Abstract
Study Objectives:
To determine the effects of sleep and sleep deprivation on plasma melatonin concentrations in humans and whether these effects are age-dependent.
Design:
At least 2 weeks of regular at-home, sleep/wake schedule followed by 3 baseline days in the laboratory and at least one constant routine (sleep deprivation).
Setting:
General Clinical Research Center (GCRC), Brigham and Women's Hospital, Boston, MA.
Participants:
In Study 1, one group (<10 lux when awake) of 19 young men (18-30 y) plus a second group (<2 lux when awake) of 15 young men (20-28 y) and 10 young women (19-27 y); in Study 2, 90 young men (18-30 y), 18 older women (65-81 y), and 11 older men (64-75 y). All participants were in good health, as determined by medical and psychological screening.
Interventions:
One to three constant routines with interspersed inversion of the sleep/wake cycle in those with multiple constant routines.
Measurements and Results:
Examination of plasma melatonin concentrations and core body temperature. Study 1. There was a small, but significant effect of sleep deprivation of up to 50 hours on melatonin concentrations (increase of 9.81 ± 3.73%, P <0.05, compared to normally timed melatonin). There was also an effect of circadian phase angle with the prior sleep episode, such that if melatonin onset occurred <8 hours after wake time, the amplitude was significantly lower (22.4% ± 4.79%, P <0.001). Study 2. In comparing melatonin concentrations during sleep to the same hours during constant wakefulness, in young men, melatonin amplitude was 6.7% ± 2.1% higher (P <0.001) during the sleep episode. In older men, melatonin amplitude was 37.0% ± 12.5% lower (P <0.05) during the sleep episode and in older women, melatonin amplitude was non-significantly 10.9% ± 8.38% lower (P = 0.13) during the sleep episode.
Conclusions:
Both sleep and sleep deprivation likely influence melatonin amplitude, and the effect of sleep on melatonin appears to be age dependent.
Citation:
Zeitzer JM; Duffy JF; Lockley SW; Dijk DJ; Czeisler CA. Plasma melatonin rhythms in young and older humans during sleep, sleep deprivation, and wake. SLEEP 2007;30(11):1437-1443.
Keywords: Melatonin, sleep deprivation, human, circadian, homeostat, body temperature, sleep, aging
INTRODUCTION
THE HYPOTHALAMIC SUPRACHIASMATIC NUCLEUS (SCN), THE CENTRAL CIRCADIAN PACEMAKER IN MAMMALS, CONTROLS OR INFLUENCES THE DAILY rhythmicity of many physiological variables, including pineal melatonin synthesis, the sleep/wake cycle, and core body temperature.1,2 The SCN output to the pineal gland is multisynaptic and controls the rhythmicity of pinealocyte arylalkylamine N-acetyltransferase activity, the rate limiting enzyme in the synthesis and the subsequent rhythm of melatonin in blood and cerebrospinal fluid.3–5 In both nocturnal and diurnal mammals, the pattern of the rhythmicity is such that plasma melatonin concentrations are high during the subjective night and low during the subjective day, even under conditions of constant darkness, forming a near square-wave pattern.2 Light, however, when applied for several minutes during the subjective night, can rapidly decrease plasma concentrations of melatonin.6–8
The rhythm of pineal melatonin production is determined primarily by the SCN and properly phased light exposure, and therefore is considered a reliable marker of both phase and amplitude of the central circadian pacemaker. Unlike other markers of the circadian clock (e.g., core body temperature), the pattern of plasma melatonin is thought to be minimally masked by exogenous factors such as stress or sleep.9,10 There is some evidence, however, that plasma melatonin concentrations may be influenced by exercise11 and posture.12–14 Plasma melatonin concentrations may also be affected by sleep deprivation, though the evidence is contradictory and difficult to interpret due to differences in those studies, including the lack of postural controls, use of stimulants to induce wakefulness, and/or the use of elevated ambient light intensities, all of which may have influenced plasma melatonin concentrations in those studies.9,15–20 Due to the frequent use of melatonin as a marker of the phase of the human circadian timing system, and the use of extended wakefulness in protocols that examine the circadian clock without the confound of sleep,21 it is of great interest to understand the interaction of sleep deprivation and plasma melatonin concentration. We therefore conducted a post hoc analysis of data from a series of studies conducted in our laboratory between 1990 and 2002 in which plasma melatonin levels were measured throughout extended episodes of wakefulness, and where the extended wake episodes began at different circadian phases. In addition, in order to better understand how aging might influence the effect of extended wake on melatonin levels, we included in our analysis data from young and older adults.
METHODS
Study 1
Two groups of healthy, young subjects were studied: Group 1 (n=19, all male) ranged in age from 18-30 years (23.1 ± 3.54 y, avg. ± SD) and Group 2 (n=25, 15 males) ranged from 19-28 years (23.2 ± 2.26 y, avg. ± SD). All subjects gave informed written consent; all experimental procedures were carried out in accordance with the principles of the Declaration of Helsinki and approved by the Brigham and Women's Hospital human research committee. For at least two weeks, subjects maintained a regular at-home sleep/wake schedule, verified by phone logs and further confirmed by actigraphy (Ambulatory Monitoring Inc., Ardsley NY) for at least 7 days before admission to the laboratory. During the prestudy screening, and throughout the study itself, subjects refrained from all prescription, nonprescription, over-the-counter, and recreational drugs, as well as caffeine and alcohol (at-home compliance verified by urinary toxicological screening upon inpatient admission).
