Abstract
Study Objectives:
This study examined the effects of liability to anxiety and stressful life events on the onset of sleep disturbances.
Design:
A 5-year longitudinal observational cohort study.
Participants:
A population sample of 16,627 men and women with undisturbed sleep and 2572 with disturbed sleep at baseline.
Interventions:
N/A.
Measurements and Results:
Liability to anxiety, as indicated by a general feeling of stressfulness (the Reeder stress inventory) and symptoms of sympathetic nervous system (SNS) hyperactivity, was assessed at baseline. The occurrence of post-baseline life events and sleep disturbances was measured at follow-up five years later. Both liability to anxiety and exposure to negative life events were strongly associated with sleep disturbances. Among the men liable to anxiety, the odds of sleep disturbances were 3.11 (95% CI 1.90-5.10) times higher for those who had experienced a severe life event within 6 months than for the others. For the men not liable to anxiety, the corresponding odds ratio was only 1.13 (95% CI 0.40-3.18). For the men and women liable to anxiety, the odds ratio for sleep disturbance 0-6 months after divorce was 2.05 (95% CI 1.38-3.05), with the corresponding odds ratio being 1.47 (95% CI 0.84-2.58) for the men and women not liable to anxiety. The effects of total or specific life events on sleep after 6 months were not dependent on liability to anxiety.
Conclusion:
This study provides prospective evidence that individuals liable to anxiety may be at particularly high risk of post-event sleep disturbances at least during the first months after the event.
Citation:
Vahtera J; Kivimäki M; Hublin C; Korkeila K; Suominen S; Paunio T; Koskenvuo M. Liability to anxiety and severe life events as predictors of new-onset sleep disturbances. SLEEP 2007;30(11):1537-1546.
Keywords: Sleep disturbance, liability, anxiety, stress, sympathetic nervous system, life events
INTRODUCTION
THE DEVELOPMENT OF CHRONIC SLEEP DISTURBANCES IS A COMPLEX PROCESS, INVOLVING PREDISPOSING, PRECIPITATING, AND PERPETUATING FACTORS IN ITS long-term evolution.1 It has been suggested that psychophysiological traits that predispose an individual to heightened arousal or reactivity increase the overall risk of poor sleep, whereas negative life events and other stressors have been assumed to act as factors precipitating the onset of a sleep disturbance.2 However, evidence from large-scale prospective studies confirming these associations and the temporal order between the hypothesized predisposing and precipitating factors is largely lacking.3,4
Studies of twins have pointed to the existence of genetic vulnerability to insomnia,5–7 and there is evidence that personality traits, such as a disposition towards neuroticism or symptoms of anxiety, may act as predisposing factors for disturbed sleep.3 People who have scored high on a questionnaire measuring the likelihood of having difficulty sleeping after stressful situations had indeed greater sleep disruption in polysomnographic assessment, and this result suggests increased vulnerability to sleep disturbances.8 Other studies have found an association between feelings of daily stress and psychiatric disorders,9 as well as between neurovegetative symptoms and psychiatric disorders.10,11 For example, palpitation, sweating, trembling, chest pain and hot flushes or cold chills are common among people liable to anxiety.11 Such liability has been suggested to be linked with vulnerability to subjective sleep disturbance,3,5,6 but to our knowledge, prospective studies to confirm its status as a predisposing factor for sleep disturbances are largely lacking.
Stressful life events are commonly believed to act as important precipitating factors in sleep disturbances.4,12 Histories furnished by patients with chronic insomnia suggest that a sleep disorder frequently begins as a stress-related phenomenon.13 According to retrospective data as well, subjective problems with sleep are common in both the immediate and long-term aftermath of exposure to traumatic events,12 accidents,14 rape and physical assault,15 and death or illness in the family.16,17 Yet, the empirical evidence available is still limited, and the time order between the hypothesized predisposing trait, the precipitating event and the development of chronic sleep disturbances is not known with certainty.1,18 Moreover, as many of the hypothesized predisposing factors are determined on the basis of clinical experience rather than on the basis of systematic research, there is a recognized need for large-scale prospective studies linking predisposing factors and precipitating events with the development of sleep disturbances.3,4
We considered that liability to anxiety involves components such as perceived daily stress and SNS activation symptoms. Hyperarousal is a main component of various proposed models of insomnia1–4 and perception of stressfulness and SNS activation symptoms are common manifestations of hyperarousal.8–11 In this study, we examined the role of liability to anxiety in the onset of sleep disturbances after exposure to stressful life events in a large population-based sample with undisturbed sleep and no exposure to such events at baseline. Vulnerability to acute sleep problems may be a marker of an increased risk of developing chronic sleep disturbances.8 Thus we hypothesized that stressful life events may be associated with new-onset sleep disturbances, not only shortly after the event (i.e., the acute situation), but also some years after the event, and that this association would be more pronounced among people liable to anxiety. We also examined whether the effects of stressful life events on sleep depended on liability to anxiety among participants with sleep disturbances prior to stressful life events.
METHODS
Population
The data were derived from the Health and Social Support (HeSSup) study, a longitudinal study on a population sample representative of the Finnish population in the following 4 age groups: 20-24, 30-34, 40-44, and 50-54 years at Time 1.19 The Time 1 postal survey was conducted in 1998, and 5 years later (Time 2), a follow-up questionnaire was sent to all of the participants still living in Finland. Of the 25,901 respondents at Time 1, 216 had died during the 5-year follow-up, 234 had moved abroad, and 969 could not be reached due to unknown addresses. Altogether, 19,629 responded (response rate 80.2%). Of these, we excluded those with incomplete responses on sleep either at Time 1 (n=135) or at Time 2 (n=246). Thus the final sample consisted of 19,199 participants (7371 men; 11,828 women). The Turku University Central Hospital Ethics Committee approved the study.
Sleep
As an indication of sleep disturbances, we used the subjective assessment of sleep quality. It was measured with a single item, “How well do you sleep in general,” with a 5-point response scale (1 = well; 2=rather well; 3=rather poorly; 4=poorly; 5=cannot say).”Cannot say” responses were coded as missing responses. The response options for sleep quality were classified as undisturbed sleep (options “well/rather well”) or disturbed sleep (options “rather poor/poor”). The 5-year test-retest reliability for sleep quality was r = 0.51.
Liability to Anxiety
Symptoms of prominent tension, worry and feelings of apprehension about everyday events and problems are common features of anxiety.11,20 Autonomic arousal is also common in people liable to anxiety.11,20 For example, the specific somatic symptoms given prominence in the diagnoses of generalized anxiety disorder include symptoms of autonomic arousal (palpitation, sweating, trembling, dry mouth), chest and abdominal symptoms (difficulty breathing, feeling of choking, chest pain, nausea), and general symptoms (hot flushes or cold chills, numbness or tingling, muscle tension, restlessness and inability to relax, difficulty swallowing).11,20 Thus we assessed liability to anxiety according to the following 2 indicators: 1) general feeling of stressfulness in daily life and 2) symptoms of sympathetic nervous system (SNS) hyperactivity, both at Time 1.
