Abstract
Sphingosine-1-phosphate (S1P) is a bioactive sphingolipid metabolite involved in many cellular processes, acting not only as an extracellular ligand to its specific G protein-coupled receptors, but also as a putative intracellular messenger with yet unidentified targets. Mast cells are tissue-dwelling pivotal early effectors of allergic responses, which produce and secrete S1P that can bind to its receptors present on mast cells to influence their activation and functions. In this review, we will first discuss the current knowledge of S1P production by two isozymes of sphingosine kinase (SphK). Mechanisms of SphK activation will be discussed, with an emphasis on experimental approaches developed to study their differential activation and biological roles in the context of mast cells. The relevance of mast cells in the etiology of allergic disorders, asthma and anaphylaxis is well established. In this review, this concept will be revisited, focusing on the contribution of S1P production and secretion to the symptoms associated with dysregulated inflammatory responses. To conclude, counteracting the proinflammatory effects of S1P could be envisioned as a therapeutic strategy to treat allergic disorders, exacerbated airway inflammation, and anaphylactic reactions, and various options will be discussed, such as the development of pharmacological tools to inhibit SphKs, S1P neutralizing monoclonal antibody, and S1P receptor antagonists.
Keywords: asthma, anaphylaxis, mast cells, immunomodulators, sphingosine-1-phosphate, sphingosine kinase
1. Introduction
It is now well accepted that sphingosine-1-phosphate (S1P) is a bioactive sphingolipid metabolite with pleiotropic actions (Spiegel & Milstien, 2003). For many years after their initial characterization, sphingolipids were only regarded as structural components of mammalian cell membranes. However, appreciation of their importance as signaling molecules grew rapidly after the discovery of high-affinity G protein-coupled receptors for S1P (Lee et al., 1998). This added to the complexity of signaling abilities of S1P as it had previously been suggested that it might be an intracellular second messenger that regulates calcium levels and cell growth and survival (Olivera & Spiegel, 2001). Therefore, it is not surprising that S1P is involved in the regulation of a variety of cellular processes, including proliferation, migration, survival, cytoskeletal organization, adherens junction assembly, morphogenesis, angiogenesis and trafficking of immune cells (Spiegel & Milstien, 2003; Cyster, 2005). Mast cells play pivotal roles in immediate-type and inflammatory allergic reactions that can result in asthma, a disease of chronic airway inflammation. Crosslinking of the high-affinity receptor for immunoglobulin E (IgE) on these cells leads to the release of many inflammatory mediators, chemokines and cytokines, as well as eicosanoids (leukotrienes and prostaglandins) and S1P (Rivera & Gilfillan, 2006). This review will recapitulate and also highlight recent exciting findings on the regulation and functions of S1P in allergic responses, their pulmonary manifestations and their systemic exacerbation defined as anaphylaxis.
2. Biosynthesis and metabolism of S1P
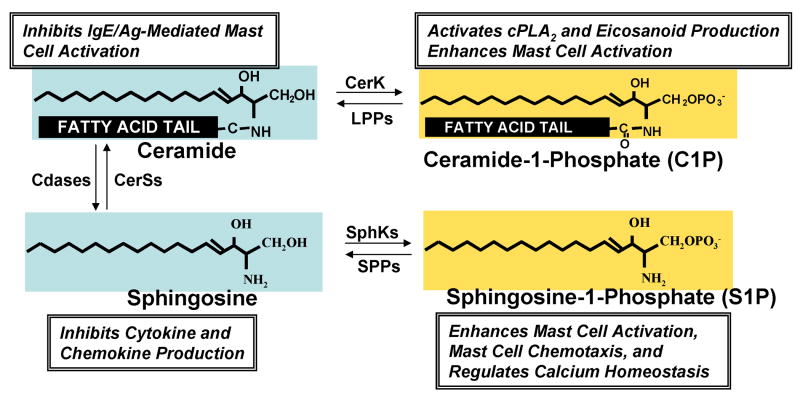
Unlike the biosynthesis of other membrane lipids such as sterols and glycerolipids, the initial steps of sphingolipid biosynthesis leading to ceramide formation take place in the cytosolic leaflet of the endoplasmic reticulum (ER), followed by transport of ceramide from the ER to the Golgi apparatus, where conversion to more complex sphingolipids takes place. The de novo pathway is initiated by the condensation of L-serine with palmitoyl-CoA to form 3-ketosphinganine, a reaction catalyzed by serine palmitoyltransferase (Hannun et al., 2001). The 3-ketosphinganine is then reduced by 3-ketosphinganine reductase in a NADPH-dependent manner to D-erythro-sphinganine (dihydrosphingosine), which is N-acylated to dihydroceramide by sphinganine N-acyltransferase and the 4-5 trans double bond then introduced by a desaturase, to finally form ceramide. The ceramide transport protein CERT, a cytoplasmic protein with a phosphatidylinositol-4-phosphate-binding domain, transports ceramide (and dihydroceramide) from the ER to the Golgi apparatus in a non-vesicular transport manner (Hanada et al., 2003). In the Golgi, ceramide and dihydroceramide are converted by sphingomyelin synthase to sphingomyelin and dihydro-sphingomyelin, on the lumenal side of the Golgi or to glucosylceramides and dihydroglucosylceramides on the cytosolic surface of the Golgi (van Meer & Holthuis, 2000). It is important to note that the sphingoid base sphingosine is not produced de novo but can only be formed from degradation of ceramide by ceramidase or turnover of plasma membrane glycosphingolipids and sphingomyelin in the endocytic recycling pathway. Sphingosine kinases (SphK1 and SphK2) catalyze the phosphorylation of sphingosine to form S1P, which can be reversibly degraded to sphingosine by two specific S1P phosphatases (SPP-1 and SPP-2) residing in the ER or irreversibly by S1P lyase. It is of interest that S1P, sphingosine and ceramide can be interconverted by the sequential actions of SPPs, ceramide synthases, ceramidases, and SphKs, respectively (Figure 1). Thus, intracellular levels of S1P are tightly regulated by the balance between synthesis and degradation.
Figure 1. Sphingolipid metabolites and their effects on mast cell functions.
The scheme shows the structures of the bioactive sphingolipid metabolites, sphingosine, sphingosine-1-phosphate, ceramide, and ceramide-1-phosphate and indicates the enzymes responsible for their interconversion. Some important actions regulated by these metabolites in mast cells are indicated.
Two mammalian isoforms of SphK have been discovered, named type 1 and 2, both widely but often differentially expressed. Furthermore, SphK1 and SphK2 display different catalytic properties, subcellular locations and substrate specificity. D-erythro-sphingosine is the preferred substrate for SphK1, whereas SphK2 phosphorylates a wider range of sphingoid base substrates, including phyto-sphingosine and dihydrosphingosine. The novel immunosuppressive compound FTY 720 (fingolimod), which is a sphingosine analogue, is phosphorylated by SphK2 to FTY 720-phosphate, an S1P mimetic that is a ligand for all of the S1P receptors except S1P2. Indeed, FTY720 causes lymphopenia in mice lacking SphK1 but not SphK2, demonstrating the requirement for SphK2 for the metabolic activation of this pro-drug (Allende et al., 2004; Kharel et al., 2005; Zemann et al., 2006).
