mice were transferred to recording cages in a sound-attenuated chamber with a 12:12 hr light:dark (LD) cycle (30 lux; lights on at 07:00 and off at 19:00) and a constant temperature (22–24°C). Mice were connected to recording cables and allowed to acclimate to running wheels for ≥10 d. On day 12, sleep/wake behavior and running wheel rotations were recorded for 24 h in the “Wheel Run” condition. On day 13, running wheels were locked and mice were allowed to adjust to this “Wheel Locked” condition for 48 h. On day 16, sleep/wake behavior was recorded for another 24 h with wheels locked. Prior reports and comparison with other recordings in our lab indicate that 2 days of locked wheels is sufficient for behavior to stabilize.5,17,28 To ensure that running behavior had not changed over time, we then unlocked the wheels on day 17. In all cases, mice readjusted to wheels within 48 h, running as much as they had on day 12 (F1,18 = 0.2, P=0.7).
EEG/EMG recordings
Ipsilateral EEG and bilateral EMG signals were acquired using Grass Model 12 amplifiers (Astro-Med, West Warwick, RI) and digitized at 128 Hz using a sleep scoring system (Sleep Sign; Kissei Comtec, Matsumoto, Japan). The EEG/EMG signals were digitally filtered (EEG, 0.3–30 Hz; EMG, 2–100 Hz) and manually scored in 10-sec epochs by a single observer blind to experimental conditions. Behavior was scored as wakefulness (low-voltage EEG with frequent EMG activity), NREM sleep (high-voltage EEG with slow waves, low voltage EMG), or REM sleep (low-voltage, theta-dominant EEG with minimal EMG activity). Behavior was scored as cataplexy only when the following criteria were met: A) Cataplexy is one or more epochs of EEG theta activity and muscle atonia immediately preceded and followed by active wakefulness.4,5,29 B) At least 40 sec of wakefulness must precede cataplexy to exclude any REM sleep that might follow a brief awakening. This criterion was established from recent work by the Nishino group showing that 40 sec of wakefulness immediately before atonia/theta correctly identified 95% of all cataplexy episodes.30 C) The animal's posture must be different than that seen during sleep (i.e., the mouse cannot be curled in a sleeping position). D) The animal's location in the cage cannot be its usual nest. Whenever behavior met criteria A, we examined integrated infrared video recordings (Sleep Sign) in detail to determine if all criteria were fulfilled.
Running Wheel Analyses
Running wheel activity was recorded using low torque, polycarbonate running wheels (Fast-Trac, Bio-Serv, Frenchtown, NJ) modified with photodetectors to record wheel rotations. This style of wheel was chosen because it does not interfere with the EEG recording cable. Wheel rotations were recorded in 10-sec epochs using a data acquisition system (Dataquest, Data Sciences International). Wheel running was analyzed using 2 approaches. First, we examined running bouts that were defined as at least 2 wheel rotations in the first 10-sec epoch of running and persistence of running for at least one additional epoch. A running bout terminated if running ceased for at least 2 consecutive 10-sec epochs. Next, we analyzed the average amount of wheel running as a function of wakefulness duration by aligning the ends and beginnings of all wakefulness bouts from the dark period and then calculating the mean amount of running that occurred in each 10-sec epoch of wakefulness. This second analysis included all wheel activity, not just the rotations that occurred in running bouts. The amount of running in each epoch appears lower with this approach because many wake bouts contain no running.
Statistics and Analysis
We analyzed the daily amounts of running using a one-way ANOVA with genotype (WT vs. KO) as the between-subjects variable. For comparisons between genotypes and time (dark phase vs. light phase), we used an omnibus 2-way, mixed design ANOVA, with genotype as the between-subjects variable and time as the repeated measure variable. We used the same technique for comparisons of genotype and wheel condition (Wheel Run vs. Wheel Locked), with wheel condition as the repeated measure variable. If the omnibus test showed significant main or interaction effects, we used uncorrected one-way ANOVA's to assess pair-wise comparisons between genotypes, time, and wheel condition. To analyze the probability of cataplexy as a function of wake bout length, we first calculated the absolute probability of transitioning from wakefulness into cataplexy for each minute of wakefulness and then used a two-way ANOVA with genotype as the between subjects variable and wake bout length as the repeated measures variable. Results are shown as means ± SEM.
RESULTS
Patterns of Wheel Running in WT and Orexin KO Mice
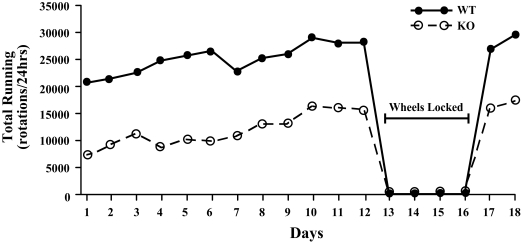
Over the first 12 days, WT (n=9) and orexin KO mice (n=11) acclimated to running wheels, establishing steady, maximal amounts of running (Figure 1). Running stabilized after 9 days as indicated by similar amounts of running on days 10, 11, and 12 in each group (F1,18 = 0.17, P = 0.2). The amount of running increased at a similar rate in both groups across the first 12 days (increases of 850 ± 231 rotations/day in WT mice and 782 ± 175 rotations/day in orexin KO mice; F1,18 = 0.2, P = 0.6).
Figure 1.
Wild type (WT) and orexin knockout (KO) mice acclimate similarly to running wheels, however, KO mice consistently run less than WT mice. Running wheels were locked on days 13 through 16. Sleep/wake EEG behavior was recorded on days 12 and 16. Wheels were unlocked on day 17, and running returned to prior levels.