The Reeder stress inventory,21 a 4-item questionnaire instrument widely used earlier, was used to measure the general feeling of stressfulness in daily life.9,22 This stress inventory consists of the following four statements: 1) “In general I am usually tense or nervous”; 2) “There is a great amount of nervous strain connected with my daily activities”; 3) “At the end of the day I am completely exhausted mentally and physically”; and 4) “My daily activities are extremely trying and stressful.” Participants indicate the extent to which each statement applies to them using a 5-point Likert format. The mean score of the 4 statements was divided into quartiles (low / medium low / medium high / high general feeling of stressfulness). The mean was 2.30 (SD 0.74), the coefficient-alpha reliability was α=0.76 and the 5-year test-retest reliability was r=0.53.
Symptoms of SNS hyperactivity were measured using an 8-item scale derived from the Finnish Twin Cohort Study.23 This measure requests the occurrence of the following 8 symptoms within the past month: 1) “Palpitation without exercise,” 2) “Irregular heartbeat,” 3) “Chest pain upon anger or emotion,” 4) “Sweating without exercise,” 5) “Flushing,” 6) “Tremor of hands,” 7) “Tremor of voice,” 8) “Muscle twitching.” The following 4 alternatives were given for each item: daily or almost daily, weekly, less often, never. The mean score of the 8 statements was divided into quartiles (low / medium low / medium high / high general feeling of stressfulness). The mean was 1.47 (SD 0.44), the coefficient-alpha reliability was α=0.77 and the 5-year test-retest reliability r=0.59. The correlation between the 2 variables measuring stress sensitivity was 0.39.
Severe Life Events
The measure of weighted life events was based on the list of 21 negative life event types derived from those used in earlier studies.24–26 The response format included the following categories (yes/no): never, within the previous 6 months, within the previous 5 years, over 5 years ago, never. The severity of each event type was classified according to the following categories: 1 = not so burdensome, 2 = burdensome, 3 = extremely burdensome. Only events that occurred after Time 1 were considered in this study. Sleep disturbances can be a symptom of illness and injury. To avoid circular causality between exposure and outcome, we excluded from the analysis 3 events that may be related to changes in the health of the participant: severe injury, illness causing work disability of over 21 days, and retirement. Because the participants were under 60 years of age at Time 2, the reason for retirement was almost exclusively ill health (in Finland the official age of retirement is 65 years).
In agreement with previous studies, we determined weights as means of squared severity ratings for each event.27 On the bases of the responses to the Time 2 survey, we calculated the sum of weighted events for each participant to obtain a cumulative severity score of all life events, separately for those events that had occurred within 6 months prior to Time 2 and those that had occurred between 6 months and 5 years before Time 2. To study specific types of events, we additionally selected the most burdensome events and classified them into 4 categories to indicate a family death or illness (5 items measuring death of spouse, death of own child, death of mother or father, severe illness of spouse, severe illness of another family member); divorce or separation (1 item); financial difficulties (1 item); and emotional, physical, or sexual violence (1 item).27 If the participant reported the occurrence of the same event at Time 1 and at Time 2, the Time 2 report for the category “within the previous 5 years” was recoded as “over 5 years ago” (i.e., before Time 1). Thus the exposure to a severe event was classified as having occurred within 6 months of Time 2, within 0.5-5 years of Time 2, before Time 1, or never.
Background Variables
Sex, age group (20-24, 30-34, 40-44, and 50-54 years), education (basic, secondary, lower tertiary, higher tertiary), and marital status (married or cohabiting, other) at Time 1 were included in the analysis as demographic variables. We also assessed Time 1 alcohol use, obesity, and chronic diseases, such as cardiovascular, pulmonary, musculoskeletal diseases, and depression, because sleep disturbances can be a symptom of any of these conditions.28,29 The participants reported their habitual frequency and the amount of beer, wine, and spirits consumed.30 They were classified as having a high alcohol intake if their weekly consumption exceeded 16 drinks (200 g of alcohol). The body mass index (BMI), calculated from self-reported weight and height, was used to measure obesity (BMI >30 kg/m2), which is a correlate of obstructive sleep apnea.31 Lifetime diagnoses of the following conditions (yes/no) were self-reports from a checklist of 29 common chronic diseases diagnosed by a physician: cardiovascular disease (hypertension, angina, myocardial infarction, or stroke); obstructive pulmonary disease (asthma or chronic obstructive bronchitis); musculoskeletal disease (sciatica, rheumatoid arthritis, osteoarthritis, fibromyalgia); and depression.32 Depression was additionally measured at Time 2.
Statistical Analysis
We used logistic regression analyses and expressed the results as odds ratios (OR) and their 95% confidence intervals (CIs). First, we studied the associations between the various background variables (demographics, high alcohol intake, obesity, cardiovascular, pulmonary, and musculoskeletal diseases, and depression) and sleep disturbances, adjusting for age and sex. Then, we explored the individual variation in the onset of sleep disturbances as a function of baseline liability to anxiety (general feeling of stressfulness and symptoms of SNS hyperactivity). The logistic regression models were adjusted for background variables at Time 1, and both indicators of liability to anxiety were entered into the same model to determine their independent effect. Next, we studied the extent to which the cumulative severity score of all negative life events, having occurred either 0-6 months or in 0.5 to 5 years prior to the measurement of sleep quality, were predictive of the onset of sleep disturbances. Correspondingly, we studied the effect of specific life events on sleep disturbances. As a means of avoiding misclassification due to recall bias, events having occurred before Time 1 (i.e., earlier than the past 5 years) were coded as missing information in these analyses. The effect of life events on the onset of sleep disturbances was modeled; first, each event separately, adjusted for demographics and additionally for liability to anxiety, health risks and chronic conditions and, then, all events in the same model to study their independent effect. Additional adjustments were made for post-event depression at Time 2 to assess the extent to which the association between a life event and disturbed sleep was caused by depression triggered by the event. The analyses were based on a combined sample of male and female subjects because there were no significant interactions between sex and liability to anxiety or sex and life events on Time 2 sleep disturbances. Finally, we analyzed whether the effect of life events on sleep was dependent on liability to anxiety by studying the interaction effects of events, general feeling of stressfulness, and symptoms of SNS hyperactivity (median split) on sleep. We expressed these results as means and their 95% CIs, adjusted for demographics. All of the analyses were stratified by Time 1 sleep quality to separately study the predictors of new-onset sleep disturbance and those of persistent sleep disturbance.
Results
Thirteen percent had sleep disturbances at Time 1 (n=2572), and new-onset sleep disturbances by Time 2 were observed in 11% of the sample (n=1795). Female sex, older age, lower educational attainment, obesity, high alcohol intake, and presence of chronic medical conditions were associated with an increased rate of preexisting and new-onset sleep disturbances. Marital status (single, divorced, or widowed) was associated with increased preexisting (but not new-onset) sleep disturbances.