Subjects spent 9, 11, or 16 days in an individual study room in the Environmental Scheduling Facility or the Intensive Physiological Monitoring Unit of the General Clinical Research Center at the Brigham and Women's Hospital (for complete details, see references 22-24). The 9-day study (n = 25, Group 2) had 3 baseline days (16 h scheduled wake/8 h scheduled sleep), a constant routine (CR, ∼50 h, see below) in which the subjects remained awake in bed in a constant, semi-recumbent posture, 1 day of an inverted timing of the sleep/wake schedule (still 16 h wake/8 h sleep), and a second CR (30 h). The 11-day study (n = 9, Group 1) had 3 baseline days (16 h wake/8 h sleep), a constant routine in which the subjects remained awake in bed in a constant, semi-recumbent posture (CR, ∼32 h, see below), 3 days of an inverted sleep/wake schedule, and a second CR (30-48 h). The 16-day study (n = 10, Group 1) was identical to the 11-day study, with the inclusion of a second set of 3 days on an inverted sleep/wake schedule and a third CR (∼48 h) (for details on the timing of the individual studies, see references 22-24). Use of multiple CRs both before and after the inversion of the sleep/wake schedule allows for an analysis of melatonin profiles that occur at various durations after waking.
For Group 1, illuminance was ∼180 lux during the wake episodes of the baseline days, <10 lux during all other wake episodes (CRs and inverted days), and <0.03 lux during all sleep episodes. The illuminance pattern was similar for Group 2, except that the illuminance was <2 lux during the last 8 hours of the third baseline day, the CRs, and the inverted day, and they experienced a high intensity experimental light pulse between the 2 CRs. Because of the potential effects of this light pulse on the post-exposure melatonin rhythm, only melatonin collected before the light pulse was used from the subjects in Group 2. As the baseline light exposure pattern in Groups 1 and 2 differed, and this might influence melatonin,25,26 these groups were analyzed separately. All lighting was produced with cool white fluorescent lamps, filtered for ultraviolet light, and the measurements made represent the maximum possible illumination at 183 cm vertical (corneal level).
All melatonin samples reported herein were obtained during the CRs and the sleep episode immediately preceding the first CR. The lighting was kept low and constant throughout the CRs (<10 lux in Group 1, <2 lux in Group 2) so as to not acutely suppress plasma melatonin concentrations or significantly affect the timing of the circadian oscillation.7 Subjects were kept continuously awake for the duration of the CR by technicians who remained with the subject. Throughout the CR, the subjects' posture was semi-recumbent, and they were restricted to sedentary activities that could be carried out while resting in bed; nutrients were provided in equal, hourly aliquots.
During the CR (Group 1 only), a rectal thermistor (YSI, Yellow Springs OH) recorded core body temperature (stored as the average of 1 min intervals). On baseline day 2 in all subjects (2 days before the first CR), an intravenous catheter, attached to a 3.7 m narrow-lumen tube that extended through a porthole to outside the room, was inserted into a forearm vein and used to collect frequent (1-6 times/h) blood samples. Catheters were replaced once every 7 days or as needed for comfort. The extended tubing length allowed for collection of blood samples from outside of the subject's room, during both wake and sleep episodes. Blood samples were spun in a centrifuge and the plasma frozen for later radioimmunoassay of melatonin (Group 1: DiagnosTech, Osceola WI; assay sensitivity of 5 pg/mL; intrassay and interassay coefficients of variation, 8% and 13%, respectively; Group 2: Brigham and Women's Hospital GCRC Core Lab; ALPCO RK-MEL assay kit; assay sensitivity of 0.2 pg/mL; intrassay and interassay coefficients of variation, 8% and 12%, respectively).
Melatonin is secreted in a near square-wave form, being at low plasma concentrations during the subjective day and elevating 10-fold or greater during the subjective night.27 We define, for the purposes of this analysis, the start and end of a melatonin secretion episode as the time at which plasma melatonin concentrations rose above (onset) and fell below (offset), respectively, 10 pg/mL (43.05 pM).28,29 For each episode that occurred during a CR, the duration that plasma melatonin concentrations were above 10 pg/mL, and the average of these concentrations, were calculated. The amplitude of each melatonin “peak” was calculated by taking the trapezoidal area under the curve for a 14-h segment encompassing the episode (from 2 h before melatonin onset until 12 h after melatonin onset). By examining a fixed time frame, the duration of integration was kept constant within and across subjects, regardless of the duration of the individual melatonin episode. Because the start or end time of integration usually did not have a corresponding blood sample taken at that precise minute, the concentration of plasma melatonin was interpolated from adjacent time points. Because of the high frequency of blood sampling (every 20-60 min), and because the interpolated concentration was at a time of typically low plasma concentrations (i.e., subjective day), interpolation can provide a good estimation of these plasma concentrations. To account for the large variation in melatonin amplitude between subjects,27 each 14-h amplitude measurement was normalized to the trapezoidal area under the curve calculated for the 8-h sleep episode that directly preceded the first CR.