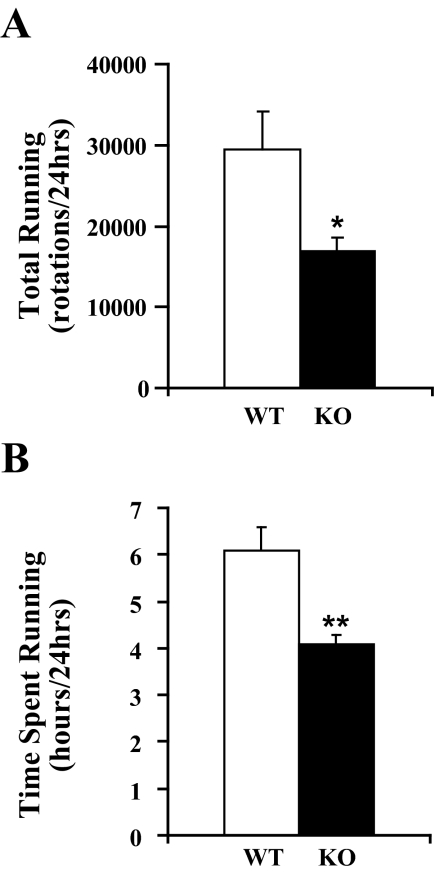
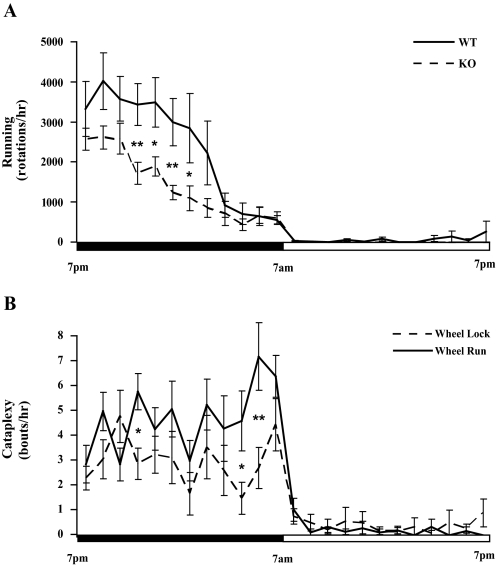
Although orexin KO mice showed a normal pattern of acclimation, they ran less than WT mice across all days (F1,18 = 9.37, P<0.01). For example, on day 12, WT mice ran 29,423 ± 4774 rotations (∼ 10.3 ± 1.7 km) whereas KO mice ran 16,985 ± 1514 rotations (∼ 6.0 ± 0.5 km; F1,18 = 7.28, P<0.02; Figure 2). Additionally, orexin KO mice spent significantly less time running than WT mice (4.1 ± 0.2 vs. 6.1 ± 0.5 hrs; F1,18 = 16.9, P < 0.001). The timing of running in KO mice resembled that seen in WT mice, with over 98% of running occurring during the dark period in both groups (F1,18 = 87.5, P < 0.001; Figure 3). Locomotor intensity (wheel rotations per hour of wakefulness 31) was reduced in orexin KO mice across the dark period (F(1,16) = 4.8, P < 0.05).
Figure 2.
Orexin KO mice run less than WT mice. A) Over 24 hours, KO mice run 42% less than WT mice. B) Orexin KO mice spend less time running than WT mice. Data shown here and on subsequent figures are from day 12, when mice were fully acclimated to wheels. *P < 0.05; **P < 0.01.
Figure 3.
A) Orexin KO mice run less than WT mice, however, the temporal pattern of running is preserved. In both groups, 98% of running occurs during the dark period. B) In orexin KO mice, cataplexy is more frequent with running wheels unlocked, but the distribution of cataplexy across the dark period is similar to that seen with locked wheels. *P < 0.05; **P < 0.01.
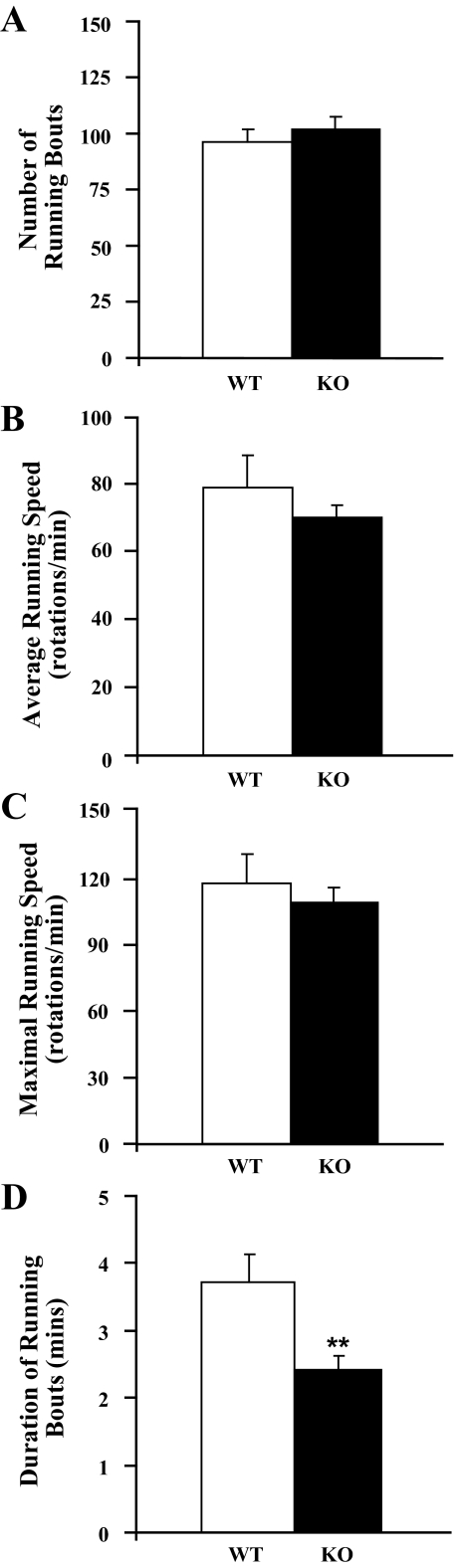
To investigate why orexin KO mice run less, we analyzed additional aspects of running during the dark period on day 12 (Figure 4). Orexin KO mice initiated running as often as WT littermates (F1,18 = 0.09, P = 0.8) and ran at similar average (F1,18 = 1.14, P = 0.3) and maximal speeds (top 10% of running epochs; F1,18 = 0.42, P = 0.5). However, the average length of a running bout was significantly shorter in orexin KO mice (F1,18 = 9.4, P = 0.01). These observations suggest that orexin KO mice have normal motivation to initiate running and they run at normal speeds, yet their total running is reduced due to their shorter running bouts.