Since SphK1 and SphK2 null mice develop and reproduce normally, it is suggested that both isoforms of SphK compensate for the lack of each other. However, double knock-outs for SphK1 and Sphk2 completely lack S1P and are embryonically lethal (Mizugishi et al., 2005), suggesting that each SphK isoform may have distinct as well as redundant functions and that S1P is crucial for life.
The concentration of S1P in plasma ranges from 0.1 to 0.6 μM, while in serum it varies from 0.4 to 1.1 μM (Yatomi et al., 1997; Caligan et al., 2000; Berdyshev et al., 2005). By comparison, tissue S1P levels are low, ranging from 0.5-75 pmol/mg (Edsall & Spiegel, 1999; Schwab et al., 2005). Therefore, a significant concentration gradient of S1P exists between blood and interstitial fluids in mammals. The main sources of S1P in the blood are hematopoietic cells. Platelets (which lack S1P lyase and possess high levels of SphK activities), neutrophils, mast cells and mononuclear cells are endowed with the ability to secrete S1P (Yatomi et al., 2001). It has recently been suggested that erythrocytes may be the major source of S1P in blood (Pappu et al., 2007). However, non-hematopoietic cells are also able to synthesize and secrete S1P. We recently examined the mechanisms involved in the secretion of S1P from mast cells and demonstrated that secretion of S1P is mediated at least in part by the ATP binding cassette (ABC) transporter ABCC1 (Mitra et al., 2006). An alternate mechanism has been proposed for the generation of extracellular S1P, which involves the secretion of SphK1 from vascular endothelial cells, which then acts as an ectokinase (Ancellin et al., 2002).
3. Mechanisms of sphingosine kinase activation
SphK activity has been shown to be increased by a plethora of external stimuli resulting in increased intracellular S1P, including ligands for GPCRs (S1P, LPA, formyl peptide, nucleotides, bradykinin, muscarinic receptor agonists), agonists of growth factor receptors (PDGF, VEGF, NGF, EGF), TGF-β, TNF-α, interleukins, calcium ionophores, phorbol ester, and cross-linking of immunoglobulin receptors (reviewed in (Spiegel & Milstien, 2003; Taha et al., 2006). Many agonists produce a rapid and transient increase of SphK1 activity accounted for by translocation to the plasma membrane and phosphorylation. However, in many cases, the involvement of SphK1 activation and subsequent formation of S1P has only been demonstrated indirectly through pharmacological approaches blocking pathways and molecular mechanisms activated by both SphK isoforms, such as pan SphK inhibitors, including D,L-threo-dihydrosphingosine and N,N-dimethylsphingosine (DMS) (Edsall et al., 1998). Much less is known of the regulation of SphK2, although it was recently shown that EGF not only activates SphK1, it also is the first example of an agonist that also activates SphK2 (Hait et al., 2005). More recently, crosslinking of the IgE receptor on mast cells was reported to activate both SphK1 and SphK2, which was important for mast cell responses (Olivera et al., 2006).
Intracellularly produced S1P can act in autocrine and/or paracrine manner to stimulate S1P receptors present at the cell surface and signal “inside-out” (Spiegel & Milstien, 2003). Thus, S1P exerts diverse cellular effects depending on the subtypes of S1P receptors that are expressed and the subsequent initiation of downstream G protein-mediated signaling, including activation of Src, focal adhesion kinases and Rac, crucial elements of cell migration. For example, binding of S1P to S1P1 or S1P3 receptors stimulates cell motility, whereas binding to S1P2 receptor generally counteracts cell movement, and rather promotes cell activation, such as mast cell degranulation (Goparaju et al., 2005; Jolly et al., 2004).
Antigen-dependent activation of mast cells is regulated by complex signaling events upon aggregation of the FcεRI high affinity receptors for IgE (Gilfillan & Tkaczyk, 2006). FcεRI is a tetrameric receptor composed of an α-chain which binds IgE, one β-chain, and a γ-chain homodimer, that initiates signaling. The Src kinase Lyn phosphorylates tyrosine residues in the β- and γ-chains in the immune-receptor tyrosine-based activation motifs (ITAMs). Once phosphorylated, the ITAMs provide high-affinity docking sites for the SH2 domains of Lyn and Syk, respectively. Both of these tyrosine kinases have been recently identified as SphK1 interacting proteins (Urtz et al., 2004), but do not phosphorylate it. However, direct interaction of SphK1 with Lyn increases the activity of both kinases and results in the recruitment of SphK1 to FcεRI and the appearance of both Lyn and SphK1 in lipid rafts (Figure 2).
Figure 2. Actions of S1P in Mast Cell Functions.
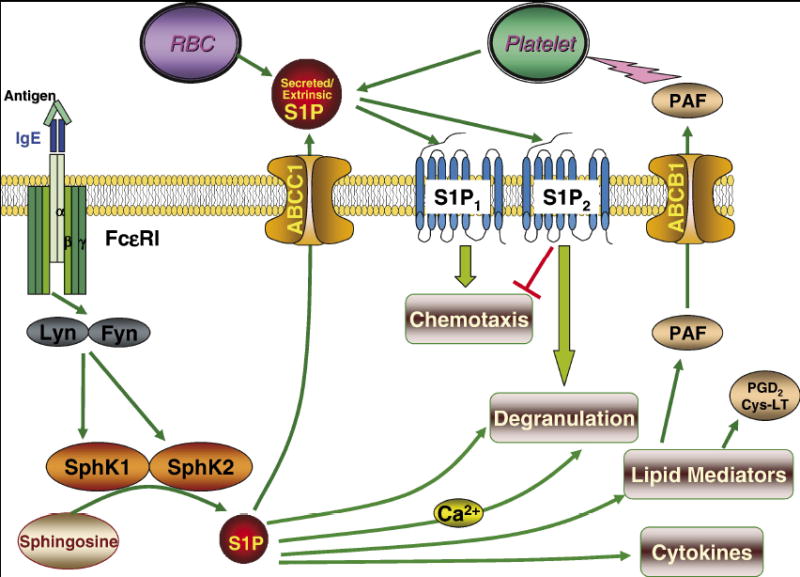
Crosslinking of FcεRI by antigen causes activation of the tyrosine kinases Lyn and Fyn that in turn stimulate SphK1 and SphK2 leading to formation of S1P. S1P formed by activation of SphK1 can then be exported by ABCC1 transporter to activate S1P receptors on the mast cell surface. S1P1 is important for mast cell migration toward Ag while S1P2 is involved in their degranulation. SphK2 acts internally to regulate calcium homeostasis and PKC, and thus regulates degranulation and release of other lipid mediators and cytokines, probably by regulating the balance between the counteracting sphingolipids, sphingosine and S1P.
A recent report showed that both SphK1 and SphK2 are activated upon FcεRI triggering in murine and human mast cells (Olivera et al., 2006). Pretreatment of IgE-sensitized murine bone marrow-derived mast cells (BMMC) with a Src-selective inhibitor prevented activation of both SphK1 and SphK2 upon Ag triggering. Pretreatment of Lyn-deficient BMMC with the Src inhibitor also abrogated SphK activation, suggesting that an additional Src protein tyrosine kinase is involved in SphK activation, likely Fyn. Indeed, BMMC from Fyn-deficient mice display a severely impaired activation of SphK1, together with complete abrogation of SphK2 activation. Similarly, Fyn kinase was an absolute requirement for KIT (the receptor for Stem Cell Factor, a pivotal mast cell growth factor)-dependent activation of SphK1 and SphK2. Both Fyn and Lyn contribute to translocation of SphK2 to the plasma membrane upon engagement of FcεRI, independently of ERK activation, calcium mobilization, and PKC activation, all signals which mediate translocation of SphK1 (Olivera et al., 2006).