Core body temperature data from Group 1 were fit with a 2-harmonic regression model30 restricted to a cycle length in the range of average circadian period (24.0 to 24.3 h).31 Data from the first 5 h of the CR were excluded. The remaining data were binned in 17-h intervals, the shortest length of time that yields accurate amplitude analysis using the 2-harmonic regression model (EB Klerman, ME Jewett, JF Duffy, personal communication). The amplitude of the fundamental component of the fitted model was used in these analyses.
Melatonin and temperature amplitude data were fit with a linear regression model (Origin 6.0, Microcal, Northampton MA) and melatonin data were tested with ANOVA or paired and unpaired, 2-tailed t-tests, as appropriate (Microsoft Excel 2002, v.10.2614.2625). Outliers in the normalized melatonin amplitude data were analyzed with a Grubbs' test (extreme studentized deviate) at a significance level of 0.01 (one detected and removed from Group 1). An additional 4 subjects (not described here) were excluded from Group 2 due to the occurrence of only a single peak of melatonin in an analysis that required 2 peaks during the same CR (one peak was partially during the sleep episode, see below).
Study 2
Three groups were studied: a group of 90 young males aged 18-30 (23.4 ± 3.52 years, avg. ± SD), a group of 11 older males aged 64-75 (67.9 ± 3.24 years) and a group of 18 older females aged 65-81 (68.7 ± 4.18 years). Each subject had the same prestudy and screening conditions as described for Study 1. As with the young subjects, all of the older subjects were in good physical and mental health with no chronic or acute medical conditions. All subjects underwent 3 standard days in the laboratory followed by a constant routine of at least 30 h. Baseline days and the constant routine are as described above for Group 1 in Study 1. A different analysis of these melatonin data has previously been reported.27 Subjects from this published data set were excluded if they had insufficient melatonin data collected during the sleep episode immediately preceding the CR (<4 blood samples assayed from the 8-h sleep episode). The average plasma melatonin concentration during this 8-h sleep episode immediately preceding the CR was calculated for each subject. The average plasma melatonin concentration during the same 8 clock hours, but occurring during the sleep-deprived wakefulness of the constant routine 24 h later, was also calculated for each subject. A simple ratio between the sleep episode melatonin and the constant routine melatonin was then determined. Data were analyzed with 2-tailed t-tests; 2-tailed, paired t-tests are noted (Microsoft Excel 2002, v.10.2614.2625). All data are presented as average ± SEM.
RESULTS
Study 1
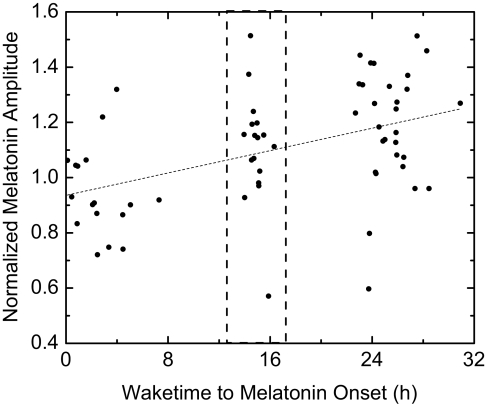
From the 19 subjects in Group 1, a total of 64 melatonin episodes collected during CRs were analyzed. One episode was excluded as an outlier. In the remaining 63 episodes, as the time between awakening and the onset of the melatonin secretion increased, the melatonin amplitude significantly increased (P < 0.005, ANOVA) (Figure 1). This increase, however, was due to the fact that melatonin onsets that occurred less than 8 h after wake time (i.e., melatonin onset during the behavioral daytime following a sleep inversion) were significantly lower in amplitude than those that occurred after at least 8 h of wakefulness (P <0.001, t-test). There was no significant effect of wake duration on melatonin amplitude once wake duration exceeded 8 h (P = 0.78, ANOVA). However, only melatonin episodes that started up to 32 h after wake onset were analyzed. To ensure that these results were not secondary to our normalization procedure, we analyzed a subset of 16 pairs of melatonin peaks (32 of the 64 total peaks) from 12 of the 19 subjects (4 of the subjects had 2 pairs from 2 separate CRs). CRs with only one melatonin peak were not included in this analysis. Each pair of melatonin peaks occurred during the same CR, with the first occurring <8 h after wake onset and the second ∼24 h later. As all the data were paired, raw amplitudes (rather than normalized) were used in the analysis (pairing removes effects of large intersubject variability in melatonin amplitude). Consistent with the total group findings using normalized amplitudes in which peaks occurring >8 h after wake onset were 22.6 % larger than those occurring closer to wake time, using this subset of raw, paired amplitudes revealed that within a given CR the earlier peaks of melatonin were 22.4% ± 4.79% smaller than the later peaks (P <0.001, paired t-test).
Figure 1.
Increase in normalized plasma melatonin amplitude with time awake. A fitted linear regression (dashed line) is shown. The dashed box indicates the normal phase position of melatonin onset in entrained, young adults (12.5-17.1 h after wake onset32). Note, 14 h of melatonin data (CR) are normalized to 8 h of melatonin data (sleep episode) for the purposes of this analysis; thus, an amplitude of 1.0 does not mean that melatonin during the constant routine was equal to melatonin during the sleep episode. Normalization is necessary given the large interindividual variation of plasma melatonin peaks.
