Abstract
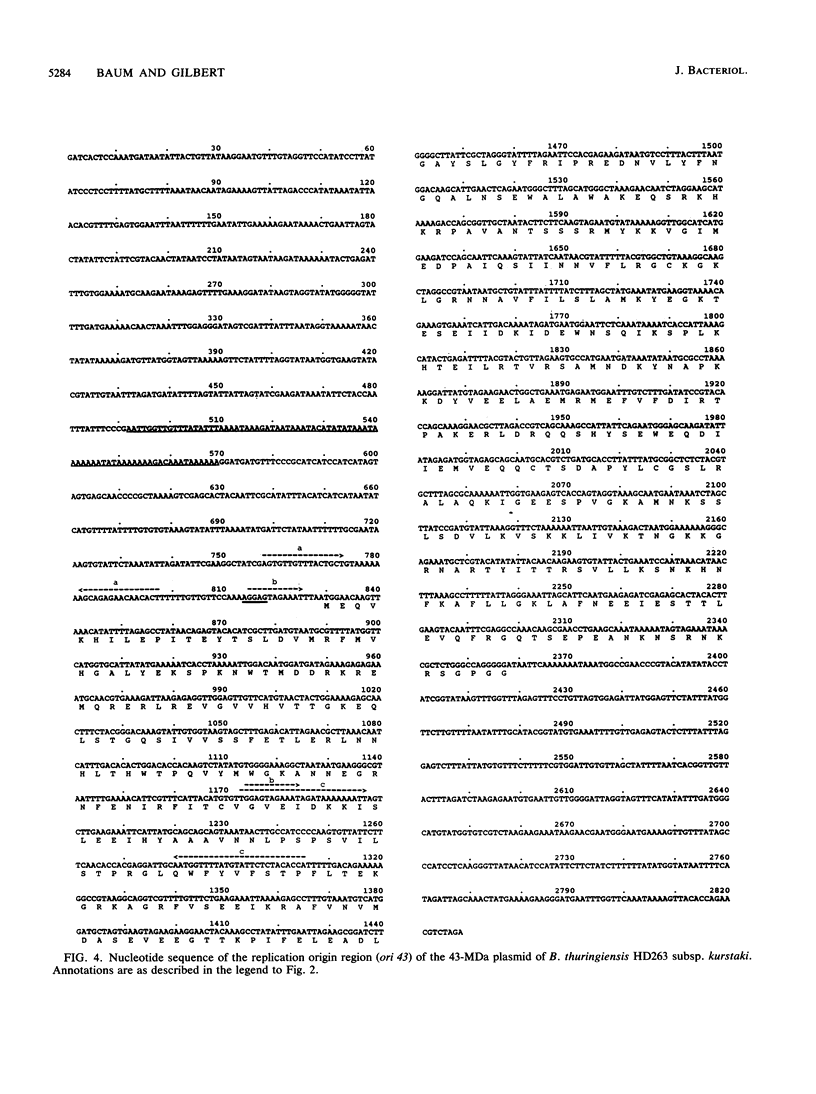
The replication origins of three large Bacillus thuringiensis plasmids, derived from B. thuringiensis HD263 subsp. kurstaki, have been cloned in Escherichia coli and sequenced. The replication origins, designated ori 43, ori 44, and ori 60, were isolated from plasmids of 43, 44, and 60 MDa, respectively. Each cloned replication origin exhibits incompatibility with the resident B. thuringiensis plasmid from which it was derived. Recombinant plasmids containing the three replication origins varied in their ability to transform strains of B. thuringiensis, Bacillus megaterium, and Bacillus subtilis. Analysis of the derived nucleotide and amino acid sequences indicates that the replication origins are nonhomologous, implying independent derivations. No significant homology was found to published sequences of replication origins derived from the single-stranded DNA plasmids of gram-positive bacteria, and shuttle vectors containing the three replication origins do not appear to generate single-stranded DNA intermediates in B. thuringiensis. The replication origin regions of the large plasmids are each characterized by a single open reading frame whose product is essential for replication in B. thuringiensis. The putative replication protein of ori 60 exhibits partial homology to the RepA protein of the Bacillus stearothermophilus plasmid pTB19. The putative replication protein of ori 43 exhibits weak but extensive homology to the replication proteins of several streptococcal plasmids, including the open reading frame E replication protein of the conjugative plasmid pAM beta 1. The nucleotide sequence of ori 44 and the amino acid sequence of its putative replication protein appear to be nonhomologous to other published replication origin sequences.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum J. A., Coyle D. M., Gilbert M. P., Jany C. S., Gawron-Burke C. Novel cloning vectors for Bacillus thuringiensis. Appl Environ Microbiol. 1990 Nov;56(11):3420–3428. doi: 10.1128/aem.56.11.3420-3428.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone E. J., Ellar D. J. Transformation of Bacillus thuringiensis by electroporation. FEMS Microbiol Lett. 1989 Apr;49(2-3):171–177. doi: 10.1016/0378-1097(89)90033-5. [DOI] [PubMed] [Google Scholar]
- Brantl S., Behnke D., Alonso J. C. Molecular analysis of the replication region of the conjugative Streptococcus agalactiae plasmid pIP501 in Bacillus subtilis. Comparison with plasmids pAM beta 1 and pSM19035. Nucleic Acids Res. 1990 Aug 25;18(16):4783–4790. doi: 10.1093/nar/18.16.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantl S., Nowak A., Behnke D., Alonso J. C. Revision of the nucleotide sequence of the Streptococcus pyogenes plasmid pSM19035 repS gene. Nucleic Acids Res. 1989 Dec 11;17(23):10110–10110. doi: 10.1093/nar/17.23.10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Dunny G. M., Schultz S. K. Characterization of three plasmid deoxyribonucleic acid molecules in a strain of Streptococcus faecalis: identification of a plasmid determining erythromycin resistance. J Bacteriol. 1974 Jan;117(1):283–289. doi: 10.1128/jb.117.1.283-289.