Figure 4.
Orexin KO mice initiate but do not sustain bouts of running. A) Orexin KO mice begin running as frequently as WT mice. B and C) The average running speed and maximal speed (top 10% of running epochs) in KO mice are similar to WT littermates. D) The mean duration of running bouts is much shorter in orexin KO mice. **P < 0.01.
Effects of Sleep/Wake Behavior on Wheel Running
Reduced running in orexin KO mice may be associated with sleepiness or cataplexy. To explore these possibilities, we examined the pattern of wheel running in relation to sleep/wake behavior during the dark phase on day 12. On average, WT mice were awake 5.5 ± 1.1 min before a running bout, whereas orexin KO mice were awake only 2.6 ± 0.3 min before running (F1,18 = 8.0, P < 0.01). After a bout of running, WT mice remained awake for 10.4 ± 2.8 min before transitioning into NREM sleep. In contrast, orexin KO mice remained awake for only 4.7 ± 0.8 min after running (F1,18 = 4.7, P < 0.05). Additionally, in orexin KO mice, 35% of all running bouts were followed by cataplexy within one minute, and 68% of these transitions occurred within ten seconds of the end of running. Occasionally, cataplexy occurred while the animal was on the running wheel, however, more typically, the mouse stumbled off of the wheel and had a cataplexy episode soon thereafter (Supplemental Movie). These observations support the hypothesis that running bouts in orexin KO mice are shorter because of imminent sleepiness or cataplexy.
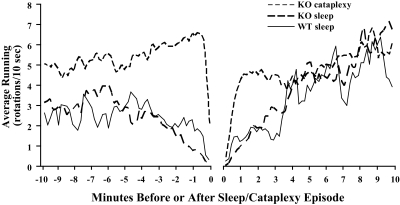
To further explore these possibilities, we analyzed wheel running around episodes of sleep and cataplexy. Prior to sleep, WT and KO mice had comparable average amounts of running and gradually decreased their running over the 5 min before sleep onset (Figure 5). After sleep, running gradually resumed, reaching steady levels within 5 to 10 min. Perhaps sleepiness leads to the gradual reduction in running before sleep and sleep inertia hinders the expression of running after sleep. If so, then KO mice show similar amounts of sleepiness and sleep inertia around episodes of sleep.
Figure 5.
The patterns of locomotor activity around sleep and cataplexy. Both WT and orexin KO mice have gradual reductions in wheel running prior to sleep, and then wheel running gradually increases after sleep. In contrast, prior to cataplexy, orexin KO mice have larger amounts of running that abruptly stop 10 to 20 seconds before cataplexy, and then they resume running within a minute after cataplexy. Graphs show the mean amount of wheel running in each 10-sec wake epoch, aligned to the end or beginning of a bout of wakefulness. (Many wake bouts contain no running, so this does not represent running speed.)
On average, KO mice ran more in the minutes prior to cataplexy than prior to sleep (F1,18 = 49.8, P < 0.001), and running persisted at high levels until ceasing immediately before episodes of cataplexy. After cataplexy, orexin KO mice rapidly resumed running, reaching steady, high amounts within 1 min. The greater amounts of running before cataplexy may reflect high emotional tone that helps trigger cataplexy, and the abrupt onset and recovery from cataplexy highlights its differences from sleep.
Together, these data indicate that orexin KO mice have short sleep latencies and rapid transitions into cataplexy after running, suggesting that sleepiness and imminent cataplexy may contribute to their short running bouts.