The difference in melatonin amplitude between the 2 groups (i.e., melatonin onset >8 h or <8 h after wake time) could be explained either by an increase in the amount of time that melatonin concentrations are elevated (i.e., the “duration” of the pulse), an increase in the average amount of melatonin during the time of elevation, or a combination of the two. Normalized melatonin amplitudes in the <8-h group averaged 0.947 ± 0.0395, while they averaged 1.16 ± 0.0305 in the >8-h group (P <0.001, t-test). The duration of the melatonin pulse was accordingly shorter in the <8-h group (8.96 ± 0.343 h) as compared to the >8-h group (10.1 ± 0.229 h) (P <0.05, t-test). The average melatonin level was also significantly different, being 89.2 ± 4.99 pM in the <8-h group and 126 ± 11.6 pM in the >8-h group (P <0.01, t-test). Thus, melatonin concentrations were both lower overall and elevated for a shorter duration when the onset of melatonin occurred less than 8 h after the onset of wakefulness.
To determine if the increase in melatonin amplitude with time awake was due to an increase in the amplitude of the circadian clock (an effect upstream from the source of melatonin production in the pineal gland), we examined the amplitude in the rhythm of core body temperature during the same CRs as were analyzed for melatonin in Group 1. Core body temperature in humans, when properly unmasked during a constant routine, has been shown to be a good marker of both the phase and amplitude of the central circadian clock.1 We found, however, no significant change in the fitted amplitude of the circadian rhythm of core body temperature relative to the duration of time awake (P = 0.41, ANOVA, n = 19).
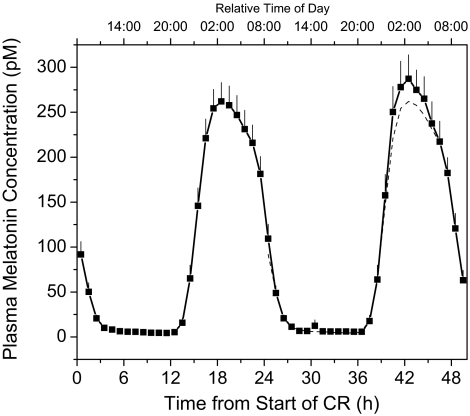
As the range of CR durations limited our previous analysis as to exclude the potential effects of very long sleep deprivations on melatonin, we expanded our analysis to a second cohort (Study 1, Group 2 in Methods) that had CRs of approximately 50 h in length in <2 lux, during which 2 full melatonin peaks occurred. The initial peak (n=25), which occurred at a normal phase angle to sleep (i.e., starting 14.6 ± 0.243 h after wake time), had an AUC of 2465 ± 254.8 pM*14 h, while the second peak, which started 38.4 ± 0.217 h after wake time, had an AUC of 2696 ± 300.1 pM*14 h (Figure 2). This represents a 9.81% ± 3.73% increase in melatonin AUC between the first and second peaks (P <0.05, paired t-test). There was, therefore, a small, but significant increase of ∼0.41%/h in the amplitude of melatonin peaks that occurred at or after a normal (12.5 h) phase relationship with wake onset, independent of the change associated with onsets that occur earlier than 8 hours after awakening.
Figure 2.
Average (±SEM) plasma melatonin during a single episode of extended (50 h) wakefulness (n=25, young men and women, Group 2 in Study 1). Data were averaged per hour within and then between subjects. The first melatonin cycle is replotted as a dashed line in the time frame of the second cycle for comparison.
Study 2
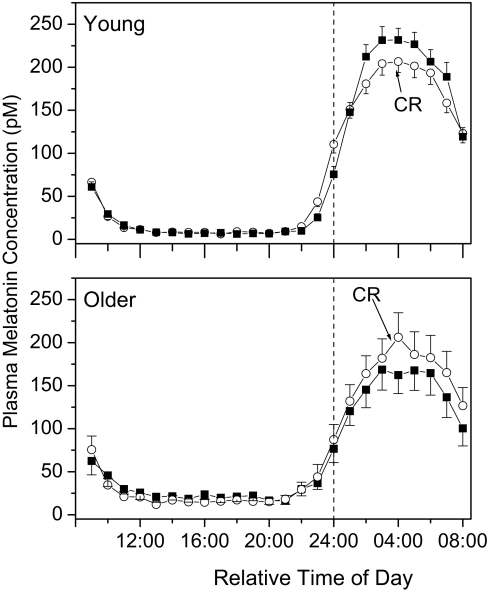
The young males (n = 90) had an average plasma melatonin concentration of 195.2 ± 11.99 pM during the sleep episode and 175.5 ± 11.00 pM during the same clock hours while awake on the constant routine 24 h later (Figure 3A), averaging 6.71% ± 2.12% higher during the sleep episode as compared to the CR (P <0.0001, paired t-test). The older subjects had an average plasma melatonin concentration of 148.7 ± 18.95 pM during the sleep episode and 166.06 ± 20.95 pM during the same clock hours on constant routine (Figure 3B), resulting in plasma melatonin concentrations that were 20.8% ± 7.30% lower during the sleep episode than during the CR (P <0.02, paired t-test). In comparing the ratio of melatonin during the sleep episode to that during the CR (i.e., relative change) between the 2 age groups, there was a significant difference between the young and older subjects (P <0.001, t-test). There was no difference between the young and older subjects in the average level of melatonin during the 8 h of the CR corresponding to the usual sleep episode (P = 0.68, t-test; 24:00-08:00 in Figure 3), which agrees with our earlier findings from the entire CR data train in these same subjects.27 There was a trend for a lower average melatonin level during the sleep episode in the older compared to the young subjects, although this did not reach significance (P = 0.052, t-test).