Regulation of SphK1 by TNF-α requires binding to TRAF2 (Xia et al., 2002). Accordingly, deletion of its TRAF2 binding consensus site abolishes binding of SphK1 to TRAF2 and subsequent stimulation of SphK1 by TNF-α, but not by phorbol ester, which also stimulates phosphorylation of SphK1 (and its translocation to the membrane) via protein kinase C and activation of extracellular signal-regulated kinase (ERK) 1/2-mediated phosphorylation of SphK1. Similarly, SphK1 is required for TNF-α-stimulated ERK1/2 activation, demonstrating intricate crosstalk between SphK1 and ERK.
IgE/Ag stimulation of human mast cells has been shown to trigger SphK1-mediated fast and transient calcium release from intracellular stores that is dependent on activation of phospholipase D (PLD) (Melendez & Khaw, 2002). It is possible that phosphatidic acid produced by activation of PLD may be a key mediator of activation and translocation of SphK1 to the plasma membrane as it is a potent stimulator of SphK activity (Olivera et al., 1996) and recombinant SphK1 has been shown to bind phosphatidic acid (Delon et al., 2004). Moreover, SphK1 and PLD1 are co-localized in cells, although direct interaction was not detected (Melendez & Khaw, 2002).
A link between activation of SphK and Ca2+ mobilization from intracellular stores in several types of cells including mast cells has clearly been established (Spiegel & Milstien, 2003). For example, Ca2+ chelators inhibited f-Met-Leu-Phe peptide- and nucleotide-triggered S1P production in HL-60 granulocytes and HEK 293 cells (Alemany et al., 2000). Moreover, it has long been known that Ca2+/calmodulin binds to SphK1 (Kohama et al., 1998). Although these data suggest that SphK could be regulated by Ca2+, direct activation by calcium has not been demonstrated, suggesting that calcium is more likely required for translocation of SphK1 to the plasma membrane. It is noteworthy that deletion of functional calmodulin-binding sites on SphK1 prevents its translocation to the plasma membrane upon stimulation with phorbol ester, even though its catalytic activity and phosphorylation are not affected (Sutherland et al., 2006).
4. Dual mode of S1P signaling: “inside-out” and intracellular effects
Intracellularly generated S1P resulting from activation of SphK can be exported out of cells and act as a ligand for the family of five S1P receptors, named S1P1-5. All bind S1P with high affinity and specificity (Spiegel & Milstien, 2000) and are coupled to different G proteins, thus enabling them to regulate a wide variety of cellular responses. Crosslinking of FcεRI in mast cells has been shown to activate SphK and increase S1P secretion, leading to autocrine/paracrine activation of S1P1, which is important for their migration toward antigen, and S1P2, which regulates their degranulation (Jolly et al., 2004). It has recently been demonstrated that intracellular S1P produced upon FcεRI crosslinking is exported out of mast cells at least in part by an ATP binding cassette transporter, ABCC1, independently of their degranulation (Mitra et al., 2006).
As mentioned above, there are also several biological effects that are driven by intracellular S1P that are not reproduced by the addition of exogenous S1P (Chalfant & Spiegel, 2005). For example, downregulating expression of SphK1 blocks the rapid increase in intracellular calcium induced by FcεRI triggering that is required for mast cell degranulation (Melendez & Khaw, 2002). Similarly, knockdown of SphK1 in macrophages inhibits the increase in intracellular calcium induced by treatment with complement anaphylatoxin C5a, and also decreases cytokine release and migratory responses to C5a (Melendez & Ibrahim, 2004). However, the relevance of these in vitro studies to the physiological role of SphK1 is unclear since macrophage mediated thioglycollate-induced peritonitis and collagen-induced arthritis are unaffected by SphK1 gene knockout (Michaud et al., 2006). These data are in sharp contrast with previous reports demonstrating a clear dependence of mast cell and macrophage functions upon reduction of SphK1 expression (Kee et al., 2005; Olivera & Rivera, 2005), suggesting possible adaptation and/or compensatory mechanisms. These issues may be more clearly resolved by the development of conditional SphK1/SphK2 knockout mice or highly selective inhibitors of SphK1 and SphK2, since complete deficiency of SphK activity (and, therefore, S1P) in the double knockout mice is embryonic lethal (Mizugishi et al., 2005).
An elegant recent report evoked a predominant role for SphK2 in the regulation of mast cell activation in vitro, while SphK1 enhances susceptibility to antigen challenge in vivo (Olivera et al., 2007). Because deletion of both SphKs is embryonically lethal, fetal liver-derived mast cells from SphK1-/-, SphK2-/-, and SphK1-/-SphK2-/- mice were utilized. By comparison of the results from each of these knockouts, SphK2 was identified as the pivotal modulator of calcium influx and downstream signaling, particularly PKCα and β, leading to degranulation and the production of eicosanoids and cytokines, with no involvement of SphK1. By contrast, passive systemic anaphylaxis was only impaired in SphK1-/- mice and plasma histamine levels were correlated with circulating levels of S1P. Altogether, their data implicate SphK2 as a determinant of intrinsic mast cell function, whereas SphK1 plays an extrinsic role (Figure 2). Nonetheless, given the differences in the gene expression profiles and responses of human and mouse mast cells, SphK1 and SphK2 may play different roles in human mast cells (Melendez & Khaw, 2002) and allergic responses, including asthma and anaphylaxis.
The finding that circulating S1P levels are primarily determined by SphK1 outside the mast cell compartment is very intriguing as these levels correlate with histamine release and anaphylaxis. A recent study demonstrated that the source of circulating S1P is mainly hematopoietic in origin with erythrocytes a major contributor, whereas lymph S1P is from a distinct radiation-resistant source, possibly lymphatic endothelium (Pappu et al., 2007). The relationship between the different sources of S1P and their contributions to allergic responses and anaphylaxis will be important to determine. However, it is tempting to speculate that upon activation of mast cells and secretion of such mediators as platelet activating factor (PAF), platelets are being activated and release additional S1P (Figure 2).
5. Pathophysiology of allergic responses, asthma, and anaphylaxis
Mast cells are resident in all normal tissues where they play a prominent role in tissue homeostasis, wound healing, and host defense (Rivera & Gilfillan, 2006). In sites of inflammation, mast cell activation initiates and/or perpetuates the pathophysiology of many disorders via their ability to differentially secrete, synthesize and release a plethora of proinflammatory mediators and cytokines (Grimbaldeston et al., 2006). In bronchial asthma, the perception of the role of mast cells has evolved to the present view that mast cells, together with T lymphocytes and eosinophils, substantially contribute to the establishment and perpetuation of bronchial inflammation. Thus, asthma and allergic diseases can be defined as inflammatory processes caused by overzealous immune responses.
Mast cells are activated by IgE/antigen crosslinking of FcεRI. However, many other stimuli unrelated to IgE can also elicit the secretion of mast cell mediators, such as neurotransmitters, Toll-like receptor ligands, basic peptides, and anaphylatoxins. Indeed, CD88, the receptor for C5a, is expressed by the subset of human lung mast cells that are double positive for tryptase and chymase and the binding of exogenous C5a to CD88 enables their activation (Oskeritzian et al., 2005).