Effects of Wheel Running on Sleep-Wake Behavior
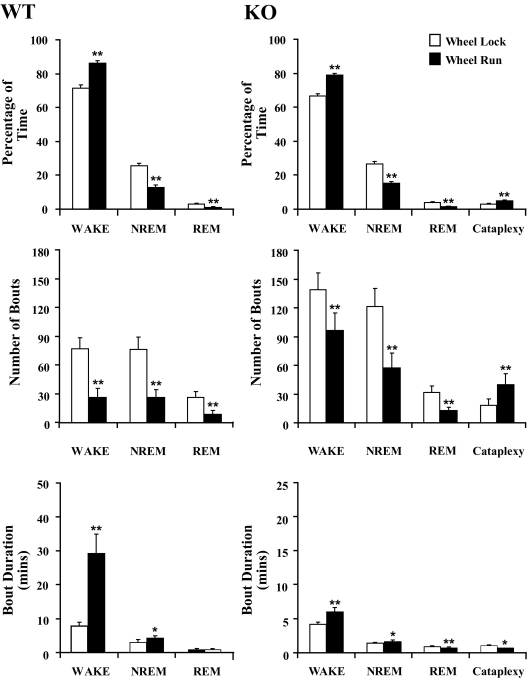
To examine the impact of running wheels on sleep/wake behavior, we compared the Wheel Run and Wheel Locked conditions. Consistent with prior observations 16–18, wheel running increased and consolidated wakefulness during the dark period but had no significant effect on sleep/wake behavior during the light period (Figure 6 and Supplemental Table). During the dark period, wheel running increased the amount of time spent awake by approximately 20% in WT and orexin KO mice (F1,18 = 68.5, P < 0.001). Thus, the increase in total wakefulness with wheel running occurs even in the absence of orexin.
Figure 6.
Wheel running consolidates sleep/wake behavior during the dark period, especially in WT mice. Wheel running in WT mice produces more wakefulness during the dark period, and wakefulness occurs in considerably longer bouts. A similar pattern occurs in orexin KO mice, though the increase in wake bout duration is smaller. Additionally, wheel running doubles the number of cataplexy episodes in orexin KO mice. *P < 0.05; **P < 0.01.
Supplemental Table 1.
Effects of Running Wheels on Sleep/Wake Behavior.
| DARK PHASE | ||||||
|---|---|---|---|---|---|---|
| % of hour |
Mean bout duration (sec) |
Number of bouts |
||||
| WR | WL | WR | WL | WR | WL | |
| Wake | ||||||
| WT | 86.0 ± 1.5** | 71.5 ± 1.9 | 1697 ± 267 ** | 435 ± 26 | 26 ± 3** | 76 ± 4 |
| KO | 78.8 ± 1.0** | 66.3 ± 1.3 | 366 ± 26 ** | 210 ± 11 | 97 ± 6 ** | 139 ± 6 |
| NREM | ||||||
| WT | 12.7 ± 1.3** | 25.4 ± 1.8 | 228 ± 21 * | 162 ± 17 | 25 ± 3** | 75.2 ± 5 |
| KO | 15.2 ± 1.0** | 26.6 ± 1.3 | 119 ± 7* | 99 ± 5 | 57 ± 6** | 121.2 ± 7 |
| REM | ||||||
| WT | 1.2 ± 0.2** | 3.1 ± 0.3 | 59 ± 5 | 59 ± 2 | 9 ± 1** | 24.4 ± 3 |
| KO | 1.3 ± 0.2** | 4.1 ± 0.4 | 44 ± 4 ** | 55 ± 3 | 13 ± 1 ** | 31.5 ± 2.5 |
| Cataplexy | ||||||
| KO | 4.7 ± 0.5** | 2.9 ± 0.4 | 52 ± 4* | 63 ± 6 | 40 ± 4** | 18 ± 2 |
| LIGHT PHASE | ||||||
|---|---|---|---|---|---|---|
| % of hour |
Mean bout duration (sec) |
Number of bouts |
||||
| WR | WL | WR | WL | WR | WL | |
| Wake | ||||||
| WT | 34.9 ± 1.5 | 37.9 ± 1.4 | 123 ± 27 | 106 ± 12 | 128 ± 10 | 161 ± 15 |
| KO | 35.3 ± 1.0 | 36.6 ± 0.9 | 90 ± 11 | 97 ± 10 | 170 ± 10 | 166 ± 6 |
| NREM | ||||||
| WT | 57.0 ± 1.3 | 54.6 ± 0.9 | 196 ± 38 | 151 ± 30 | 130 ± 10 | 161 ± 15 |
| KO | 56.3 ± 1.0 | 55.3 ± 0.8 | 145 ± 26 | 145 ± 18 | 173 ± 9 | 166 ± 7 |
| REM | ||||||
| WT | 8.0 ± 0.3 | 7.4 ± 0.8 | 64 ± 5 | 72 ± 19 | 54 ± 2 | 47 ± 4 |
| KO | 8.2 ± 0.4 | 7.6 ± 0.4 | 57 ± 57 | 69 ± 30 | 74 ± 4 | 64 ± 4 |
| Cataplexy | ||||||
| KO | 0.3 ± 0.1 | 0.4 ± 0.1 | 56 ± 23 | 92 ± 60 | 2 ± 1 | 2 ± 1 |
Running wheels increased and consolidated wakefulness during the dark period but had no effects on behavior during the light period. The total amount of wakefulness increased in both WT and orexin KO mice, but KO mice continued to have relatively short bouts of wakefulness even with running wheels. In orexin KO mice, running wheels doubled the number of bouts of cataplexy. *, p < 0.05; **, p < 0.01.