Liability to Anxiety as a Predictor of Sleep Disturbances
The level of general feeling of stressfulness was slightly higher for the men than for the women (2.35 vs. 2.27, P <0.0001), while the women had more often had symptoms of SNS hyperactivity (1.48 vs. 1.44, P <0.0001). A general feeling of stressfulness and symptoms of SNS hyperactivity were strong predictors of disturbed sleep (Table 2). After adjustment for demographics, the odds ratio for new-onset sleep disturbances in the highest versus lowest quartile of general feeling of stressfulness was 2.4 (95% CI 2.0-2.7). The corresponding odds ratio for those with symptoms of SNS hyperactivity was 2.2 (95% CI 1.9-2.5). When both indicators of liability to anxiety were modeled simultaneously, these ratios were attenuated by 31%-44%, but both general feeling of stressfulness and symptoms of SNS hyperactivity remained independent predictors of sleep disturbances (Model 3, Table 2). The effect of liability to anxiety on sleep was not dependent on sex (for all tests of interaction, P >0.20)
Table 2.
Association Between General Feeling of Stressfulness and Symptoms of SNS Hyperactivity at Time 1 and Sleep Disturbances at Time 2 by Self-Reported Sleep Quality at Time 1
| Indicator of Liability to Anxiety | TIME 1: Preexisting Sleep Disturbance |
TIME 1: No Preexisting Sleep Disturbance |
||||||
|---|---|---|---|---|---|---|---|---|
| N (%) | Model 1 | Model 2 | Model 3 | N (%) | Model 1 | Model 2 | Model 3 | |
| General feeling of stressfulness (quartile) | ||||||||
| Lowest | 322 (13) | 1.00 | 1.00 | 1.00 | 5,042 (30) | 1.00 | 1.00 | 1.00 |
| Second | 274 (11) | 0.69 (0.50-0.97) | 0.68 (0.48-0.95) | 0.65 (0.46-0.91) | 3,165 (19) | 1.06 (0.90-1.25) | 1.06 (0.90-1.25) | 1.01 (0.86-1.19) |
| Third | 824 (32) | 0.85 (0.65-1.11) | 0.86 (0.65-1.12) | 0.80 (0.61-1.05) | 5,378 (33) | 1.60 (1.40-1.83) | 1.59 (1.39-1.81) | 1.45 (1.27-1.66) |
| Highest | 1,140 (44) | 1.12 (0.87-1.46) | 1.10 (0.85-1.43) | 0.98 (0.75-1.29) | 2,974 (18) | 2.35 (2.04-2.71) | 2.22 (1.93-2.57) | 1.93 (1.42-1.91) |
| Symptoms of SNS hyperactivity (quartile) | ||||||||
| Lowest | 430 (17) | 1.00 | 1.00 | 1.00 | 5,475 (33) | 1.00 | 1.00 | 1.00 |
| Second | 230 (9) | 0.90 (0.64-1.25) | 0.93 (0.67-1.29) | 0.92 (0.66-1.28) | 2,316 (14) | 1.16 (0.97-1.38) | 1.12 (0.94-1.34) | 1.08 (0.90-1.29) |
| Third | 701 (27) | 0.96 (0.75-1.23) | 0.96 (0.75-1.24) | 0.95 (0.74-1.23) | 5,183 (31) | 1.64 (1.44-1.88) | 1.56 (1.36-1.78) | 1.43 (1.25-1.64) |
| Highest | 1,194 (47) | 1.46 (1.16-1.80) | 1.45 (1.14-1.84) | 1.41 (1.10-1.80) | 3,567 (22) | 2.17 (1.90-2.49) | 1.97 (1.72-2.27) | 1.65 (1.42-1.91) |
Results are presented as odds ratios and 95% confidence intervals, adjusted for baseline characteristics (sex, age group and education (Model 1); Model 1 + high alcohol intake (>16 drinks/wk), obesity (BMI>30) and chronic diseases (Model 2); Model 2 + another indicator of liability to anxiety (Model 3). All of the associations were independent of sex (test for interaction P >0.21).
A participant was considered to be positive for sleep disturbance if he or she assessed the overall quality of sleep as being rather poor or poor. Baseline general feeling of stressfulness was assessed by the Reeder stress inventory and symptoms of SNS (sympathetic nervous system) hyperactivity by the average experience of palpitation and sweating without exercise, irregular heartbeat, flushing, chest pain upon emotion, tremor of hands or voice, or muscle twitching.
Table 1.
Odds Ratios (95% Confidence Intervals) of Prevalent and Incident Sleep Disturbances by Time 1 Covariates
| Covariate | All Participants |
Participants without Sleep Disturbance at Time 1 |
||
|---|---|---|---|---|
| Number% | Prevalent Sleep Disturbances at Time 1 OR (95% CI)a | Number% | Incident Sleep Disturbance at Time 2 OR (95% CI)a | |
| Sex | ||||
| Men | 7,371 (38) | 1.00 | 6,414 (39) | 1.00 |
| Women | 11,828 (62) | 1.14 (1.05-1.23) | 10,213 (61) | 1.19 (1.08-1.32) |
| Age group | ||||
| 20-34 years | 4,803 (25) | 1.00 | 4,465 (27) | 1.00 |
| 30-34 years | 4,370 (23) | 1.37 (1.21-1.56) | 3,865 (23) | 1.23 (1.06-1.43) |
| 40-44 years | 4,767 (25) | 2.05 (1.83-2.30) | 4,061 (24) | 1.59 (1.38-1.84) |
| 50-54 years | 5,259 (27) | 2.63 (2.35-2.94) | 4,236 (26) | 1.70 (1.48-1.96) |
| Education | ||||
| Higher tertiary | 2,694 (14) | 1.00 | 2,354 (14) | 1.00 |
| Lower tertiary | 6,244 (33) | 1.18 (1.04-1.34) | 5,466 (33) | 1.17 (0.99-1.38) |
| Secondary | 4,165 (22) | 1.34 (1.17-1.53) | 3,604 (22) | 1.26 (1.05-1.50) |
| Basic | 5,895 (31) | 1.52 (1.34-1.73) | 5,024 (31) | 1.48 (1.26-1.75) |
| Marital status | ||||
| Married or cohabiting | 13,184 (31) | 1.00 | 11,410 (31) | 1.00 |
| Other | 5,995 (69) | 1.21 (1.11-1.32) | 5,201 (69) | 1.08 (0.96-1.21) |
| Health risks and chronic conditions | ||||
| Obesity (BMI30) | 1,846 (10) | 1.38 (1.23-1.55) | 1,494 (9) | 1.38 (1.18-1.61) |
| High alcohol intake (16 drinks/week) | 1,678 (9) | 1.79 (1.59-2.03) | 1,300 (8) | 1.47 (1.24-1.74) |
| Cardiovascular disease | 1,465 (8) | 1.52 (1.34-1.73) | 1,132 (7) | 1.38 (1.15-1.64) |
| Obstructive pulmonary disease | 2,442 (13) | 1.49 (1.35-1.66) | 1,956 (12) | 1.30 (1.13-1.49) |
| Musculoskeletal disease | 3,967 (21) | 1.66 (1.52-1.82) | 3,065 (19) | 1.47 (1.30-1.65) |
| Depression | 2,128 (11) | 2.64 (2.39-2.92) | 1,439 (9) | 2.08 (1.81-2.40) |
A participant was considered to be positive for insomnia if he or she assessed the overall quality of sleep as being rather poor or poor. aAdjusted for age group and sex.
Table 2 shows that liability to anxiety may increase the risk that preexisting sleep disturbance develop into persistent sleep disturbances. Participants in the highest quartile of symptoms of SNS hyperactivity had 1.5 (95% CI 1.2-1.8) times higher odds of sleep disturbances persisting at Time 2 than those in the lowest quartile.