Mast cells secrete autocoid mediators, such as histamine, prostaglandin D2 (PGD2), and leukotriene C4 (LTC4), which induce increased vascular permeability, tissue edema, bronchoconstriction, massive recruitment of inflammatory cells, and mucous secretion, all features of asthma. Mast cells can also secrete preformed and/or de novo synthesized proinflammatory cytokines, able to regulate IgE synthesis and the development of inflammation, and several profibrogenic cytokines, including TGF-β and basic fibroblast growth factor. The serine proteases, tryptase, chymase and carboxypeptidase, whose secretion depends on the phenotype of the human mast cell, constitute major secretory products as well and can interact with numerous cell types via protease activated receptors and alternate processes. Clinically, these interactions are also important in acute systemic exacerbation of allergic reactions or anaphylaxis, which will be discussed below.
Mast cells of asthmatic patients micro-localize within airway smooth muscle (ASM) cells, the airway mucous glands, and the bronchial epithelium. Thus, the disordered airway physiology and wall remodeling features of asthma are consequences of inflammation and bronchial hyperresponsiveness (BHR). Furthermore, abnormal ASM function is fundamentally important in the pathophysiology of asthma and there is a positive correlation between ASM mast cell numbers and BHR (Brightling et al., 2002), evoking a functional interaction between these two cell types. Symptom exacerbation occurs in the presence of environmental allergens, likely through the release of mast cell-derived mediators, which are all potent agonists for ASM contraction and can stimulate their proliferation (Berger et al., 2001). Mast cells also infiltrate the bronchial epithelium in asthma, strategically placed at the entry level of potential allergens. Upon allergen-dependent activation, mast cells release inflammatory mediators that influence bronchial epithelial function. In addition, there is also a significant correlation between the density of mast cells within mucous glands and the degree of mucous obstruction in the airways. Numerous mast cell-derived cytokines are likely to contribute to many features of asthma. Among these, TNF-α is a pivotal cytokine that orchestrates many of the asthmatic symptoms. Increased TNF-α expression has recently been shown in patients with refractory asthma (Berry et al., 2006) and may be part of a positive autocrine feedback loop on the mast cell population, since human lung mast cell-derived tumor necrosis factor-α (TNF-α) can, in turn, induce mast cell mediator release (Coward et al., 2002).
Anaphylaxis is a systemic syndrome representing an extreme and acute form of allergic reaction that consists of a sensitization phase during which allergen-specific IgE is produced followed by an acute effector phase triggered by allergen-induced degranulation of mast cells. Anaphylaxis is a typical type I hypersensitivity reaction, sharing common mechanisms with asthma. However, a mast cell-independent mouse model of anaphylaxis has been described, in which symptoms correlate with an increase in vascular permeability, probably by effects on the endothelial barrier. Interestingly, in addition to canonical IgE-mast cell mediated mechanisms, an alternative pathway involving IgG1 and FcγRIII has been shown to trigger anaphylaxis in mice (Finkelman et al., 2005; Galli et al., 2005; Rivera & Gilfillan, 2006). Moreover, genetically mast cell-deficient mice can exhibit acute allergic responses (Choi et al., 1998). B cell lymphoma 10 (Bcl10) and mucosa-associated lymphoid tissue 1 (Malt1) have recently been identified as novel key regulators of mast cell signaling (Klemm et al., 2006). Mice deficient for either protein display severely impaired IgE-dependent late phase anaphylactic reactions. Although their mast cells showed normal degranulation and leukotriene secretion, the NF-κB signaling pathway was severely disabled with the subsequent absence of mast cell-derived TNF-α and IL-6.
Importantly, mouse models and clinical patient observations are not always in agreement. Thus, although mouse “models” of asthma have provided insights into immunological processes, mice do not have asthma, much less chronic asthma. However, a model has recently been developed in which mice sensitized by repetitive and long-term intra-nasal challenge with Ag (ovalbumin) in the absence of artificial adjuvant develop mast-cell dependent AHR with many features of chronic asthma (Yu et al., 2006). A discussion of the limitations of mouse models is beyond the scope of this review but this is thoroughly reviewed elsewhere (Wenzel & Holgate, 2006).
6. Relevance of S1P in the etiology of allergic disorders
An initial clue that S1P might be an important component of the mast cell-dependent inflammatory cascade of events observed in allergic reactions and asthma was provided by the observations that S1P levels are increased in bronchoalveolar lavage (BAL) fluid of asthmatics after challenge with Ag (Ammit et al., 2001). This led to studies showing that cross-linking of FcεRI on rodent mast cells activates SphK, increasing production of S1P (Jolly et al., 2004). Interestingly, S1P levels correlated with eosinophil numbers in the BAL of asthmatic subjects (Ammit et al., 2001). The recent discovery that S1P is secreted by mast cells via ABCC1 transporters (Mitra et al., 2006), an active transport system, further supports the notion that mast cells may utilize S1P to regulate migration of inflammatory cells to sites of inflammation (Figure 3).
Figure 3. Multiple Functions of Mast Cell-Derived S1P in Allergic Inflammation.
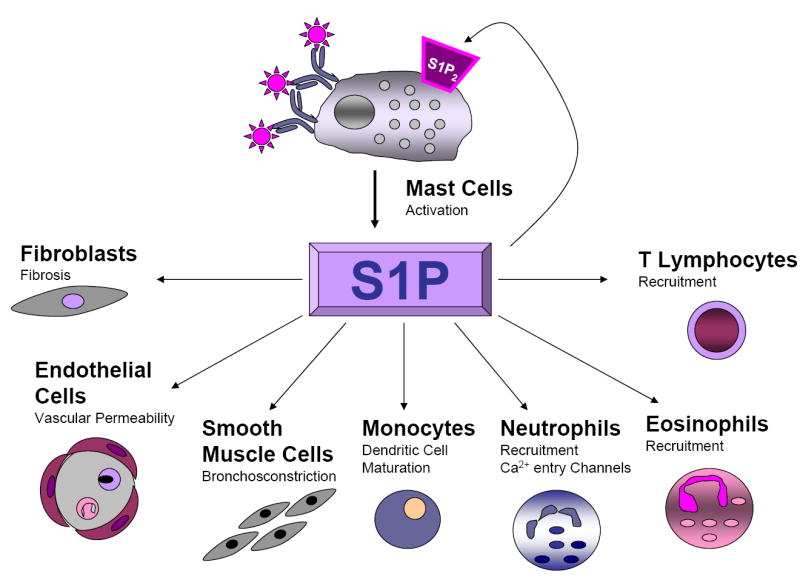
Secretion of S1P by activated mast cells can promote allergic reactions on a broad scale by activating and recruiting many types of immune cells involved in allergic and inflammatory responses, including eosinophils (Roviezzo et al., 2004), Th2 lymphocytes (Sawicka et al., 2003) and neutrophils. S1P also profoundly affects endothelial cell function and promotes adhesion molecule expression in endothelial cells (Garcia et al., 2001; Lee et al., 1999; Wang et al., 1999; Xia et al., 1998), induces contraction and proliferation of airway smooth muscle cells (Ammit et al., 2001), fibroblast proliferation, and shifts maturing dendritic cell-induced polarization of T cells into a Th2 phenotype (Idzko et al., 2002).