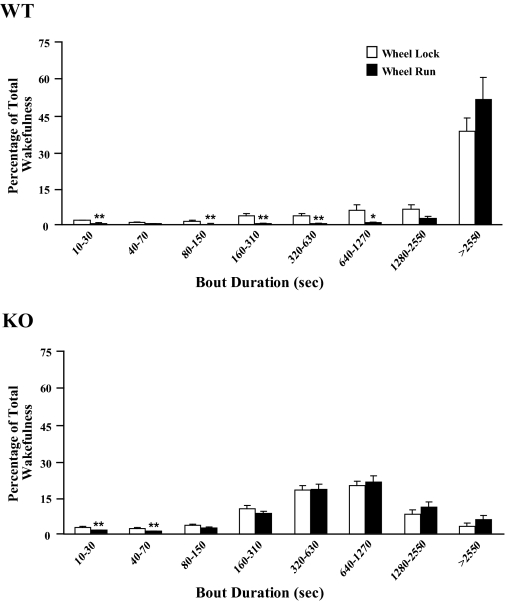
Wheel running also consolidated wakefulness. The number of wake bouts was reduced in both groups (F1,18 = 21.8, P < 0.001), and the average duration of wake bouts increased by 75% in orexin KO mice and by 290% in WT mice (F1,18 = 30.3, P < 0.001). Wheel running shifted the distribution of wakefulness into longer wake bouts, with approximately half of all wakefulness occurring in very long bouts (longer than 42.5 min) in WT mice (Supplemental Figure). Orexin deficiency disrupts the production of long wake bouts,5 and this shift to longer bouts was less prominent in KO mice.
Supplemental Figure 1.
Running wheels consolidate wakefulness during the dark period. These time-weighted frequency histograms show that with running wheels unlocked, less wakefulness occurs in very short bouts and more wakefulness occurs in long to very long bouts. Note that even with unlocked running wheels, the wakefulness of orexin KO mice mainly occurs in mid-length bouts. *, p < 0.05; **, p < 0.01.
In both groups, wheel running reduced the amount of dark period NREM sleep by 40%-50% (F1,18 = 60.2, P < 0.001) and decreased the amount of REM sleep by 60%-70% (F1,18 = 82.2, P < 0.001) mainly through a reduction in the number of NREM and REM bouts. This reduction in sleep had little effect on measures of sleep homeostasis. EEG delta power during either dark or light period NREM sleep was not increased (F1,18 = 3.0, P = 0.1) and there were no significant increases in the amounts of NREM (F1,18 = 3.4, P = 0.08) or REM (F1,18 = 2.8, P = 0.11) sleep during the light period.
Compared to WT mice, orexin KO mice still had fragmented wakefulness and sleep, despite the wake-promoting effects of wheel running (Figure 6). During the dark period, KO mice had slightly less wakefulness (F1,18 = 14.2, P < 0.001) and much shorter wake bouts than WT mice (F1,18 = 40.6, P < 0.001). Additionally, the durations of NREM and REM bouts were shorter in KO mice (NREM, F1,18 = 28.4, P < 0.001; REM, F1,18 = 16.3, P < 0.001), though the total amounts of each sleep state were similar in both groups. Overall, wheel running increased the total amount of wakefulness but only partially improved the fragmented sleep/wake behavior of orexin KO mice.
Effects of Running Wheels on Cataplexy
Wheel running substantially increased cataplexy in orexin KO mice. Compared to the Wheel Locked condition, orexin KO mice with unlocked wheels spent nearly twice as much time in cataplexy (F1,18 = 11.6, P < 0.01). This increase was caused by a doubling in the number of cataplexy bouts during the dark period (F1,18 = 22.2, P < 0.001) with only a slight decrease in cataplexy duration. Most cataplexy bouts (78%) were preceded by some wheel running in the prior minute. During the light period, cataplexy remained rare and unaffected by the presence of a functional running wheel despite less dark period REM sleep.
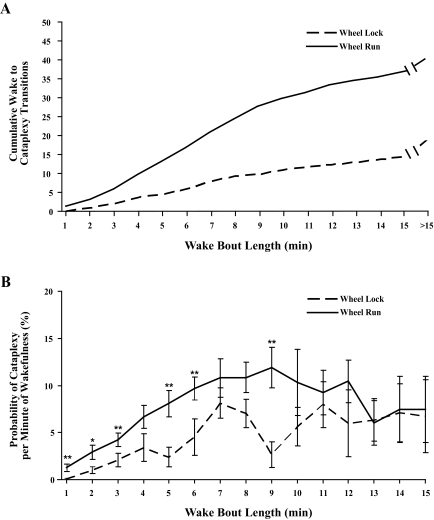
To examine whether the increase in cataplexy with wheel running is a consequence of prolonged wakefulness, we analyzed cataplexy as a function of wake bout length. With running wheels locked, nearly all episodes of cataplexy occurred during the first 15 min of wakefulness, and on average, orexin KO mice had about 18 episodes of cataplexy during the dark period (Figure 7). With running wheels unlocked, most cataplexy still occurred during the first 15 min of wakefulness, but it occurred more frequently, resulting in an average of 40 episodes of cataplexy during the dark period. With locked wheels, the absolute probability of transitioning from wakefulness into cataplexy in any minute gradually rose over the first 10 min of wakefulness and then remained roughly constant. However, when running wheels were unlocked, the probability of entering cataplexy was generally higher during the first 10 min of wakefulness (F1,18 = 5.65, P<0.05). In addition, for both the Wheel Run and the Wheel Locked conditions, the longer a KO mouse was awake, the more likely it was to experience a cataplexy episode (F15,119 = 60.95, P<0.001). Thus, at least 2 factors contribute to the increase in cataplexy with wheel running: a time-dependent increase in cataplexy probability (cataplexy is more likely with prolonged wakefulness); and a time-independent increase in the probability of cataplexy (cataplexy is generally more likely with wheel running).
Figure 7.