Figure 3.
Average (±SEM) plasma melatonin in young (top, n=90) and older (bottom, n=29) subjects during a normally phased sleep episode (closed boxes) and a constant routine where they remained awake at the same clock hours (open circles). Data were aligned such that each subject's wake time was graphically adjusted to 08:00 and the data from the baseline day and night and from the CR expressed relative to wake time; sleep time is from 24:00 to 08:00. Melatonin data were averaged hourly within and then across subjects.
Our groups of young and old subjects were not balanced for sex (all men in the young group, 38% men in the older group), so we also examined the possibility that a sex difference could explain the observed difference in sleep vs. CR melatonin level. Older men had melatonin concentrations of 119.4 ± 34.40 pM during their sleep episode and 139.8 ± 34.84 pM during the same clock hours during the CR (37.0% ± 12.5% increase, P <0.05 paired t-test); the sleep:CR ratio was 0.76 ± 0.063. Older women had melatonin concentrations of 166.6 ± 21.85 pM during their sleep episode and 182.1 ± 26.24 pM during the same clock hours during the CR (nonsignificant 10.9% ± 8.38% increase, P = 0.13, paired t-test); the sleep:CR ratio was 0.94 ± 0.074. There were no significant differences between older men and women in the average melatonin during the sleep episode (P = 0.23, t-test), during the same 8 clock hours during the constant routine (P = 0.34, t-test), or in the sleep:CR ratio (P = 0.07, t-test), although this last comparison does near significance. In comparing the older women to the young men, there was no significant difference between the sleep episode (P = 0.32, t-test) or constant routine (P = 0.81, t-test) melatonin averages, but the sleep:CR ratio was significantly higher in young males (P <0.05, t-test), as was observed for the total data set. In comparing the older men to the young men, there was no significant difference in the constant routine melatonin average (P = 0.29, t-test), but the sleep:CR ratio was significantly lower in the older men (P <0.001, t-test) and the average melatonin level during the eight hour sleep episode was also significantly lower in the older men (P <0.05, t-test), which was not observed in the total data set comparison.
DISCUSSION
Our data indicate that melatonin concentrations are significantly affected by both sleep and sleep deprivation, and also quite strongly by the phase angle of melatonin onset to the offset of the prior sleep episode. In examining normalized melatonin data in young men, when melatonin onset occurs within 8 h of wake time (an unusual phase relationship that might occur during travel or shift work), the duration of the elevated plasma levels and average concentration of melatonin during this time are ∼22% shorter and smaller than they would have been had the melatonin onset occurred at a normal phase angle. When melatonin onset occurs at a normal phase angle (n.b., normally, melatonin onset occurs 12.5-17.1 h after wake time in healthy, young men32) or any time up to 30 h after wake time, there is no significant association of melatonin amplitude with duration of wake. When melatonin onset occurs after 36 h of wake time (i.e., a second melatonin peak during the same continuous episode of wakefulness), the amplitude is significantly increased by ∼10% over the melatonin peak that occurred earlier in the wake episode.
The observed reduction in melatonin episode duration and average level associated with melatonin onsets that occur within 8 h of waking does not appear to be due to a change in the amplitude of the central circadian pacemaker, because no associated change in temperature amplitude was observed in the same subjects. It is likely that there is either a residual effect of sleep on the pineal gland, or that there is a homeostatic phenomenon such that after 8 h of wakefulness, there is a near plateau of homeostatic pressure on pineal production of melatonin. Another possibility is that the photoreceptors responsible for transmitting light information from the retina to the SCN have been dark adapted during the 8 hours of scheduled sleep in darkness25 and are sensitive enough to evoke a suppression of melatonin early, but not late, in the course of the CR. A final possibility is that the changes in the sympathetic and parasympathetic activity that accompany awakening diminish pineal production of melatonin.
Besides this possibly indirect effect of sleep on melatonin, sleep has a direct effect on melatonin in young men. In these individuals, plasma melatonin during sleep is elevated compared to the same clock hours during wakefulness under CR conditions. Previous investigators have shown either no difference between melatonin during a sleep episode and melatonin during the same clock hours, but with sleep deprivation,9,17,33 or have reported elevated melatonin during the sleep deprivation episode.15,19 Those earlier studies, however, failed to control for the effects of light capable of partially suppressing melatonin concentrations during the sleep deprivation 7,34 and did not take into account postural or activity level differences between the sleep and sleep deprivation conditions. Under our conditions of controlled dim light exposure, low activity levels, and controlled posture, we observed a small increase in melatonin during sleep, as compared to sleep deprivation. This difference may be due to any number of factors that differ between the sleep episode and the CR, including sleep itself, posture, food intake, activity, and even the very low light level. Prior to the onset of the sleep episode, subjects change from the standing to the supine posture, which has been shown to affect melatonin concentrations.12,13 During the CR in Study 2, subjects have been in a semi-recumbent posture for ∼16 h before the melatonin episode occurs. Therefore, one would expect that a change in melatonin associated with a shift in posture would be most prominent at the beginning of the sleep episode, when compared with the CR data. As can be seen in Figure 3, however, it is not until the second hour of the sleep episode that changes in melatonin are manifested, and these changes last until the end of the melatonin secretory episode. Thus, not only is there a delay from the change in posture to a change in melatonin, but the effects last longer that would be expected with a postural change; though a change in posture having an influence remains a possibility.