Of interest, T lymphocytes comprise an important cell type in inflammatory infiltrates. Convincing evidence from studies with S1P1-deficient mice have demonstrated that ligation of the S1P1 receptor by S1P is pivotal for the recirculation of T lymphocytes from secondary lymphoid organs to peripheral tissues where inflammation takes place (Matloubian et al., 2004). Consequently, the novel immunomodulator FTY720, a chemical derivative of the fungal metabolite myriocin and a sphingosine analogue, which can be phosphorylated by SphK2 to FTY720-phosphate, an S1P mimetic, was shown to modulate lymphocyte trafficking by binding to S1P1 (Graler & Goetzl, 2004; Matloubian et al., 2004; Wei et al., 2005). Thus, FTY720-phosphate produced in vivo from FTY720 blocks the egress of lymphocytes into the medullary sinus of lymph nodes and efferent lymphatics, leading not only to an overall decrease in circulating T and B lymphocytes, or lymphopenia, but also to inhibition of lymphocyte influx into sites of inflammation. These long-lasting effects from a single low dose make FTY720 a good candidate drug for suppressing transplant rejection and T cell-mediated inflammatory diseases as well as for treatment of multiple sclerosis (Brinkmann et al., 2002).
Of note, it was recently found that neither FTY720 nor FTY720-phosphate, despite its similarity to S1P, affect mast cell degranulation, yet they significantly reduced Ag-induced secretion of PGD2 and cysteinyl-LT. FTY720 was discovered to be a directinhibitor of cytosolic phospholipase A2, the rate-limiting enzyme in the production of arachidonic acid, the precursor of all eicosanoids (Payne et al., 2007). Indeed, oral treatment of mice with FTY720 inhibits AHR induced by adoptive transfer of Th1 and Th2 cells and asthma induced by active immunization and challenge with ovalbumin (Sawicka et al., 2003). In addition, inhaled FTY720 prior to or during ongoing allergen challenge suppresses Th2-dependent eosinophilic airway inflammation and bronchial AHR by inhibition of migration of lung dendritic cells to the mediastinal lymph nodes, thus preventing the formation of allergen-specific Th2 cells in the lymph nodes (Idzko et al., 2006).
Mast cells express both S1P1 and S1P2 receptors on their surface and similar to T lymphocytes, S1P1 is pivotal for mast cell chemotaxis. Conversely, S1P2 mediates mast cell activation and degranulation. Importantly, S1P2 levels are upregulated after activation by Ag (Jolly et al., 2004). Since mast cells secrete S1P, it can act on its receptors expressed on the mast cell surface in a positive feedback loop to amplify mast cell functions (Figure 3).
Furthermore, S1P has been shown to induce smooth muscle contraction and increased intracellular calcium by activating the S1P2 receptor (Watterson et al., 2005). Recently, it was shown that prolonged exposure to low concentrations of S1P markedly enhanced methacholine-induced contraction of bronchial smooth muscles by Rho-mediated myosin phosphatase inactivation, and therefore, calcium sensitization (Kume et al., 2007). Moreover, another report showed that S1P triggers AHR in ovalbumin-sensitized mice, using isolated bronchi and whole lungs. Mechanistically, increased AHR was coupled with enhanced expression not only of SphK1 and SphK2, but also of S1P2 (and S1P3) receptors. Therefore, S1P and its receptors constitute a multifaceted immunomodulatory system (Rosen & Goetzl, 2005) (Figure 3).
The regulatory effects of S1P on vascular endothelial barrier functions are also important to examine, since asthma is more and more unambiguously recognized as a disease consecutive to the rupture of endothelial barrier. In this regard, it has long been known that S1P promotes endothelial adherens junction assembly (Lee et al., 1999). This is relevant to the hyperpermeability issues associated with lung injury and pulmonary edema (Bhattacharya, 2004), as adherens junctions critically determine endothelial barrier properties. Enhanced junction assembly in lung endothelial cells augments barrier functions and prevents pulmonary edema formation. Administration of selective S1P1 competitive antagonists to mice induces disruption of barrier integrity in pulmonary endothelium (Rosen et al., 2007; Sanna et al., 2006). Interestingly, endothelial S1P1 expression is finely regulated by the existence of a large caveolar reservoir of receptors and the ability of agonists to differentially induce receptor ubiquitination and turnover (Rosen et al., 2007). An important observation in relation to barrier functions of S1P was that a single intravenous injection of S1P had protective effects against lung injury caused by high-volume mechanical ventilation and intratracheal endotoxin instillation in animal models (McVerry & Garcia, 2004). The sustained protective effects were attributed to expression of S1P1 by endothelial cells (Rosen & Goetzl, 2005). However, S1P administration via the airways but not via the vasculature induces lung leakage (Gon et al., 2005). Using receptor-null mice, it was demonstrated that activation of S1P3 in alveolar epithelium results in increased permeability via tight junction opening and loss of ZO-1, an essential component of the cytoplasmic plaque associated with tight junctions (Gon et al., 2005). Thus, spatially and mechanistically distinct S1P receptor subtypes can have opposing effects on pulmonary epithelial and endothelial barriers. Therefore, regulation of endothelial and epithelial cell functions by S1P constitutes another facet through which it might influence the vascular permeability changes seen in asthma and anaphylaxis. It is well established that lung tissues constitute a likely source of S1P. Thus, immunolocalization of SphK1 has been examined in human lung samples and shown to be associated with the apical surface of pseudostratified epithelial cells of the bronchi and underlying smooth muscle cells, submucosal serous glands, type II alveolar macrophages (secreting surfactant), and endothelial cells of blood vessels. Altogether, these findings suggest potential roles for SphK1-mediated synthesis of S1P in altering pulmonary functions (Johnson et al., 2005)
The observation that TNF-α is overproduced in asthmatic subjects is also of interest since TNF-α is a potent activator of SphK and subsequent production of S1P. SphK1 is activated in many cell types relevant to allergic disorders and occurs principally in response to inflammatory cytokines, such as TNF-α and IL-1β. Moreover, prostaglandins play an important role in airway inflammation and are produced by cycloxygenases (COX) whose activation is mediated by SphK1 (Pettus et al., 2003). Gene knockout and pharmacological approaches have demonstrated that S1P induces COX-2, a process that requires Gα12 (Ki et al., 2007). Moreover, treatment with S1P induced COX-2 in the lungs (and livers) of mice and increased plasma PGE2, effects that were prevented by Gα12 deficiency (Ki et al., 2007).
7. S1P as potential therapeutic target for treatment of allergic disorders
To date, asthma therapy is mainly targeted at suppressing the symptoms that are consequences of airway inflammation and ASM contraction. As described above, a number of S1P-mediated events are relevant to the pathophysiology of allergic responses, asthma and the exacerbated anaphylactic reaction. Given its multifaceted roles in asthma and allergy pathophysiology, inhibitors of SphK that block formation of S1P could also inhibit IgE/Ag-dependent cysteinyl-LT and cytokine production by preventing activation of the ERK pathway and AP-1-mediated transcription. SphK inhibitors have already been utilized to suppress tumor growth in mouse xenograft models and to improve chemosensitivity (French et al., 2006). The development of more specific inhibitors with improved aqueous solubility will be important for their potential therapeutic use in human allergic disorders. Similarly, selective S1P receptor antagonists could also constitute a relevant strategy for the modulation of pulmonary and allergic disorders. Of note, administration of a monoclonal anti-S1P antibody to neutralize circulating S1P substantially reduced tumor progression in several murine xenograft and allograft models (Visentin et al., 2006). Use of a neutralizing anti-S1P antibody in allergic disorders merits evaluation.