Wheel running increase the probability of cataplexy. A) With running wheels locked, the cumulative number of transitions into cataplexy gradually increases over the first 15 min of wakefulness. With wheels unlocked, the number of transitions accumulates more rapidly. In both conditions, a few additional episodes of cataplexy occur after 15 min of wakefulness. B) The probability of transitioning into cataplexy in any minute of wakefulness is higher with running wheels unlocked. This difference is most apparent in the first 10 min of wakefulness and disappears by 15 min, probably because of a time-dependent increase in cataplexy.
DISCUSSION
The current studies demonstrate that running is reduced in orexin KO mice because their running bouts are considerably shorter than normal. Transitions into sleep or cataplexy occur shortly after running in KO mice, and running bouts may be shortened by sensations of sleepiness or imminent cataplexy. In addition, wheel running doubles the amount of cataplexy, suggesting that cataplexy is triggered by high levels of activity or possibly heightened emotional tone associated with running.
Possible Causes of Reduced Wheel Running in Orexin KO Mice
Orexin KO mice run much less than WT littermates, largely due to shortened running bouts. Sleepiness and cataplexy are two obvious explanations. After a bout of running, orexin KO mice fall asleep more quickly than WT mice, and 35% of all running bouts are followed by cataplexy within 1 min. These associations do not prove that sleepiness or cataplexy directly truncate running bouts, but these hypotheses could be tested by examining wheel running in orexin KO mice treated with cataplexy-suppressing or wake-promoting drugs that have little effect on locomotor activity (e.g. modafinil31,32).
Alternatively, orexin KO mice may run less because they are less motivated to continue running or they find running less rewarding. Orexin KO mice appear motivated to initiate running as often as WT mice (normal number of running bouts), however, their failure to sustain running could reflect an inability to remain motivated. Wheel running can be viewed as a naturally rewarding and potentially addictive behavior that is dependent on the release of dopamine from mesolimbic projections to the nucleus accumbens.26,27 Orexin neurons are activated in anticipation of a food or drug reward,10 and they innervate and excite neurons in the VTA and nucleus accumbens.20,33 Direct injection of orexin into the nucleus accumbens increases locomotor activity,12 and the locomotor-enhancing effects of orexin are blocked by dopamine antagonists.9 Considered together, these studies demonstrate that orexin strengthens reward signals by enhancing signaling in the VTA and nucleus accumbens, and suggest that orexin KO mice may run less because they find running less rewarding.
Orexin has also been implicated in other systems that may directly influence running. The orexin neurons innervate areas that intimately control motor behavior such as the substantia nigra, midbrain locomotor region, and motor neurons.20 Injection of orexin into the midbrain locomotor region of decerebrate cats reduces the amount of electrical stimulation needed to trigger locomotion,34 and when applied near motor neurons, orexin increases muscle tone.35,36 However, direct effects of orexin on motor systems seem an unlikely explanation for reduced running because orexin KO mice have normal running speeds and initiate running as frequently as WT mice.
Overall, our experiments suggest that orexin KO mice run less due to sleepiness and cataplexy. Future studies could examine whether reduced motivation or decreased activation of motor pathways also contribute to this deficit.
The Effects of Running Wheels on Wakefulness
The behavioral response of WT and orexin KO mice to wheel running confirms that physical activity promotes arousal. In healthy people, exercise promotes wakefulness, whereas forced bed rest often results in more sleep and shorter bouts of wakefulness.14,15 Consistent with prior studies,16–18 we found that wheel running increases the total amount of wakefulness during the dark period by about 20% and lengthens the duration of wake bouts in both WT and orexin KO mice. The orexin neurons are innervated by brain regions that influence locomotor activity including the substantia nigra,19 and the orexin neurons are especially active during periods of robust locomotor activity.21–25 These observations suggested that orexin might be required for the wake-promoting effects of wheel running, but this mechanism now appears unlikely as both WT and orexin KO mice showed proportionately similar increases in total wakefulness in the presence of running wheels. Although orexin is necessary for producing long periods of wakefulness,5 the arousing effects of wheel running may be mediated by other signaling molecules such as norepinephrine.37
The Effects of Running Wheels on Cataplexy
Wheel running doubles the amount of cataplexy in orexin KO mice, in part, due to an increase in the amount of wakefulness and longer bouts of wakefulness. However, even after correcting for these changes, wheel running increases the probability of cataplexy per minute of wakefulness and running often precedes cataplexy. These results suggest that cataplexy can be triggered by running or the internal states that accompany running. Vigorous physical activity itself is unlikely to be the trigger because cataplexy is uncommon during unexciting exercise in people with narcolepsy, though this should be tested in animal experiments using forced exercise. Laughter, mirth, and surprise frequently trigger cataplexy in people with narcolepsy,38 and play and highly palatable food often trigger cataplexy in narcoleptic dogs.39 Thus far, there has been little evidence that positive emotions trigger cataplexy in mice, although cataplexy is sometimes preceded by behaviors that could be associated with positive emotions such as climbing and burrowing.4 Wheel running is rewarding for mice as they will work to access wheels,40 and though it is speculative, it is possible that wheel running induces strong, positive emotions that trigger cataplexy.