Stressful Life Events as Predictors
Altogether 13,180 participants had a stressful event within 0.5-5 years of Time 2, and for 5642 such an event occurred within 0-6 months (table 3). In the latter group, 3984 (71%) were exposed to 1 event, 1156 (20%) experienced 2 events, and 502 had 3-8 events, the mean sum of the weighted events being 7.91 (range 0-52).
Table 3.
Frequency and Severity Rating of the Life Events Among the Participants (N=19 199)
| Life Event | Time Since Event |
Weight | ||
|---|---|---|---|---|
| 0-6 months | >0.5-5 years | >5 years | ||
| Death of own child | 12 (0.1) | 79 (0.5) | 396 (2.3) | 7.42 |
| Death of spouse | 19 (0.1) | 158 (0.9) | 312 (1.8) | 7.01 |
| Emotional, physical or sexual violence | 278 (1.6) | 736 (4.2) | 1,309 (7.4) | 6.16 |
| Severe illness in a family member | 705 (4.0) | 2,641 (14.0) | 3,349 (19.0) | 5.53 |
| Death of mother | 182 (1.0) | 1,245 (6.9) | 3,598 (19.8) | 5.04 |
| Major increase in marital problems | 1,100 (6.2) | 2,456 (13.9) | 1,704 (9.6) | 4.92 |
| Divorce or separation | 377 (2.1) | 1,908 (10.6) | 3,407 (18.8) | 4.75 |
| Severe conflicts with supervisor | 551 (3.1) | 1,276 (7.2) | 983 (5.6) | 4.63 |
| Severe financial difficulties | 1,120 (6.3) | 2,063 (11.6) | 2,094 (11.7) | 4.37 |
| Death of father | 194 (1.1) | 1,501 (8.1) | 6,806 (36.8) | 4.25 |
| aSevere injury | 137 (0.8) | 554 (3.1) | 779 (4.4) | 4.24 |
| Severe conflicts with coworkers | 539 (3.1) | 998 (5.7) | 587 (3.3) | 4.16 |
| Miscarriage (own or partner) | 103 (0.6) | 621 (3.5) | 2,065 (11.8) | 4.14 |
| aIllness causing work disability over 21 days | 814 (4.6) | 2,025 (11.4) | 1,431 (8.0) | 4.05 |
| Death of close friend | 502 (2.9) | 1,719 (9.8) | 2,335 (13.3) | 4.01 |
| Loss of job | 448 (2.5) | 1,309 (7.3) | 1,786 (10.0) | 3.95 |
| Abortion (own or partner) | 48 (0.3) | 411 (2.4) | 1,761 (10.1) | 3.66 |
| Breakup of long-term friendship | 515 (2.9) | 1,779 (10.0) | 1,662 (9.4) | 3.56 |
| Death of another close relative | 1,046 (5.9) | 4,675 (26.2) | 6,658 (37.3) | 3.41 |
| Unemployment of spouse | 519 (2.9) | 1,276 (7.2) | 1,275 (7.2) | 2.74 |
| aRetirement | 143 (0.8) | 471 (2.7) | 394 (2.2) | 2.72 |
The response format for life events included the following categories (yes / no): never, during the previous 6 months, during the previous 5 years, earlier, never. Weights were determined as means of squared severity ratings (1 = not so burdensome, 2 = burdensome; 3 = extremely burdensome) for each event.
Not used in the study
Stressful events within 0.5-5 years and within 0-6 months were predictive of the onset of sleep disturbances by Time 2 among the participants with no preexisting sleep disturbances (Table 4). There was a linear association between the severity of cumulative event exposure and the risk of sleeping problems. The participants who had experienced severe event(s) 0.5-5 years or 0-6 months before the measurement of sleep had 2 times higher odds of disturbed sleep than the unexposed participants. Adjustment for other predictors of sleep disturbances, such as liability to anxiety, high consumption of alcohol, obesity, and chronic disease, had relatively little effect on these findings (Model 2 in 4).
Table 4.
Association Between the Cumulative Severity Score of All Life Events and Sleep Disturbances at Time 2 by Self-Reported Sleep Quality at Time 1
| Cumulative severity score for the events | TIME 1: Preexisting Sleep Disturbances |
TIME 1: No Preexisting Sleep Disturbances |
||||
|---|---|---|---|---|---|---|
| N | Model 1 | Model 2 | N | Model 1 | Model 2 | |
| 0.5-5 years since event | ||||||
| 0 | 588 | 1.00 | 1.00 | 4,704 | 1.00 | 1.00 |
| 1-4.9 | 603 | 1.00 (0.79-1.26) | 0.96 (0.75-1.22) | 4,291 | 1.28 (1.11-1.48) | 1.25 (1.07-1.44) |
| 5-9.9 | 618 | 1.13 (0.89-1.44) | 1.11 (0.87-1.41) | 3,973 | 1.50 (1.30-1.73) | 1.41 (1.22-1.64) |
| 10-52 | 652 | 1.15 (0.91-1.46) | 1.07 (0.84-1.37) | 3,043 | 1.99 (1.72-2.30) | 1.76 (1.51-2.04) |
| 0-6 months since event | ||||||
| 0 | 1,550 | 1.00 | 1.00 | 10,859 | 1.00 | 1.00 |
| 1-4.9 | 489 | 1.17 (0.94-1.45) | 1.12 (0.90-1.40) | 2,971 | 1.35 (1.19-1.54) | 1.29 (1.14-1.47) |
| 5-9.9 | 238 | 1.30 (0.97-1.73) | 1.23 (0.91-1.65) | 1,383 | 1.42 (1.19-1.68) | 1.32 (1.11-1.57) |
| 10-35 | 100 | 1.80 (1.16-2.80) | 1.58 (1.00-2.45) | 461 | 2.06 (1.60-2.65) | 1.80 (1.39-2.33) |
Results are presented as odds ratios and 95% confidence intervals, adjusted for baseline characteristics (sex, age group and education (Model 1); Model 1 + high alcohol intake (>16 drinks /wk), obesity (BMI>30), chronic diseases, general feeling of stressfulness, and symptoms of SNS hyperactivity (Model 2). Life events and their timing were measured at follow-up. Weights were determined as means of squared severity ratings for each event. All of the associations were independent of sex (test for interaction P >0.05).
A participant was considered to be positive for sleep disturbance if he or she assessed the overall quality of sleep as being rather poor or poor. The baseline general feeling of stressfulness was assessed by the Reeder stress inventory and symptoms of SNS (sympathetic nervous system) hyperactivity by the average experience of palpitation and sweating without exercise, irregular heartbeat, flushing, chest pain upon emotion, tremor of hands or voice, or muscle twitching.