8. Conclusions
In sum, the proinflammatory effects of S1P in the airways result from its ability to induce secretion of chemoattractants contributing to the inflammatory cell infiltrate and its actions on S1P receptors expressed at the surface of mast cells, which are also able to secrete S1P, acting then in a positive autocrine amplification loop. There is increasing evidence that SphKs, S1P and S1P receptors are also pivotal in the regulation of immune cell trafficking, activation and inflammation, which makes them excellent candidates to target for the treatment and/or the relief of allergic manifestations.
Acknowledgments
Carole A. Oskeritzian was supported by NIH grant KO1 AR053186, Sarah Spiegel was supported by NIH grant RO1 AI50094, and Sheldon Milstien was supported by the Intramural Research Program of the National Institute of Mental Health.
Abbreviations
- ABC
ATP binding cassette
- Ag
antigen
- AHR
airway hyperresponsiveness
- ASM
airway smooth muscle
- BAL
bronchoalveolar lavage
- BMMC
bone marrow-derived mast cells
- COX
cyclooxygenase
- ER
endoplasmic reticulum
- ERK
extracellular signal regulated kinase
- IgE
immunoglobulin E
- IL-1
interleukin 1
- LT
leukotriene
- PG
prostaglandin
- PKC
protein kinase C
- PLD
phospholipase D
- S1P
sphingosine-1-phosphate
- SphK
sphingosine kinase
- SPP
S1P phosphatase
- TNF
tumor necrosis factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Carole A. Oskeritzian, Department of Biochemistry and Molecular Biology, Virginia Commonwealth University School of Medicine, Richmond, VA 23298
Sheldon Milstien, NIMH, NIH, Bethesda, MD 20892.
Sarah Spiegel, Department of Biochemistry and Molecular Biology, Virginia Commonwealth University School of Medicine, Richmond, VA 23298.
References
- Alemany R, Sichelschmidt B, zu Heringdorf DM, Lass H, van Koppen CJ, Jakobs KH. Stimulation of sphingosine-1-phosphate formation by the P2Y(2) receptor in HL-60 cells: Ca(2+) requirement and implication in receptor-mediated Ca(2+) mobilization, but not MAP kinase activation. Mol Pharmacol. 2000;58:491–497. doi: 10.1124/mol.58.3.491. [DOI] [PubMed] [Google Scholar]
- Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, Hajdu R, Rosenbach M, Keohane CA, Mandala S, Spiegel S, Proia RL. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem. 2004;279:52487–52492. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- Ammit AJ, Hastie AT, Edsall LC, Hoffman RK, Amrani Y, Krymskaya VP, Kane SA, Peters SP, Penn RB, Spiegel S, Panettieri RA., Jr Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. FASEB J. 2001;15:1212–1214. doi: 10.1096/fj.00-0742fje. [DOI] [PubMed] [Google Scholar]
- Ancellin N, Colmont C, Su J, Li Q, Mittereder N, Chae SS, Steffansson S, Liau G, Hla T. Extracellular export of sphingosine kinase-1 enzyme: Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J Biol Chem. 2002;277:6667–6675. doi: 10.1074/jbc.M102841200. [DOI] [PubMed] [Google Scholar]
- Berdyshev EV, Gorshkova IA, JG NG, Natarajan V, Hubbard WC. Quantitative analysis of sphingoid base-1-phosphates as bisacetylated derivatives by liquid chromatography-tandem mass spectrometry. Anal Biochem. 2005;339:129–136. doi: 10.1016/j.ab.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Berger P, Perng DW, Thabrew H, Compton SJ, Cairns JA, McEuen AR, Marthan R, Tunon De Lara JM, Walls AF. Tryptase and agonists of PAR-2 induce the proliferation of human airway smooth muscle cells. J Appl Physiol. 2001;91:1372–1379. doi: 10.1152/jappl.2001.91.3.1372. [DOI] [PubMed] [Google Scholar]
- Berry MA, Hargadon B, Shelley M, Parker D, Shaw DE, Green RH, Bradding P, Brightling CE, Wardlaw AJ, Pavord ID. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med. 2006;354:697–708. doi: 10.1056/NEJMoa050580. [DOI] [PubMed] [Google Scholar]
- Bhattacharya J. Lung injury: sphingosine-1-phosphate to the rescue. Am J Respir Crit Care Med. 2004;170:928–929. doi: 10.1164/rccm.2408008. [DOI] [PubMed] [Google Scholar]
- Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. The immune modulator, FTY 720, targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- Caligan TB, Peters K, Ou J, Wang E, Saba J, Merrill AH., Jr A high-performance liquid chromatographic method to measure sphingosine 1-phosphate and related compounds from sphingosine kinase assays and other biological samples. Anal Biochem. 2000;281:36–44. doi: 10.1006/abio.2000.4555. [DOI] [PubMed] [Google Scholar]
- Chalfant CE, Spiegel S. Sphingosine 1-phosphate and ceramide 1-phosphate: expanding roles in cell signaling. J Cell Sci. 2005;118:4605–4612. doi: 10.1242/jcs.02637. [DOI] [PubMed] [Google Scholar]
- Choi IH, Shin YM, Park JS, Lee MS, Han EH, Chai OH, Im SY, Ha TY, Lee HK. Immunoglobulin E-dependent active fatal anaphylaxis in mast cell-deficient mice. J Exp Med. 1998;188:1587–1592. doi: 10.1084/jem.188.9.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coward WR, Okayama Y, Sagara H, Wilson SJ, Holgate ST, Church MK. NF-kappa B and TNF-alpha: a positive autocrine loop in human lung mast cells? J Immunol. 2002;169:5287–5293. doi: 10.4049/jimmunol.169.9.5287. [DOI] [PubMed] [Google Scholar]
- Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–159. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- Delon C, Manifava M, Wood E, Thompson D, Krugmann S, Pyne S, Ktistakis NT. Sphingosine kinase 1 is an intracellular effector of phosphatidic acid. J Biol Chem. 2004;279:44763–44774. doi: 10.1074/jbc.M405771200. [DOI] [PubMed] [Google Scholar]
- Edsall LC, Spiegel S. Enzymatic measurement of sphingosine 1-phosphate. Anal Biochem. 1999;272:80–86. doi: 10.1006/abio.1999.4157. [DOI] [PubMed] [Google Scholar]
- Edsall LC, Van Brocklyn JR, Cuvillier O, Kleuser B, Spiegel S. N,N-Dimethylsphingosine is a potent competitive inhibitor of sphingosine kinase but not of protein kinase C: modulation of cellular levels of sphingosine 1-phosphate and ceramide. Biochemistry. 1998;37:12892–12898. doi: 10.1021/bi980744d. [DOI] [PubMed] [Google Scholar]
- Finkelman FD, Rothenberg ME, Brandt EB, Morris SC, Strait RT. Molecular mechanisms of anaphylaxis: lessons from studies with murine models. J Allergy Clin Immunol. 2005;115:449–457. doi: 10.1016/j.jaci.2004.12.1125. [DOI] [PubMed] [Google Scholar]