Alternatively, the increase in cataplexy could be caused by increased wakefulness with reduced sleep and increased REM sleep pressure. First, cataplexy occurs during wakefulness, and with wheel running, mice are simply awake more, with more time at risk for a transition into cataplexy. However, this explanation appears insufficient as wheel running doubles the amount of cataplexy, yet the total amount of wakefulness increases only 20%. A second explanation is that cataplexy increases with wheel running because wheel running lengthens wake bouts and the probability of cataplexy increases with the duration of wakefulness; thus, with wheel running, mice spend more time at risk in long wake bouts. However, the lengthening of wake bouts with wheel running is relatively small in orexin KO mice. A third perspective is that the reduction in dark period sleep with wheel running could increase the pressure for cataplexy because cataplexy is more frequent in dogs with less REM sleep41 and clinical observations suggest that sleep deprivation worsens cataplexy perhaps by increasing REM sleep pressure.42 Still, the reduction in dark period sleep with wheel running appears not to generate much sleep pressure as there is no significant increase in light period sleep, delta power in NREM sleep during the dark and light periods is not higher, and the distribution of cataplexy across the dark period is unaltered (no increase in cataplexy towards the end of the dark period as one might expect with rising sleep pressure). The increased risk of cataplexy per minute of wakefulness suggests that positive emotions could trigger cataplexy with wheel running, although long bouts of wakefulness and less sleep may also contribute.
Implications and Future Directions
These experiments demonstrate that orexin KO mice run less because their running bouts are shorter. Sleepiness, cataplexy, or reduced motivation likely underlie this inability to sustain running, and future studies could test the importance of these factors by treating KO mice with drugs that improve alertness and suppress cataplexy and by studying motivation and reward in formal operant conditions. An improved understanding of these factors should provide new perspectives on the mild obesity that is common in people with narcolepsy.
Wheel running doubles the amount of cataplexy in orexin KO mice, and thus on a practical level, wheel running provides a simple method for increasing cataplexy that should be useful in future cataplexy experiments. Whether this increase in cataplexy is caused by physical activity or positive emotions remains uncertain, but ongoing studies suggest that murine cataplexy can be triggered by other rewards such as anticipation of food, especially highly palatable food 43. If so, this should provide new opportunities to understand the neurobiology of positive emotions and how emotions trigger cataplexy in people with narcolepsy.
ACKNOWLEDGMENTS
This study was supported by NIH grants NS055367 and HL60292 and Sleep Research Society grant 002WN06. Orexin KO mice were a kind gift from Takeshi Sakurai of the University of Tsukuba. Cecilia Diniz Behn provided helpful advice on calculating state transition probabilities.
Work performed at: Beth Israel Deaconess Medical Center
Disclosures: This research was supported by grants from the NIH and Sleep Research Society
Supplemental Movie 1.
A short episode of cataplexy that occurs during wheel running. An orexin KO mouse runs quickly, climbs on top of the running wheel, and then slides off with hind legs still on the wheel. This cataplexy bout lasts only 20-30 sec, and the mouse then resumes running. During cataplexy, the EEG contains theta activity (top trace), and the EMG shows very low tone with some EKG artifact (lower trace). Scoring of behavior: W, wakefulness; C, cataplexy; C*, cataplexy with artifact (excluded from EEG spectral analysis). (Movie file available on journalsleep.org)
Footnotes
Disclosure Statement
This is not an industry supported study. Dr. Scammell has received research support from Takeda and Jazz and has participated in speaking engagements for Cephalon, Jazz, Alexza, Lundbeck, and Takeda. The other authors have reported no financial conflicts of interest.
REFERENCES
- 1.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nature Med. 2000;6:991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 2.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–74. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crocker A, Espana RA, Papadopoulou M, et al. Concomitant loss of dynorphin, NARP, and orexin in narcolepsy. Neurology. 2005;65:1184–8. doi: 10.1212/01.wnl.0000168173.71940.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 5.Mochizuki T, Crocker A, McCormack S, Yanagisawa M, Sakurai T, Scammell TE. Behavioral State Instability in Orexin Knock-Out Mice. J Neurosci. 2004;24:6291–300. doi: 10.1523/JNEUROSCI.0586-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hara J, Yanagisawa M, Sakurai T. Difference in obesity phenotype between orexin-knockout mice and orexin neuron-deficient mice with same genetic background and environmental conditions. Neurosci Lett. 2005;380:239–42. doi: 10.1016/j.neulet.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 7.Mieda M, Williams SC, Sinton CM, Richardson JA, Sakurai T, Yanagisawa M. Orexin neurons function in an efferent pathway of a food-entrainable circadian oscillator in eliciting food-anticipatory activity and wakefulness. J Neurosci. 2004;24:10493–501. doi: 10.1523/JNEUROSCI.3171-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang S, Zeitzer JM, Sakurai T, Nishino S, Mignot E. Sleep/wake fragmentation disrupts metabolism in a mouse model of narcolepsy. J Physiol. 2007;581:649–63. doi: 10.1113/jphysiol.2007.129510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura T, Uramura K, Nambu T, et al. Orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Brain Res. 2000;873:181–7. doi: 10.1016/s0006-8993(00)02555-5. [DOI] [PubMed] [Google Scholar]