A difference in activity is also unlikely to have a large impact on our finding of a higher melatonin level during sleep than during wakefulness at the same clock hours in young men. The studies in which locomotion has been shown to have an acute effect on melatonin concentrations used an exercise-condition locomotion. Subjects in our study were either lying in bed attempting to sleep (in the sleep condition) or were limited to activities that could be done while confined to bed during the CR (and a member of the technical staff remained in the room with them during the CR to ensure that only very low levels of activity occurred). It is possible that the hourly aliquots of food and water during the CR (as opposed to the absence of food and water intake during the sleep episode) influenced melatonin, but there was no unusual composition to the food that would obviously account for the change (e.g., high tryptophan).
It is also possible that the low light levels during the CR were suppressing melatonin levels in the young males, though we did not observe consistent suppression of melatonin with light of this low an illuminance 7,34 nor did we observe a significant difference in melatonin levels in CRs conducted in <10 lux (Study 1, Group 1) and in <2 lux (Study 1, Group 2) (P = 0.26, t-test). There has been observed, however, a correlation between lower melatonin concentrations in dim light (5-13 lux) and phase shift responses to the dim light,35 indicating that even dim light might have the ability to both affect the circadian pacemaker and slightly suppress plasma melatonin concentrations.
Another likely possibility is that sleep itself caused an augmentation of the melatonin amplitude. It is possible that changes in sympathetic (superior cervical ganglion) or parasympathetic (sphenopalatine ganglion) innervation of the pineal gland during sleep elicit this alteration. Alternatively, central brain mechanisms (e.g., decrease in monoaminergic tone) or peripheral organs (e.g., decreased catabolism by liver during sleep) may be involved in this mechanism. These types of changes may also underlie the change in melatonin amplitude that occurs when melatonin occurs before 8 h of wakefulness have elapsed.
Paradoxically, in older men and women, melatonin concentrations are lower during the sleep episode than during the same 8 h that occur during the wakefulness of a CR. Rather than being ∼7% higher during the sleep episode (as in the young men), melatonin concentrations in older men and women were 21% lower during the sleep episode as compared with the same clock hours during the CR. This appears mainly due to results from the older men, who had melatonin concentrations that were 37% lower during the sleep episode. In contrast, the older women had only an 11% decline during the sleep episode. As with the elevated melatonin levels observed during the sleep episode of the young men, the mechanism resulting in lower melatonin during the sleep episode of older men and women is unknown. It does further indicate that there may be significant sex-related differences in age-related changes in sleep physiology.36
The finding that melatonin levels during the wakefulness of the CR are higher in older subjects and lower in younger subjects, both in comparison to their respective sleep episode values, has important implications for the debate over whether melatonin concentrations decline with healthy aging. Since 1979, there have been more than 63 primary manuscripts addressing changes in melatonin with age. Of those papers, 34 indicated that melatonin was lower in older individuals, 22 showed no change with age, 6 had mixed results, and 1 described an increase in melatonin levels with age. All but 2 of the studies analyzed melatonin rhythms in the presence of a sleep-wake cycle. In our current analysis, we observed a significant difference between sleep episode melatonin in the young and older men, though not between the young men and older women. As we reported earlier using this same subject group but comparing the entire CR melatonin profile between the age groups,27 we did not find a significant difference with age in the circadian amplitude of melatonin concentrations during the wakefulness of a CR. By examining melatonin during the sleep episode in the present report, we found a decline in melatonin level in the older men, but not the older women, as compared to the young men. In the present study we also examined melatonin during the nighttime hours of the CR when the subjects remained awake, and again there was no decline in either the older men or women, as compared to the young men. Our experimental protocol, using 2 weeks of at-home sleep/wake stabilization, 3 baseline days in the laboratory, and a CR, was designed to examine the underlying circadian rhythm of melatonin. This also tries to answer the question: does the human pineal change with age such that it no longer has the capacity to function (i.e., produce melatonin) when compared with a younger pineal? There are, obviously, changes with aging, likely associated with changes in response to sleep, that are able to independently affect plasma melatonin levels. While the capacity of the system to produce melatonin appears not to change significantly, there do appear to be changes with aging, possibly the well-described change in sleep architecture or continuity, that can affect plasma melatonin concentrations. It will be critical in future experiments to determine the cause of these changes and whether they have a significant clinical impact upon sleep.