Table 5 shows that all of the specific categories of stressful events were predictive of the onset of sleep disturbances. The participants who had experienced an event within 0.5-5 years had 1.4-1.9 times higher odds of disturbed sleep than the unexposed participants did; for those who had experienced an event within 6 months, the corresponding odds varied between 1.5 and 2.1. These associations were 14%-33% lower for the events within 0.5-5 years and 4%-39% lower for the events within 6 months after further adjustment for obesity, high alcohol intake, chronic diseases, and liability to anxiety. The decrease was greatest for the association between violence and disturbed sleep. When all of the life events were modeled simultaneously a death or illness in the family, divorce and financial difficulties proved to be independent predictors of new-onset sleep disturbances within 0.5-5 years (Model 3 in Table 5). Only financial difficulties were independently associated with sleep problems within 0-6 months. The effect of life events on sleep was independent of sex in all cases (test of interaction, P >0.23).
Table 5.
The Association Between Specific Stressful Life Events and Sleep Disturbances at Time 2 by Self-Reported Sleep Quality at Time 1
| Life Event | TIME 1: Preexisting Sleep Disturbances |
TIME 1: No Preexisting Sleep Disturbances |
||||||
|---|---|---|---|---|---|---|---|---|
| N | Model 1 | Model 2 | Model 3 | N | Model 1 | Model 2 | Model 3 | |
| Death or illness in the family | ||||||||
| Never | 561 | 1.00 | 1.00 | 1.00 | 5,616 | 1.00 | 1.00 | 1.00 |
| 0.5-5 years since event | 480 | 1.18 (0.88-1.58) | 1.10 (0.81-1.48) | 1.12 (0.74-1.70) | 2,895 | 1.46 (1.24-1.72) | 1.39 (1.17-1.64) | 1.32 (1.07-1.63) |
| 0-6 months since event | 152 | 1.46 (0.98-2.16) | 1.43 (0.95-2.14) | 1.09 (0.60-1.97) | 922 | 1.52 (1.22-1.90) | 1.42 (1.13-1.78) | 1.17 (0.86-1.60) |
| Divorce | ||||||||
| Never | 1,455 | 1.00 | 1.00 | 1.00 | 10,937 | 1.00 | 1.00 | 1.00 |
| 0.5-5 years since event | 197 | 0.82 (0.60-1.11) | 0.78 (0.57-1.08) | 0.49 (0.27-0.88) | 1,283 | 1.41 (1.18-1.70) | 1.34 (1.11-1.61) | 1.47 (1.13-1.91) |
| 0-6 months since event | 56 | 1.28 (0.73-2.27) | 1.21 (0.68-2.16) | 1.23 (0.54-2.81) | 321 | 1.81 (1.32-2.49) | 1.78 (1.29-2.46) | 1.46 (0.91-2.35) |
| Financial difficulties | ||||||||
| Never | 1,323 | 1.00 | 1.00 | 1.00 | 11,263 | 1.00 | 1.00 | 1.00 |
| 0.5-5 years since event | 174 | 1.21 (0.87-1.70) | 1.10 (0.78-1.56) | 2.61 (1.20-5.70) | 828 | 1.88 (1.54-2.30) | 1.76 (1.43-2.15) | 1.91 (1.39-2.62) |
| 0-6 months since event | 217 | 1.62 (1.19-2.22) | 1.47 (1.06-2.03) | 1.28 (0.71-2.29) | 903 | 2.06 (1.70-2.49) | 1.80 (1.48-2.19) | 2.06 (1.55-2.73) |
| Violence | ||||||||
| Never | 1,812 | 1.00 | 1.00 | 1.00 | 13,524 | 1.00 | 1.00 | 1.00 |
| 0.5-5 years since event | 93 | 1.15 (0.74-1.78) | 1.09 (0.70-1.69) | 1.11 (0.41-2.99) | 458 | 1.69 (1.30-2.20) | 1.47 (1.13-1.92) | 1.08 (0.69-1.69) |
| 0-6 months since event | 63 | 2.04 (1.16-3.59) | 1.83 (1.03-3.25) | 2.17 (0.89-5.29) | 215 | 1.66 (1.13-2.42) | 1.40 (0.96-2.06) | 1.35 (0.74-2.47) |
Results are presented as odds ratios and 95% confidence intervals, adjusted for baseline characteristics (sex, age group and education (Model 1); Model 1 + high alcohol intake (>16 drinks /wk), obesity (BMI>30), chronic diseases, general feeling of stressfulness, and symptoms of SNS hyperactivity (Model 2); Model 2 + all other events (Model 3).
Life events and their timing were measured at follow-up. Only events which occurred after baseline measurements were considered. All of the associations were independent of sex (test for interaction P >0.23).
A participant was considered to be positive for sleep disturbance if he or she assessed the overall quality of sleep as being rather poor or poor. The baseline general feeling of stressfulness was assessed by the Reeder stress inventory and symptoms of SNS (sympathetic nervous system) hyperactivity by the average experience of palpitation and sweating without exercise, irregular heartbeat, flushing, chest pain upon emotion, tremor of hands or voice, or muscle twitching.
Among those with preexisting sleep disturbances, exposure to severe life events in general, as well as to specific events, such as financial difficulties and violence, was associated with 1.6-2.0 times higher odds of disturbed sleep persisting, but only if the event had occurred within 0-6 months (Tables 4 and 5).
The effect of life events on sleep quality were not explained by depression, as adjustment for post-event depression in addition to demographics and pre-event depression lowered the associations between a life event and sleep disturbances only to a small extent. Among the participants with no preexisting sleep disturbances, controlling for post-event depression reduced the odds of disturbed sleep by 11%-20% for death or illness in the family, 21%-25% for divorce, and 21%-22% for financial difficulties. In relation to violence, the odds of disturbed sleep were reduced more substantially: from 1.56 to 1.31 for events within 0.5-5 years and from 1.54 to 1.33 for events within 0-6 months (data not shown).
Predisposing Vulnerability, Negative Life Events, and New-Onset Sleep Disturbances
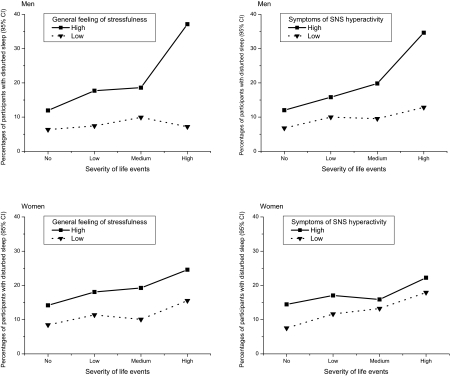
Finally, we studied the effect of life events on sleep by taking into account individual liability to anxiety. Among the men, the data seem to indicate an interaction between baseline liability to anxiety and increasing severity of event exposure on future risk of developing sleep disturbances in relation to events that occurred within 0-6 months, although the test of interaction were not significant. As shown in Figure 1, general feeling of stressfulness and symptoms of SNS hyperactivity increased the risk of sleep disturbances, if the cumulative severity score of life events was high. For the men liable to anxiety, the odds of sleep disturbances 0-6 months after exposure to severe life events were 3.11 (95% CI 1.90-5.10) times higher in combination with a high general feeling of stressfulness and 2.88 (95% CI 1.69-4.91) times higher in combination with symptoms of SNS hyperactivity than for those with no exposure to life events. For the men not liable to anxiety, the odds of new-onset sleep disturbances after severe life events were not significantly elevated. For the women, these odds ratios varied from 1.54 to 2.38 (95% CI 1.07-4.13). The effect of life events on sleep disturbances within 0.5-5 years was not dependent on liability to anxiety for either sex (data not shown).
