Abstract
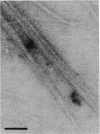
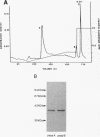
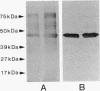
Porphyromonas (Bacteroides) gingivalis adheres to gram-positive bacteria, such as Actinomyces viscosus, when colonizing the tooth surface. However, little is known of the adhesins responsible for this interaction. A series of experiments were performed to determine whether P. gingivalis fimbriae function in its coadhesion with A. viscosus. Fimbriae typical of P. gingivalis were isolated from strain 2561 (ATCC 33277) by the method of Yoshimura et al. (F. Yoshimura, K. Takahashi, Y. Nodasaka, and T. Suzuki, J. Bacteriol. 160:949-957, 1984) in fractions enriched with a 40-kDa subunit, the fimbrillin monomer, P. gingivalis-A. viscosus coaggregation was inhibited by purified rabbit antifimbrial immunoglobulin G (IgG) at dilutions eightfold higher than those of preimmune IgG, providing indirect evidence implicating P. gingivalis fimbriae in coadhesion. Three types of direct binding assays further supported this observation. (i) Mixtures of isolated P. gingivalis fimbriae and A. viscosus WVU627 cells were incubated for 1 h, washed vigorously with phosphate-buffered saline (pH 7.2), and subjected to electrophoresis. Transblots onto nitrocellulose were probed with antifimbrial antiserum. Fimbrillin labeled positively on these blots. No reaction occurred with the control protein, porcine serum albumin, when blots were exposed to anti-porcine serum albumin, (ii) A. viscosus cells incubated with P. gingivalis fimbriae were agglutinated only after the addition of antifimbrial antibodies. (iii) Binding curves generated from an enzyme immunoassay demonstrated concentration-dependent binding of P. gingivalis fimbriae to A. viscosus cells. From these lines of evidence, P. gingivalis fimbriae appear to be capable of binding to A. viscosus and mediating the coadhesion of these species.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourgeau G., Mayrand D. Aggregation of Actinomyces strains by extracellular vesicles produced by Bacteroides gingivalis. Can J Microbiol. 1990 May;36(5):362–365. doi: 10.1139/m90-063. [DOI] [PubMed] [Google Scholar]
- Boyd J., McBride B. C. Fractionation of hemagglutinating and bacterial binding adhesins of Bacteroides gingivalis. Infect Immun. 1984 Aug;45(2):403–409. doi: 10.1128/iai.45.2.403-409.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D. P., Kubiniec M. A., Yoshimura F., Genco R. J. Molecular cloning and sequencing of the gene encoding the fimbrial subunit protein of Bacteroides gingivalis. J Bacteriol. 1988 Apr;170(4):1658–1665. doi: 10.1128/jb.170.4.1658-1665.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Buivids I. A., Simardone J. R. Actinomyces viscosus fibril antigens detected by immunogold electron microscopy. Infect Immun. 1989 Apr;57(4):1327–1331. doi: 10.1128/iai.57.4.1327-1331.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Grove D. A. Bacteroides gingivalis vesicles bind to and aggregate Actinomyces viscosus. Infect Immun. 1989 May;57(5):1618–1620. doi: 10.1128/iai.57.5.1618-1620.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Schwarz-Faulkner S., Grove D. A. Coaggregation among periodontal pathogens, emphasizing Bacteroides gingivalis--Actinomyces viscosus cohesion on a saliva-coated mineral surface. Can J Microbiol. 1988 Mar;34(3):299–306. doi: 10.1139/m88-055. [DOI] [PubMed] [Google Scholar]
- GIBBONS R. J., MACDONALD J. B. Hemin and vitamin K compounds as required factors for the cultivation of certain strains of Bacteroides melaninogenicus. J Bacteriol. 1960 Aug;80:164–170. doi: 10.1128/jb.80.2.164-170.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier D., Mayrand D. Functional characterization of extracellular vesicles produced by Bacteroides gingivalis. Infect Immun. 1987 Jan;55(1):111–117. doi: 10.1128/iai.55.1.111-117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley P. S., Tipler L. S. An electron microscope survey of the surface structures and hydrophobicity of oral and non-oral species of the bacterial genus Bacteroides. Arch Oral Biol. 1986;31(5):325–335. doi: 10.1016/0003-9969(86)90047-6. [DOI] [PubMed] [Google Scholar]