ACKNOWLEDGMENTS
The authors wish to thank Jessica E. Daniels for helping with data analysis, the subjects who participated in these studies, and the research technicians and staff of the Brigham and Women's Hospital GCRC and the Division of Sleep Medicine, and George C. Brainard, PhD, and colleagues at Thomas Jefferson University, Philadelphia, without whom these studies could not have been performed. These studies were supported in part by grants NIA-1-PO1-AG09975 (CAC) and NIA-1-R01-AG06072 (CAC) from the National Institute on Aging; grant NIMH-1-R01-MH45130 (CAC) from the National Institute of Mental Health; grant NINDS-1-R01-NS36590 (Brainard) from the National Institute of Neurological Disorders and Stroke; grant NAGW-4033 (CAC) from the National Aeronautics and Space Administration; and General Clinical Research Center grant NCRR-GCRC-M01-RR-02635 (Williams). JMZ was supported by training grant 5-T32-DA07282-03 from the National Institute on Drug Abuse.
Footnotes
Disclosure Statement
This is not an industry supported study. Dr. Dijk has received research support from H. Lundbeck A/S and has performed consulting/advisory services fro Phillips Lighting, H. Lundbeck A/S, Cephalon, Merck, Sanofi-Aventis, and GlaxoSmithKline. Dr. Czeisler has received research support from Cephalon and Pfizer; has consulted for or served on the advisory board of Actelion, Avera, Cephalon, Coca-Cola, Hypnion, Morgan Stanley, Sleep Multimedia, Respironics, Takeda, Vanda, and Warburg-Pincus; has financial interests in Hypnion, Lifetrac, and Vanda; and has participated in speaking engagements for Cephalon, Sanofi-Aventis, and Takeda. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Jewett ME, Kronauer RE, Czeisler CA. Phase-amplitude resetting of the human circadian pacemaker via bright light: a further analysis. J Biol Rhythms. 1994;9:295–314. doi: 10.1177/074873049400900310. [DOI] [PubMed] [Google Scholar]
- 2.Klein DC. The mammalian melatonin rhythm generating system. In: Wetterberg L, editor. Light and biological rhythms in man. New York: Pergamon Press; 1993. pp. 55–70. [Google Scholar]
- 3.Bogumil RJ. Pulsatile variations in hormone levels. In: Ferin EM, Halberg F, Richart R, Vande Wiele R, editors. Biorhythms and human reproduction. New York: John Wiley and Sons; 1974. pp. 107–31. [Google Scholar]
- 4.Coon SL, Roseboom PH, Baler R, et al. Pineal serotonin N-acetyltransferase: expression cloning and molecular analysis. Science. 1995;270:1681–3. doi: 10.1126/science.270.5242.1681. [DOI] [PubMed] [Google Scholar]
- 5.Zeitzer JM, Ayas NT, Shea SA, Brown R, Czeisler CA. Absence of detectable melatonin and preservation of cortisol and thyrotropin rhythms in tetraplegia. J Clin Endocrinol Metab. 2000;85:2189–96. doi: 10.1210/jcem.85.6.6647. [DOI] [PubMed] [Google Scholar]
- 6.McIntyre IM, Norman TR, Burrows GD, Armstrong SM. Human melatonin response to light at different times of the night. Psychoneuroendocrinol. 1989;14:187–93. doi: 10.1016/0306-4530(89)90016-4. [DOI] [PubMed] [Google Scholar]
- 7.Zeitzer JM, Dijk D-J, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanematsu N, Honma S, Katsuno Y, Honma K. Immediate response to light of rat pineal melatonin rhythm: analysis by in vivo microdialysis. Am J Physiol. 1994;266:R1849–55. doi: 10.1152/ajpregu.1994.266.6.R1849. [DOI] [PubMed] [Google Scholar]
- 9.Morris M, Lack L, Barrett J. The effect on sleep/wake state on nocturnal melatonin excretion. J Pineal Res. 1990;9:133–8. doi: 10.1111/j.1600-079x.1990.tb00701.x. [DOI] [PubMed] [Google Scholar]
- 10.Parfitt AG, Klein DC. Sympathetic nerve endings in the pineal gland protect against acute stress-induced increase in N-acetyltransferase (EC 2.3.1.5) activity. Endocrinol. 1976;99:840–51. doi: 10.1210/endo-99-3-840. [DOI] [PubMed] [Google Scholar]
- 11.Reiter RJ, Richardson BA. Some perturbations that disturb the circadian melatonin rhythm. Chronobiol Intl. 1992;9:314–21. doi: 10.3109/07420529209064541. [DOI] [PubMed] [Google Scholar]
- 12.Deacon S, Arendt J. Posture influences melatonin concentrations in plasma and saliva in humans. Neurosci Letters. 1994;167:191–4. doi: 10.1016/0304-3940(94)91059-6. [DOI] [PubMed] [Google Scholar]
- 13.Nathan PJ, Jeyaseelan AS, Burrows GD, Norman TR. Modulation of plasma melatonin concentrations by changes in posture. J Pineal Res. 1998;24:219–23. doi: 10.1111/j.1600-079x.1998.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 14.Deacon S, Arendt J, English J. Posture: a possible masking factor of the melatonin circadian rhythm. In: Touitou Y, Arendt J, Pévet P, editors. Melatonin and the pineal gland: from basic science to clinical application. Amsterdam: Elsevier Science Publishers B.V.; 1993. pp. 387–90. [Google Scholar]