Figure 1.
Effect of general feeling of stressfulness in daily life and symptoms of sympathetic nervous system (SNS) hyperactivity on the association between the severity of life events and the onset of disturbances
Percentages of participants with sleep disturbances (95% confidence intervals) derived from a logistic regression analysis adjusted for age group and education. A participant was considered to be positive for sleep disturbance if he or she assessed the overall quality of sleep as being rather poor or poor. The baseline general feeling of stressfulness was assessed by the Reeder stress inventory (median split) and symptoms of SNS hyperactivity by the average experience of palpitation and sweating without exercise, irregular heartbeat, flushing, chest pain upon emotion, tremor of hands or voice, or muscle twitching (median split). Life events and their timing were measured at follow-up. Weights were determined as means of squared severity ratings for each event.
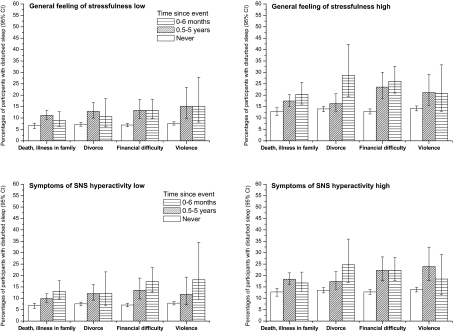
Figure 2 shows corresponding tests for specific life events. Of the 8 tests of interaction, only one was significant—divorce in combination with a general feeling of stressfulness (P = 0.042). Compared with the unexposed participants, those who had experienced divorce had 2.05 (95% CI 1.38-3.05) times higher odds of sleep disturbances within 0-6 months after the divorce in combination with a high general feeling of stressfulness, while, in combination with a low general feeling of stressfulness, the corresponding odds ratios were nonsignificant. The findings related to the effects of life events in combination with symptoms of SNS hyperactivity were in agreement with those obtained for a general feeling of stressfulness. The risk of sleep problems did not vary according to baseline liability to anxiety in relation to events within 0.5-5 years (P >0.10). Liability to anxiety did not increase the risk of preexisting sleep disturbances developing into persistent sleep disturbances after a stressful event (data not shown).
Figure 2.
Effect of general feeling of stressfulness in daily life and symptoms of sympathetic nervous system (SNS) hyperactivity on the association between life events and the onset of sleep disturbances
Percentages of participants with sleep disturbances (95% confidence intervals) derived from a logistic regression analysis adjusted for age group and education. A participant was considered to be positive for sleep disturbance if he or she assessed the overall quality of sleep as being rather poor or poor. The baseline general feeling of stressfulness was assessed by the Reeder stress inventory (median split) and symptoms of SNS hyperactivity by the average experience of palpitation and sweating without exercise, irregular heartbeat, flushing, chest pain upon emotion, tremor of hands or voice, or muscle twitching. Life events and their timing were measured at follow-up. Only events that occurred after the baseline measurements were considered.
DISCUSSION
This 5-year follow-up of a population sample of 19,199 Finns showed that exposure to severe stressful events can trigger sleep disturbances in people with undisturbed sleep before the event. Those liable to anxiety before the event seemed to be at a higher risk of post-event sleep disturbances compared with those not liable to anxiety. This heightened vulnerability was evident, however, only 0-6 months after the event. The strength of this study is a study design that allowed the timing of pre-event predisposing traits and the occurrence of specific stressful events precipitating the onset of sleep disturbances. Control for a large number of potential confounding factors suggest that the observed associations were not explained by socioeconomic position, obesity, high alcohol intake, or chronic medical conditions at study entry.28,29
Our study partially agrees with retrospective evidence suggesting that some people are more vulnerable to the evolution of sleep disturbances.3,33 Both a general feeling of stressfulness in daily life and symptoms of SNS hyperactivity (hypothesized predisposing components of liability to anxiety) were strong predictors of sleep disturbances irrespective of sex. This association was observable even after control for a wide set of other precipitating and perpetuating factors, such as stressful life events, high alcohol consumption, obesity, depression, and other medical conditions. We found a similar strong and robust association between negative life events (hypothesized precipitating factors) and subsequent sleep disturbances. Earlier research has found stable differences between people in the way they experience the environment as a source of negative emotions and the way they respond with mood changes after stressors in their daily life.34,35 Such behavioral predispositions reflect the degree of arousal that adverse stimuli may evoke.3,8 Studies on twins suggest that a disposition towards neuroticism and symptoms of anxiety and depression may not only increase a person's sensitivity to stressful life events, but also the risk of exposure to stressful events in the realm of getting along with other people, such as marital difficulties.36 Genes have been found to be associated with the tendency to develop negative emotions in response to minor environmental experiences. For example, functional variants in the serotonin transporter gene (5-HTTLPR) may affect the way people continually respond to, and cope with, stressful life events and minor environmental experiences in daily life.37
Our finding of the higher vulnerability to sleep disturbances among those with the hypothesized psychological predisposition in comparison with those with no such predisposition is in line with the literature. Importantly, we found a significant interactions between liability to anxiety and life events with respect to sleep disturbances. People with a tendency to experience daily life as highly stressful are more likely to react to some life events, such as divorce, by developing sleep problems than those with no such tendency. However, this increased vulnerability may not be an explanation to long-term sleep disturbances triggered by life events, as it was observable only 0-6 months after the event. In fact, the relative risk of sleep disturbances measured 0.5-5 years after an event was equally high among those with and those without the hypothesized psychological predisposition.
We chose to measure the concept of liability to anxiety in a relatively new fashion by two separate components, perception of stressfulness and SNS activation symptoms.21,23 Our findings showed that the 2 components captured dimensions that were only moderately correlated with each other (r=0.39). In multivariate models, they independently predicted sleep disturbances. Obviously, there is individual variation in the extent to which liability to anxiety is expressed by psychological symptoms, such as prominent tension, worry, and feelings of apprehension about everyday problems; and physiological symptoms, such as palpitation, chest pain, sweating, trembling, flushing, or muscle twitching. The measurement of both psychological and physiological symptoms of anxiety seems to detect individuals with anxiety-related vulnerability to sleep disturbances more accurately than the often-used scales measuring psychological symptoms only.21,38
Our measure of sleep disturbances was a 1-item survey question concerning the overall quality of sleep. In this study, the baseline prevalence of rather poor and poor quality of sleep was 13.4%, and the corresponding incidence at follow-up was 10.8%. These figures are well in line with those reported for other measures of sleep problems elsewhere.18 For example, in the 2002 “Sleep in America” poll, 27% of the respondents categorized their sleep quality as fair or poor.3 Our findings of older age, low education, obesity, alcohol abuse, and several medical conditions (such as depression and pulmonary, cardiovascular, and musculoskeletal diseases) as risk factors for sleep disturbances also agree with the results of earlier research.1 Similarly, our findings are in line with literature reporting an association between traumatic events and poor sleep.12–14
Our measure of sleep disturbances refers to dissatisfaction with sleep. We did not measure the lifetime history of sleep disturbances for individuals without sleep disturbances at baseline. Prior studies have shown that people who suffer from sleep disturbances differ as to whether they are satisfied or dissatisfied with sleep; sleep disturbances among sleep-dissatisfied persons are more severe.38 We do not know the type of sleep disorder reported by the participants, as our survey did not specifically enquire about various sleep disorders. Although we took into account obesity, a correlate of obstructive sleep apnea,31 and self-reports of pulmonary and musculoskeletal diseases diagnosed by a physician, we cannot rule out the possibility of reported sleep disturbances not only resulting from insomnia, but also from other sleep disorders, such as periodic limb movements, restless legs syndrome, and narcolepsy. However, we do not believe that such a case would have severely biased our study as we are aware of no evidence suggesting that liability to anxiety or exposure to stressful events could be a risk factor for sleep disorders other than insomnia.