- French KJ, Upson JJ, Keller SN, Zhuang Y, Yun JK, Smith CD. Antitumor activity of sphingosine kinase inhibitors. J Pharmacol Exp Ther. 2006;318:596–603. doi: 10.1124/jpet.106.101345. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nature Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- Gon Y, Wood MR, Kiosses WB, Jo E, Sanna MG, Chun J, Rosen H. S1P3 receptor-induced reorganization of epithelial tight junctions compromises lung barrier integrity and is potentiated by TNF. Proc Natl Acad Sci USA. 2005;102:9270–9275. doi: 10.1073/pnas.0501997102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Goparaju SK, Jolly PS, Watterson KR, Bektas M, Alvarez S, Sarkar S, Mel L, Ishii I, Chun J, Milstien S, Spiegel S. The S1P2 receptor negatively regulates platelet-derived growth factor-induced motility and proliferation. Mol Cell Biol. 2005;25:4237–4249. doi: 10.1128/MCB.25.10.4237-4249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G protein-coupled receptors. FASEB J. 2004;18:551–553. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- Grimbaldeston MA, Metz M, Yu M, Tsai M, Galli SJ. Effector and potential immunoregulatory roles of mast cells in IgE-associated acquired immune responses. Curr Opin Immunol. 2006;18:751–760. doi: 10.1016/j.coi.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Hait NC, Sarkar S, Le Stunff H, Mikami A, Maceyka M, Milstien S, Spiegel S. Role of sphingosine kinase 2 in cell migration towards epidermal growth factor. J Biol Chem. 2005;280:29462–29469. doi: 10.1074/jbc.M502922200. [DOI] [PubMed] [Google Scholar]
- Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–809. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Luberto C, Argraves KM. Enzymes of sphingolipid metabolism: from modular to integrative signaling. Biochemistry. 2001;40:4893–4903. doi: 10.1021/bi002836k. [DOI] [PubMed] [Google Scholar]
- Idzko M, Hammad H, van Nimwegen M, Kool M, Muller T, Soullie T, Willart MA, Hijdra D, Hoogsteden HC, Lambrecht BN. Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function. J Clin Invest. 2006;116:2935–2944. doi: 10.1172/JCI28295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idzko M, Panther E, Corinti S, Morelli A, Ferrari D, Herouy Y, Dichmann S, Mockenhaupt M, Gebicke-Haerter P, Di Virgilio F, Girolomoni G, Norgauer J. Sphingosine 1-phosphate induces chemotaxis of immature and modulates cytokine-release in mature human dendritic cells for emergence of Th2 immune responses. FASEB J. 2002;16:625–627. doi: 10.1096/fj.01-0625fje. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Johnson KY, Crellin HG, Ogretmen B, Boylan AM, Harley RA, Obeid LM. Immunohistochemical distribution of sphingosine kinase 1 in normal and tumor lung tissue. J Histochem Cytochem. 2005;53:1159–1166. doi: 10.1369/jhc.4A6606.2005. [DOI] [PubMed] [Google Scholar]
- Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, Milstien S, Spiegel S. Transactivation of sphingosine-1-phosphate receptors by Fc{epsilon}RI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med. 2004;199:959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee TH, Vit P, Melendez AJ. Sphingosine kinase signalling in immune cells. Clin Exp Pharmacol Physiol. 2005;32:153–161. doi: 10.1111/j.1440-1681.2005.04166.x. [DOI] [PubMed] [Google Scholar]
- Kharel Y, Lee S, Snyder AH, Sheasley-O’neill SL, Morris MA, Setiady Y, Zhu R, Zigler MA, Burcin TL, Ley K, Tung KS, Engelhard VH, Macdonald TL, Lynch KR. Sphingosine kinase 2 is required for modulation of lymphocyte traffic by FTY720. J Biol Chem. 2005;280:36865–36872. doi: 10.1074/jbc.M506293200. [DOI] [PubMed] [Google Scholar]
- Ki SH, Choi MJ, Lee CH, Kim SG. Galpha 12 specifically regulates COX-2 induction by sphingosine 1-phosphate: Role for JNK-dependent ubiquitination and degradation of Ikappa Balpha. J Biol Chem. 2007;282:1938–1947. doi: 10.1074/jbc.M606080200. [DOI] [PubMed] [Google Scholar]
- Klemm S, Gutermuth J, Hultner L, Sparwasser T, Behrendt H, Peschel C, Mak TW, Jakob T, Ruland J. The Bcl10-Malt1 complex segregates Fc epsilon RI-mediated nuclear factor kappa B activation and cytokine production from mast cell degranulation. J Exp Med. 2006;203:337–347. doi: 10.1084/jem.20051982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohama T, Olivera A, Edsall L, Nagiec MM, Dickson R, Spiegel S. Molecular cloning and functional characterization of murine sphingosine kinase. J Biol Chem. 1998;273:23722–23728. doi: 10.1074/jbc.273.37.23722. [DOI] [PubMed] [Google Scholar]
- Kume H, Takeda N, Oguma T, Ito S, Kondo M, Ito Y, Shimokata K. Sphingosine 1-phosphate causes airway hyperreactivity by Rho-mediated myosin phosphatase inactivation. J Pharmacol Exp Ther. 2007;320:766–773. doi: 10.1124/jpet.106.110718. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha’afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- McVerry BJ, Garcia JG. Endothelial cell barrier regulation by sphingosine 1-phosphate. J Cell Biochem. 2004;92:1075–1085. doi: 10.1002/jcb.20088. [DOI] [PubMed] [Google Scholar]
- Melendez AJ, Ibrahim FB. Antisense knockdown of sphingosine kinase 1 in human macrophages inhibits c5a receptor-dependent signal transduction, Ca2+ signals, enzyme release, cytokine production, and chemotaxis. J Immunol. 2004;173:1596–1603. doi: 10.4049/jimmunol.173.3.1596. [DOI] [PubMed] [Google Scholar]
- Melendez AJ, Khaw AK. Dichotomy of Ca2+ signals triggered by different phospholipid pathways in antigen stimulation of human mast cells. J Biol Chem. 2002;277:17255–17262. doi: 10.1074/jbc.M110944200. [DOI] [PubMed] [Google Scholar]
- Michaud J, Kohno M, Proia RL, Hla T. Normal acute and chronic inflammatory responses in sphingosine kinase 1 knockout mice. FEBS Lett. 2006;580:4607–4612. doi: 10.1016/j.febslet.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci USA. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera A, Mizugishi K, Tikhonova A, Ciaccia L, Odom S, Proia RL, Rivera J. The sphingosine kinase-sphingosine-1-phosphate axis Is a determinant of mast cell function and anaphylaxis. Immunity. 2007 doi: 10.1016/j.immuni.2007.02.008. in press. [DOI] [PubMed] [Google Scholar]
- Olivera A, Rivera J. Sphingolipids and the balancing of immune cell function: lessons from the mast cell. J Immunol. 2005;174:1153–1158. doi: 10.4049/jimmunol.174.3.1153. [DOI] [PubMed] [Google Scholar]
- Olivera A, Rosenthal J, Spiegel S. Effect of acidic phospholipids on sphingosine kinase. J Cell Biochem. 1996;60:529–537. doi: 10.1002/(sici)1097-4644(19960315)60:4<529::aid-jcb9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Olivera A, Spiegel S. Sphingosine kinase: a mediator of vital cellular functions. Prostaglandins. 2001;64:123–134. doi: 10.1016/s0090-6980(01)00108-3. [DOI] [PubMed] [Google Scholar]