- 10.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–9. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 11.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Thorpe AJ, Kotz CM. Orexin A in the nucleus accumbens stimulates feeding and locomotor activity. Brain Res. 2005;1050:156–62. doi: 10.1016/j.brainres.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 13.Narita M, Nagumo Y, Hashimoto S, et al. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonnet MH, Arand DL. Sleepiness as measured by modified multiple sleep latency testing varies as a function of preceding activity. Sleep. 1998;21:477–83. [PubMed] [Google Scholar]
- 15.Campbell SS. Duration and placement of sleep in a “disentrained” environment. Psychophysiology. 1984;21:106–13. doi: 10.1111/j.1469-8986.1984.tb02327.x. [DOI] [PubMed] [Google Scholar]
- 16.Welsh D, Richardson GS, Dement WC. Effect of running wheel availability on circadian patterns of sleep and wakefulness in mice. Physiol Behav. 1988;43:771–77. doi: 10.1016/0031-9384(88)90375-7. [DOI] [PubMed] [Google Scholar]
- 17.Edgar DM, Kilduff TS, Martin CE, Dement WC. Influence of running wheel activity on free-running sleep/wake and drinking circadian rhythms in mice. Physiol Behav. 1991;50:373–8. doi: 10.1016/0031-9384(91)90080-8. [DOI] [PubMed] [Google Scholar]
- 18.Vyazovskiy VV, Ruijgrok G, Deboer T, Tobler I. Running wheel accessibility affects the regional electroencephalogram during sleep in mice. Cereb Cortex. 2006;16:328–36. doi: 10.1093/cercor/bhi110. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–61. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Estabrooke IV, McCarthy MT, Ko E, et al. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–62. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida Y, Fujiki N, Nakajima T, et al. Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur J Neurosci. 2001;14:1075–81. doi: 10.1046/j.0953-816x.2001.01725.x. [DOI] [PubMed] [Google Scholar]
- 23.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–98. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–20. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.España RA, Valentino RJ, Berridge CW. Fos immunoreactivity in hypocretin-synthesizing and hypocretin-1 receptor-expressing neurons: effects of diurnal and nocturnal spontaneous waking, stress and hypocretin-1 administration. Neuroscience. 2003;121:201–17. doi: 10.1016/s0306-4522(03)00334-8. [DOI] [PubMed] [Google Scholar]
- 26.Lett BT, Grant VL, Koh MT, Flynn G. Prior experience with wheel running produces cross-tolerance to the rewarding effect of morphine. Pharmacol Biochem Behav. 2002;72:101–5. doi: 10.1016/s0091-3057(01)00722-5. [DOI] [PubMed] [Google Scholar]
- 27.Werme M, Messer C, Olson L, et al. Delta FosB regulates wheel running. J Neurosci. 2002;22:8133–8. doi: 10.1523/JNEUROSCI.22-18-08133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanagasioglu M, Borbely AA. Effect of voluntary locomotor activity on sleep in the rat. Behav Brain Res. 1982;4:359–68. doi: 10.1016/0166-4328(82)90060-2. [DOI] [PubMed] [Google Scholar]
- 29.Willie JT, Chemelli RM, Sinton CM, et al. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–30. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 30.Fujiki N, Cheng T, Yoshino F, Nishino S. Specificity of direct transitions from wake to REM sleep in orexin/ataxin-3 narcoleptic mice. Sleep. 2006;29:A225. doi: 10.1016/j.expneurol.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edgar DM, Seidel WF. Modafinil induces wakefulness without intensifying motor activity or subsequent rebound hypersomnolence in the rat. J Pharmacol Exp Ther. 1997;283:757–69. [PubMed] [Google Scholar]
- 32.Willie JT, Renthal W, Chemelli RM, et al. Modafinil more effectively induces wakefulness in orexin-null mice than in wild-type littermates. Neuroscience. 2005;130:983–95. doi: 10.1016/j.neuroscience.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takakusaki K, Takahashi K, Saitoh K, et al. Orexinergic projections to the cat midbrain mediate alternation of emotional behavioural states from locomotion to cataplexy. J Physiol. 2005;568:1003–20. doi: 10.1113/jphysiol.2005.085829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamuy J, Fung SJ, Xi M, Chase MH. Hypocretinergic control of spinal cord motoneurons. J Neurosci. 2004;24:5336–45. doi: 10.1523/JNEUROSCI.4812-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peever JH, Lai YY, Siegel JM. Excitatory effects of hypocretin-1 (orexin-A) in the trigeminal motor nucleus are reversed by NMDA antagonism. J Neurophysiol. 2003;89:2591–600. doi: 10.1152/jn.00968.2002. [DOI] [PubMed] [Google Scholar]
- 37.Dishman RK. Brain monoamines, exercise, and behavioral stress: animal models. Med Sci Sports Exerc. 1997;29:63–74. doi: 10.1097/00005768-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Anic-Labat S, Guilleminault C, Kraemer HC, Meehan J, Arrigoni J, Mignot E. Validation of a cataplexy questionnaire in 983 sleep-disorders patients. Sleep. 1999;22:77–87. [PubMed] [Google Scholar]
- 39.Nishino S, Mignot E. Pharmacological aspects of human and canine narcolepsy. Prog Neurobiol. 1997;52:27–78. doi: 10.1016/s0301-0082(96)00070-6. [DOI] [PubMed] [Google Scholar]
- 40.Kagan J, Berkun M. The reward value of running activity. J Comp Physiol Psychol. 1954;47:108. doi: 10.1037/h0058877. [DOI] [PubMed] [Google Scholar]
- 41.Nishino S, Bliesath J, Honda K, Mignot E. The occurence of cataplexy in relation to preceding REM sleep. Sleep. 2004;27:A248. [Google Scholar]
- 42.Gelb M, Guilleminault C, Kraemer H, et al. Stability of cataplexy over several months--information for the design of therapeutic trials. Sleep. 1994;17:265–73. doi: 10.1093/sleep/17.3.265. [DOI] [PubMed] [Google Scholar]
- 43.Clark E, Mochizuki T, Scammell T. Anticipation of food worsens murine cataplexy. Annual Meeting of the Society for Neuroscience. 2006:361–26. [Google Scholar]