We cannot rule out the possibility that our results were influenced by recall and the reporting of stressful life events. This is a potential explanation for the absence of anxiety-related vulnerability to sleep problems in relation to stressors that occurred 6 months to 5 years prior to the follow-up survey. Supporting the operation of recall bias, the number of events reported for the 0.5-5 years preceding the follow-up survey was lower than would have been expected from the figures reported for the preceding 0-6 months. This trend was especially evident for financial difficulties and violence. A part of this discrepancy may be related to the fact that we recoded the follow-up report for the event category “within the previous 5 years” as “over 5 years ago” if the participant reported the occurrence of the same event in the baseline survey. This recording decreased “false positive” reports for the specified time period, but it also led to an underestimate of the cases of repeated events, such as violence against women, typically committed repeatedly by their intimate partner.39 A complementary explanation is that the events recalled as having occurred 0.5-5 years earlier, such as illnesses and financial difficulties, were more severe than the same events recalled within a few months after the event. This possibility would explain our finding of an association that was as strong or even stronger between sleep disturbances and an event that occurred 0.5-5 years earlier than for the same event that had occurred more recently.
We are not aware of studies directly testing the validity of the 2 measures we used to measure liability to anxiety, the Reeder stress inventory and symptoms of SNS hyperactivity.21,23 However, the scales requested information on psychological symptoms of prominent tension; worry and feelings of apprehension about everyday events and problems; and physiological symptoms of autonomic arousal. All these symptoms are included in the diagnostic criteria for anxiety disorders in the ICD-10 and the DSM-IV indicating that these measures satisfy the criteria for a good face validity. The items in these 2 scales also overlap with those in validated scales to measure anxiety by symptoms20,41 and both these symptoms and clinically assessed anxiety have been shown to be associated with increased risk of health problems including, e.g., cardiovascular disease.21,42–45 Thus we feel confident that our measure is indeed a valid instrument in detecting individuals liable to anxiety.
CONCLUSIONS
People liable to anxiety may be at higher risk of post-event sleep disturbances than those not liable to anxiety. This heightened vulnerability, however, was observable only within 6 months after the event but not necessarily later. Thus, partial support was found for the hypothesis that predisposing traits would increase the risk of sleep disturbances in the aftermath of stressful life events.
ACKNOWLEDGMENTS
Jussi Vahtera and Mika Kivimäki were supported by the Academy of Finland (projects 105195 and 117604).
Footnotes
Disclosure Statement
This is not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Sateia MJ, Nowell PD. Insomnia. Lancet. 2004;364:1959–73. doi: 10.1016/S0140-6736(04)17480-1. [DOI] [PubMed] [Google Scholar]
- 2.Spielman AJ, Caruso LS, Glovinsky BP. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10:541–53. [PubMed] [Google Scholar]
- 3.Van Dongen HP, Vitellaro KM, Dinges DF. Individual differences in adult human sleep and wakefulness: leitmotif for a research agenda. Sleep. 2005;28:479–96. doi: 10.1093/sleep/28.4.479. [DOI] [PubMed] [Google Scholar]
- 4.Roth T, Drake C. Evolution of insomnia: current status and future direction. Sleep Med. 2004;5(Suppl 1):S23–30. doi: 10.1016/s1389-9457(04)90004-4. [DOI] [PubMed] [Google Scholar]
- 5.Partinen M, Kaprio J, Koskenvuo M, Putkonen P, Langinvainio H. Genetic and environmental determination of human sleep. Sleep. 1983;6:179–85. doi: 10.1093/sleep/6.3.179. [DOI] [PubMed] [Google Scholar]
- 6.Heath AC, Kendler KS, Eaves LJ, Martin NG. Evidence for genetic influences on sleep disturbance and sleep pattern in twins. Sleep. 1990;13:318–35. doi: 10.1093/sleep/13.4.318. [DOI] [PubMed] [Google Scholar]
- 7.Watson NF, Goldberg J, Arguelles L, Buchwald D. Genetic and environmental influences on insomnia, daytime sleepiness, and obesity in twins. Sleep. 2006;29:645–9. doi: 10.1093/sleep/29.5.645. [DOI] [PubMed] [Google Scholar]
- 8.Drake C, Richardson G, Roehrs T, Scofield H, Roth T. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 2004;27:285–91. doi: 10.1093/sleep/27.2.285. [DOI] [PubMed] [Google Scholar]
- 9.Macleod J, Davey Smith G, Heslop P, Metcalfe C, Carroll D, Hart C. Psychological stress and cardiovascular disease: empirical demonstration of bias in a prospective observational study of Scottish men. BMJ. 2002;324:1247–51. doi: 10.1136/bmj.324.7348.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson J, Krishnan R, France R, Pelton S. Neurovegetative symptoms in chronic pain and depression. J Affect Disord. 1985;9:213–8. doi: 10.1016/0165-0327(85)90050-3. [DOI] [PubMed] [Google Scholar]
- 11.Tyrer B, Baldwin D. Generalised anxiety disorder. Lancet. 2006;368:2156–66. doi: 10.1016/S0140-6736(06)69865-6. [DOI] [PubMed] [Google Scholar]
- 12.Lavie P. Sleep disturbances in the wake of traumatic events. N Engl J Med. 2001;345:1825–32. doi: 10.1056/NEJMra012893. [DOI] [PubMed] [Google Scholar]
- 13.Healey ES, Kales A, Monroe LJ, Bixler EO, Chamberlin K, Soldatos CR. Onset of insomnia: role of life-stress events. Psychosom Med. 1981;43:439–51. doi: 10.1097/00006842-198110000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Theorell T, Leymann H, Jodko M, Konarski K, Norbeck HE, Eneroth P. “Person under train” incidents: medical consequences for subway drivers. Psychosom Med. 1992;54:480–8. doi: 10.1097/00006842-199207000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Nishith P, Resick PA, Mueser KT. Sleep difficulties and alcohol use motives in female rape victims with posttraumatic stress disorder. J Trauma Stress. 2001;14:469–79. doi: 10.1023/A:1011152405048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds CF, 3rd, Hoch CC, Buysse DJ, et al. Sleep after spousal bereavement: a study of recovery from stress. Biol Psychiatry. 1993;34:791–7. doi: 10.1016/0006-3223(93)90068-o. [DOI] [PubMed] [Google Scholar]