- 15.Åkerstedt T, Fröberg JE, Friberg Y, Wetterberg L. Melatonin excretion, body temperature and subjective arousal during 64 hours of sleep deprivation. Psychoneuroendocrinol. 1979;4:219–25. doi: 10.1016/0306-4530(79)90005-2. [DOI] [PubMed] [Google Scholar]
- 16.Åkerstedt T, Gillberg M, Wetterberg L. The circadian covariation of fatigue and urinary melatonin. Biol Psychiatry. 1982;17:547–54. [PubMed] [Google Scholar]
- 17.Salín-Pascual RJ, Ortega-Soto H, Huerto-Delgadillo L, Camacho-Arroyo I, Roldán-Roldán G, Tamarkin L. The effect of total sleep deprivation on plasma melatonin and cortisol in healthy human volunteers. Sleep. 1988;11:362–9. doi: 10.1093/sleep/11.4.362. [DOI] [PubMed] [Google Scholar]
- 18.von Treuer K, Norman TR, Armstrong SM. Overnight human plasma melatonin, cortisol, prolactin, TSH, under conditions of normal sleep, sleep deprivation, and sleep recovery. J Pineal Res. 1996;20:7–14. doi: 10.1111/j.1600-079x.1996.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 19.Brun J, Chamba G, Khalfallah Y, et al. Effect of modafinil on plasma melatonin, cortisol and growth hormone rhythms, rectal temperature and performance in healthy subjects during a 36 h sleep deprivation. J Sleep Res. 1998;7:105–14. doi: 10.1046/j.1365-2869.1998.00100.x. [DOI] [PubMed] [Google Scholar]
- 20.Lakin-Thomas PL. A beginner's guide to limit cycles, their uses and abuses. Biol Rhythm Res. 1995;26:216–32. [Google Scholar]
- 21.Duffy JF, Dijk D-J. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms. 2002;17:4–13. doi: 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]
- 22.Duffy JF, Kronauer RE, Czeisler CA. Phase-shifting human circadian rhythms: Influence of sleep timing, social contact and light exposure. J Physiol. 1996;495:289–97. doi: 10.1113/jphysiol.1996.sp021593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeitzer JM, Kronauer RE, Czeisler CA. Photopic transduction implicated in human circadian entrainment. Neurosci Letters. 1997;232:135–8. doi: 10.1016/s0304-3940(97)00599-5. [DOI] [PubMed] [Google Scholar]
- 24.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88:4502–5. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 25.Smith KA, Schoen MW, Czeisler CA. Adaptation of human pineal melatonin suppression by recent photic history. J Clin Endocrinol Metab. 2004;89:3610–4. doi: 10.1210/jc.2003-032100. [DOI] [PubMed] [Google Scholar]
- 26.Hébert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeitzer JM, Daniels JE, Duffy JF, et al. Plasma melatonin concentration: does it decline with age? Am J Med. 1999;107:432–6. doi: 10.1016/s0002-9343(99)00266-1. [DOI] [PubMed] [Google Scholar]
- 28.Lewy AJ, Sack RL, Blood ML, Bauer VK, Cutler NL, Thomas KH. Melatonin marks circadian phase position and resets the endogenous circadian pacemaker in humans. In: Waterhouse JM, editor. Circadian clocks and their adjustment. Chichester, UK: Wiley; 1995. pp. 303–21. [DOI] [PubMed] [Google Scholar]
- 29.McArthur AJ, Lewy AJ, Sack RL. Non-24-hour sleep-wake syndrome in a sighted man: circadian rhythm studies and efficacy of melatonin treatment. Sleep. 1996;19:544–53. doi: 10.1093/sleep/19.7.544. [DOI] [PubMed] [Google Scholar]
- 30.Brown EN, Czeisler CA. The statistical analysis of circadian phase and amplitude in constant-routine core-temperature data. J Biol Rhythms. 1992;7:177–202. doi: 10.1177/074873049200700301. [DOI] [PubMed] [Google Scholar]
- 31.Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–81. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 32.Duffy JF, Zeitzer JM, Rimmer DW, Klerman EB, Dijk D-J, Czeisler CA. Peak of circadian melatonin rhythm occurs later within the sleep of older subjects. Am J Physiol. 2002;282:E297–303. doi: 10.1152/ajpendo.00268.2001. [DOI] [PubMed] [Google Scholar]
- 33.Strassman RJ, Peake GT, Qualls CR, Lisansky EJ. A model for the study of the acute effects of melatonin in man. J Clin Endocrinol Metab. 1987;65:847–52. doi: 10.1210/jcem-65-5-847. [DOI] [PubMed] [Google Scholar]
- 34.Zeitzer JM, Khalsa SBS, Duffy JF, et al. Dose-dependent response of the human circadian system to photic stimulation during the late biological night. Am J Physiol. 2005;289:R839–44. doi: 10.1152/ajpregu.00232.2005. [DOI] [PubMed] [Google Scholar]
- 35.Cajochen C, Jewett ME, Dijk D-J. Human circadian melatonin rhythm phase delay during a fixed sleep-wake schedule interspersed with nights of sleep deprivation. J Pineal Res. 2003;35:149–57. doi: 10.1034/j.1600-079x.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 36.Dijk D-J. Sleep of aging women and men: back to basics. Sleep. 2006;29:12–3. doi: 10.1093/sleep/29.1.12. [DOI] [PubMed] [Google Scholar]