- Olivera A, Urtz N, Mizugishi K, Yamashita Y, Gilfillan AM, Furumoto Y, Gu H, Proia RL, Baumruker T, Rivera J. IgE-dependent activation of sphingosine kinases 1 and 2 and secretion of sphingosine 1-phosphate requires Fyn kinase and contributes to mast cell responses. J Biol Chem. 2006;281:2515–2525. doi: 10.1074/jbc.M508931200. [DOI] [PubMed] [Google Scholar]
- Oskeritzian CA, Zhao W, Min HK, Xia HZ, Pozez A, Kiev J, Schwartz LB. Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J Allergy Clin Immunol. 2005;115:1162–1168. doi: 10.1016/j.jaci.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007 doi: 10.1126/science.1139221. in press. [DOI] [PubMed] [Google Scholar]
- Payne SG, Oskeritzian CA, Griffiths R, Subramanian P, Barbour SE, Chalfant CE, Milstien S, Spiegel S. The immunosuppressant drug FTY720 inhibits cytosolic phospholipase A2 independently of sphingosine-1-phosphate receptors. Blood. 2007;109:1077–1085. doi: 10.1182/blood-2006-03-011437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettus BJ, Bielawski J, Porcelli AM, Reames DL, Johnson KR, Morrow J, Chalfant CE, Obeid LM, Hannun YA. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. FASEB J. 2003;17:1411–1421. doi: 10.1096/fj.02-1038com. [DOI] [PubMed] [Google Scholar]
- Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nature Rev Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- Rosen H, Sanna MG, Cahalan SM, Gonzalez-Cabrera PJ. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends Immunol. 2007;28:102–107. doi: 10.1016/j.it.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Roviezzo F, Del Galdo F, Abbate G, Bucci M, D’Agostino B, Antunes E, De Dominicis G, Parente L, Rossi F, Cirino G, De Palma R. Human eosinophil chemotaxis and selective in vivo recruitment by sphingosine 1-phosphate. Proc Natl Acad Sci USA. 2004;101:11170–11175. doi: 10.1073/pnas.0401439101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, Wei SH, Parker I, Jo E, Cheng WC, Cahalan MD, Wong CH, Rosen H. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P(1) antagonist in vivo. Nature Chem Biol. 2006;2:434–441. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- Sawicka E, Zuany-Amorim C, Manlius C, Trifilieff A, Brinkmann V, Kemeny DM, Walker C. Inhibition of Th1- and th2-mediated airway inflammation by the sphingosine 1-phosphate receptor agonist FTY720. J Immunol. 2003;171:6206–6214. doi: 10.4049/jimmunol.171.11.6206. [DOI] [PubMed] [Google Scholar]
- Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Functions of a new family of sphingosine-1-phosphate receptors. Biochim Biophys Acta. 2000;1484:107–116. doi: 10.1016/s1388-1981(00)00010-x. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nature Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Sutherland CM, Moretti PA, Hewitt NM, Bagley CJ, Vadas MA, Pitson SM. The calmodulin-binding site of sphingosine kinase and its role in agonist-dependent translocation of sphingosine kinase 1 to the plasma membrane. J Biol Chem. 2006;281:11693–11701. doi: 10.1074/jbc.M601042200. [DOI] [PubMed] [Google Scholar]
- Taha TA, Hannun YA, Obeid LM. Sphingosine kinase: biochemical and cellular regulation and role in disease. J Biochem Mol Biol. 2006;39:113–131. doi: 10.5483/bmbrep.2006.39.2.113. [DOI] [PubMed] [Google Scholar]
- Urtz N, Olivera A, Bofill-Cardona E, Csonga R, Billich A, Mechtcheriakova D, Bornancin F, Woisetschlager M, Rivera J, Baumruker T. Early activation of sphingosine kinase in mast cells and recruitment to FcepsilonRI are mediated by its interaction with Lyn kinase. Mol Cell Biol. 2004;24:8765–8777. doi: 10.1128/MCB.24.19.8765-8777.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G, Holthuis JC. Sphingolipid transport in eukaryotic cells. Biochim Biophys Acta. 2000;1486:145–170. doi: 10.1016/s1388-1981(00)00054-8. [DOI] [PubMed] [Google Scholar]
- Visentin B, Vekich JA, Sibbald BJ, Cavalli AL, Moreno KM, Matteo RG, Garland WA, Lu Y, Yu S, Hall HS, Kundra V, Mills GB, Sabbadini RA. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–238. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Wang F, Van Brocklyn JR, Hobson JP, Movafagh S, Zukowska-Grojec Z, Milstien S, Spiegel S. Sphingosine 1-phosphate stimulates cell migration through a G(i)- coupled cell surface receptor. Potential involvement in angiogenesis. J Biol Chem. 1999;274:35343–35350. doi: 10.1074/jbc.274.50.35343. [DOI] [PubMed] [Google Scholar]
- Watterson KR, Ratz PH, Spiegel S. The role of sphingosine-1-phosphate in smooth muscle contraction. Cell Signal. 2005;17:289–298. doi: 10.1016/j.cellsig.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Wei SH, Rosen H, Matheu MP, Sanna MG, Wang SK, Jo E, Wong CH, Parker I, Cahalan MD. Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nature Immunol. 2005;6:1215–1216. doi: 10.1038/ni1269. [DOI] [PubMed] [Google Scholar]
- Wenzel S, Holgate ST. The mouse trap: It still yields few answers in asthma. Am J Respir Crit Care Med. 2006;174:1173–1176. doi: 10.1164/rccm.2609002. [DOI] [PubMed] [Google Scholar]
- Xia P, Gamble JR, Rye KA, Wang L, Hii CST, Cockerill P, Khew-Goodall Y, Bert AG, Barter PJ, Vadas MA. Tumor necrosis factor-a induces adhesion molecule expression through the sphingosine kinase pathway. Proc Natl Acad Sci USA. 1998;95:14196–14201. doi: 10.1073/pnas.95.24.14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P, Wang L, Moretti PA, Albanese N, Chai F, Pitson SM, D’Andrea RJ, Gamble JR, Vadas MA. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-alpha signaling. J Biol Chem. 2002;277:7996–8003. doi: 10.1074/jbc.M111423200. [DOI] [PubMed] [Google Scholar]
- Yatomi Y, Igarashi Y, Yang L, Hisano N, Qi R, Asazuma N, Satoh K, Ozaki Y, Kume S. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J Biochem. 1997;121:969–973. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- Yatomi Y, Ozaki Y, Ohmori T, Igarashi Y. Sphingosine 1-phosphate: synthesis and release. Prostaglandins Other Lipid Mediat. 2001;64:107–122. doi: 10.1016/s0090-6980(01)00103-4. [DOI] [PubMed] [Google Scholar]
- Yu M, Tsai M, Tam SY, Jones C, Zehnder J, Galli SJ. Mast cells can promote the development of multiple features of chronic asthma in mice. J Clin Invest. 2006;116:1633–1641. doi: 10.1172/JCI25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemann B, Kinzel B, Muller M, Reuschel R, Mechtcheriakova D, Urtz N, Bornancin F, Baumruker T, Billich A. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood. 2006;107:1454–1458. doi: 10.1182/blood-2005-07-2628. [DOI] [PubMed] [Google Scholar]