- 17.Vahtera J, Pentti J, Helenius H, Kivimaki M. Sleep disturbances as a predictor of long-term increase in sickness absence among employees after family death or illness. Sleep. 2006;29:673–82. doi: 10.1093/sleep/29.5.673. [DOI] [PubMed] [Google Scholar]
- 18.Sateia MJ, Doghramji K, Hauri PJ, Morin CM. Evaluation of chronic insomnia. An American Academy of Sleep Medicine review. Sleep. 2000;23:243–308. [PubMed] [Google Scholar]
- 19.Korkeila K, Suominen S, Ahvenainen J, et al. Non-response and related factors in a nation-wide health survey. Eur J Epidemiol. 2001;17:991–9. doi: 10.1023/a:1020016922473. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton M. The assessment of anxiety states by rating. BR J Med Psychol. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 21.Reeder LG, Schrama PG, Dirken JM. Stress and cardiovascular health: an international cooperative study. Soc Sci Med. 1973;7:573–84. doi: 10.1016/0037-7856(73)90026-7. [DOI] [PubMed] [Google Scholar]
- 22.Haynes SG, Levine S, Scotch N, Feinleib M, Kannel WB. The relationship of psychosocial factors to coronary heart disease in the Framingham study. Methods and risk factors. Am J Epidemiol. 1978;107:362–83. doi: 10.1093/oxfordjournals.aje.a112556. [DOI] [PubMed] [Google Scholar]
- 23.Hublin C, Kaprio J, Partinen M, Koskenvuo M, Heikkila K. The Ullanlinna Narcolepsy Scale: validation of a measure of symptoms in the narcoleptic syndrome. J Sleep Res. 1994;3:52–9. doi: 10.1111/j.1365-2869.1994.tb00104.x. [DOI] [PubMed] [Google Scholar]
- 24.Holmes TH, Rahe RH. The social readjustment rating scale. J Psychosom Res. 1967;11:213–8. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- 25.Dohrenwend BS, Krasnoff L, Askenasy AR, Dohrenwend BP. The psychiatric epidemiology research interview life events scale. In: Goldberg L, Breznitz S, editors. Handbook of stress: theoretical and clinical aspects. New York: Free Press; 1982. pp. 332–63. [Google Scholar]
- 26.Korkeila K, Korkeila J, Vahtera J, et al. Childhood adversities, adult risk factors and depressiveness: a population study. Soc Psychiatry Psychiatr Epidemiol. 2005;40:700–6. doi: 10.1007/s00127-005-0969-x. [DOI] [PubMed] [Google Scholar]
- 27.Kivimaki M, Vahtera J, Elovainio M, Lillrank B, Kevin MV. Death or illness of a family member, violence, interpersonal conflict, and financial difficulties as predictors of sickness absence: longitudinal cohort study on psychological and behavioral links. Psychosom Med. 2002;64:817–25. [PubMed] [Google Scholar]
- 28.Fabsitz RR, Sholinsky P, Goldberg J. Correlates of sleep problems among men: the Vietnam Era Twin Registry. J Sleep Res. 1997;6:50–6. doi: 10.1046/j.1365-2869.1997.00026.x. [DOI] [PubMed] [Google Scholar]
- 29.Harvey AG. Insomnia: symptom or diagnosis? Clin Psychol Rev. 2001;21:1037–59. doi: 10.1016/s0272-7358(00)00083-0. [DOI] [PubMed] [Google Scholar]
- 30.Kaprio J, Koskenvuo M, Langinvainio H, Romanov K, Sarna S, Rose RJ. Genetic influences on use and abuse of alcohol: a study of 5638 adult Finnish twin brothers. Alcohol Clin Exp Res. 1987;11:349–56. doi: 10.1111/j.1530-0277.1987.tb01324.x. [DOI] [PubMed] [Google Scholar]
- 31.Flemons WW. Clinical practice. Obstructive sleep apnea. N Engl J Med. 2002;347:498–504. doi: 10.1056/NEJMcp012849. [DOI] [PubMed] [Google Scholar]
- 32.Vahtera J, Poikolainen K, Kivimaki M, Ala-Mursula L, Pentti J. Alcohol intake and sickness absence: a curvilinear relation. Am J Epidemiol. 2002;156:969–76. doi: 10.1093/aje/kwf138. [DOI] [PubMed] [Google Scholar]
- 33.Vgontzas AN, Tsigos C, Bixler EO, et al. Chronic insomnia and activity of the stress system: a preliminary study. J Psychosom Res. 1998;45(1 Spec No):21–31. doi: 10.1016/s0022-3999(97)00302-4. [DOI] [PubMed] [Google Scholar]
- 34.Watson D, Clark LA, Harkness AR. Structures of personality and their relevance to psychopathology. J Abnorm Psychol. 1994;103:18–31. [PubMed] [Google Scholar]
- 35.van Eck M, Nicolson NA, Berkhof J. Effects of stressful daily events on mood states: relationship to global perceived stress. J Pers Soc Psychol. 1998;75:1572–85. doi: 10.1037//0022-3514.75.6.1572. [DOI] [PubMed] [Google Scholar]
- 36.Kendler KS, Kessler RC, Walters EE, et al. Stressful life events, genetic liability, and onset of an episode of major depression in women. Am J Psychiatry. 1995;152:833–42. doi: 10.1176/ajp.152.6.833. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs N, Kenis G, Peeters F, Derom C, Vlietinck R, van Os J. Stress-related negative affectivity and genetically altered serotonin transporter function: evidence of synergism in shaping risk of depression. Arch Gen Psychiatry. 2006;63:989–96. doi: 10.1001/archpsyc.63.9.989. [DOI] [PubMed] [Google Scholar]
- 38.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (form Y) Palo Alto: Consulting Psychologists Press Inc; 1983. [Google Scholar]
- 39.Ohayon MM, Caulet M, Priest RG, Guilleminault C. DSM-IV and ICSD-90 insomnia symptoms and sleep dissatisfaction. Br J Psychiatry. 1997;171:382–8. doi: 10.1192/bjp.171.4.382. [DOI] [PubMed] [Google Scholar]
- 40.Coker AL, Smith PH, McKeown RE, King MJ. Frequency and correlates of intimate partner violence by type: physical, sexual, and psychological battering. Am J Public Health. 2000;90:553–9. doi: 10.2105/ajph.90.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 42.Eaker ED, Pinsky J, Castelli WP. Myocardial infarction and coronary death among women: psychosocial predictors from a 20-year follow-up of women in the Framingham Study. Am J Epidemiol. 1992;135(854):64. doi: 10.1093/oxfordjournals.aje.a116381. [DOI] [PubMed] [Google Scholar]
- 43.Kubzansky LD, Kawachi I, Spiro A, III, Weiss ST, Vokonas PS, Sparrow D. Is worrying bad for your heart? A prospective study of worry and coronary heart disease in the Normative Aging Study. Circulation. 1997;95(818):24. doi: 10.1161/01.cir.95.4.818. [DOI] [PubMed] [Google Scholar]
- 44.Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol. 2005;45:637–51. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Goodwin RD, Cox BJ, Clara I. Neuroticism and physical disorders among adults in the community: results from the National Comorbidity Survey. J Behav Med. 2006;29:229–38. doi: 10.1007/s10865-006-9048-5. [DOI] [PubMed] [Google Scholar]