- Isogai H., Isogai E., Yoshimura F., Suzuki T., Kagota W., Takano K. Specific inhibition of adherence of an oral strain of Bacteroides gingivalis 381 to epithelial cells by monoclonal antibodies against the bacterial fimbriae. Arch Oral Biol. 1988;33(7):479–485. doi: 10.1016/0003-9969(88)90028-3. [DOI] [PubMed] [Google Scholar]
- Kinder S. A., Holt S. C. Characterization of coaggregation between Bacteroides gingivalis T22 and Fusobacterium nucleatum T18. Infect Immun. 1989 Nov;57(11):3425–3433. doi: 10.1128/iai.57.11.3425-3433.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee J. Y., Sojar H. T., Bedi G. S., Genco R. J. Porphyromonas (Bacteroides) gingivalis fimbrillin: size, amino-terminal sequence, and antigenic heterogeneity. Infect Immun. 1991 Jan;59(1):383–389. doi: 10.1128/iai.59.1.383-389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Ellen R. P., Hoover C. I., Felton J. R. Association of proteases of Porphyromonas (Bacteroides) gingivalis with its adhesion to Actinomyces viscosus. J Dent Res. 1991 Feb;70(2):82–86. doi: 10.1177/00220345910700021501. [DOI] [PubMed] [Google Scholar]
- Li J., Ellen R. P. Relative adherence of Bacteroides species and strains to Actinomyces viscosus on saliva-coated hydroxyapatite. J Dent Res. 1989 Sep;68(9):1308–1312. doi: 10.1177/00220345890680090301. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Buivids I. A., Ellen R. P. Adhesion of Actinomyces viscosus to Porphyromonas (Bacteroides) gingivalis-coated hexadecane droplets. J Bacteriol. 1991 Apr;173(8):2581–2589. doi: 10.1128/jb.173.8.2581-2589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Hanley J., Clubb L., Fanning S. Detection of pilus subunits (pilins) and filaments by using anti-P pilin antisera. Infect Immun. 1988 Sep;56(9):2330–2335. doi: 10.1128/iai.56.9.2330-2335.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S., Ellen R. P., Grove D. A. Bacteroides gingivalis-Actinomyces viscosus cohesive interactions as measured by a quantitative binding assay. Infect Immun. 1987 Oct;55(10):2391–2397. doi: 10.1128/iai.55.10.2391-2397.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh U., Grenier D., McBride B. C. Bacteroides gingivalis vesicles mediate attachment of streptococci to serum-coated hydroxyapatite. Oral Microbiol Immunol. 1989 Dec;4(4):199–203. doi: 10.1111/j.1399-302x.1989.tb00252.x. [DOI] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempro P., Cassels F., Siraganian R., Hand A. R., London J. Use of adhesin-specific monoclonal antibodies to identify and localize an adhesin on the surface of Capnocytophaga gingivalis DR2001. Infect Immun. 1989 Nov;57(11):3418–3424. doi: 10.1128/iai.57.11.3418-3424.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Weiss E. I., Kolenbrander P. E., London J., Hand A. R., Andersen R. N. Fimbria-associated proteins of Bacteroides loescheii PK1295 mediate intergeneric coaggregations. J Bacteriol. 1987 Sep;169(9):4215–4222. doi: 10.1128/jb.169.9.4215-4222.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. I., London J., Kolenbrander P. E., Andersen R. N., Fischler C., Siraganian R. P. Characterization of monoclonal antibodies to fimbria-associated adhesins of Bacteroides loescheii PK1295. Infect Immun. 1988 Jan;56(1):219–224. doi: 10.1128/iai.56.1.219-224.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Takahashi K., Nodasaka Y., Suzuki T. Purification and characterization of a novel type of fimbriae from the oral anaerobe Bacteroides gingivalis. J Bacteriol. 1984 Dec;160(3):949–957. doi: 10.1128/jb.160.3.949-957.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Takasawa T., Yoneyama M., Yamaguchi T., Shiokawa H., Suzuki T. Fimbriae from the oral anaerobe Bacteroides gingivalis: physical, chemical, and immunological properties. J Bacteriol. 1985 Aug;163(2):730–734. doi: 10.1128/jb.163.2.730-734.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Watanabe K., Takasawa T., Kawanami M., Kato H. Purification and properties of a 75-kilodalton major protein, an immunodominant surface antigen, from the oral anaerobe Bacteroides gingivalis. Infect Immun. 1989 Nov;57(11):3646–3652. doi: 10.1128/iai.57.11.3646-3652.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]