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan W. P., Gonzalez J. M., Jr, Gilbert M. P., Dankocsik C. Isolation and characterization of EG2158, a new strain of Bacillus thuringiensis toxic to coleopteran larvae, and nucleotide sequence of the toxin gene. Mol Gen Genet. 1988 Nov;214(3):365–372. doi: 10.1007/BF00330468. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R., Scher B., Cirigliano C. Fate of transforming deoxyribonucleic acid after uptake by competent Bacillus subtilis: phenotypic characterization of radiation-sensitive recombination-deficient mutants. J Bacteriol. 1973 Apr;114(1):273–286. doi: 10.1128/jb.114.1.273-286.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M. Q., Yoshikawa H., Ogasawara N. Structure of the dnaA region of Pseudomonas putida: conservation among three bacteria, Bacillus subtilis, Escherichia coli and P. putida. Mol Gen Genet. 1989 Feb;215(3):381–387. doi: 10.1007/BF00427033. [DOI] [PubMed] [Google Scholar]
- González J. M., Jr, Dulmage H. T., Carlton B. C. Correlation between specific plasmids and delta-endotoxin production in Bacillus thuringiensis. Plasmid. 1981 May;5(3):352–365. doi: 10.1016/0147-619x(81)90010-x. [DOI] [PubMed] [Google Scholar]
- Gruss A., Ehrlich S. D. The family of highly interrelated single-stranded deoxyribonucleic acid plasmids. Microbiol Rev. 1989 Jun;53(2):231–241. doi: 10.1128/mr.53.2.231-241.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T. J., Contente S., Dubnau D. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol. 1978 Apr;134(1):318–329. doi: 10.1128/jb.134.1.318-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfte H., Whiteley H. R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989 Jun;53(2):242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanaka T., Ishikawa H., Aiba S. Complete nucleotide sequence of the low copy number plasmid pRAT11 and replication control by the RepA protein in Bacillus subtilis. Mol Gen Genet. 1986 Oct;205(1):90–96. doi: 10.1007/BF02428036. [DOI] [PubMed] [Google Scholar]
- Kronstad J. W., Schnepf H. E., Whiteley H. R. Diversity of locations for Bacillus thuringiensis crystal protein genes. J Bacteriol. 1983 Apr;154(1):419–428. doi: 10.1128/jb.154.1.419-428.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kües U., Stahl U. Replication of plasmids in gram-negative bacteria. Microbiol Rev. 1989 Dec;53(4):491–516. doi: 10.1128/mr.53.4.491-516.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lereclus D., Arantès O., Chaufaux J., Lecadet M. Transformation and expression of a cloned delta-endotoxin gene in Bacillus thuringiensis. FEMS Microbiol Lett. 1989 Jul 15;51(1):211–217. doi: 10.1016/0378-1097(89)90511-9. [DOI] [PubMed] [Google Scholar]
- Macaluso A., Mettus A. M. Efficient transformation of Bacillus thuringiensis requires nonmethylated plasmid DNA. J Bacteriol. 1991 Feb;173(3):1353–1356. doi: 10.1128/jb.173.3.1353-1356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson L., Préfontaine G., Brousseau R. Transformation of Bacillus thuringiensis vegetative cells by electroporation. FEMS Microbiol Lett. 1989 Aug;51(3):273–277. doi: 10.1016/0378-1097(89)90409-6. [DOI] [PubMed] [Google Scholar]
- Mettus A. M., Macaluso A. Expression of Bacillus thuringiensis delta-endotoxin genes during vegetative growth. Appl Environ Microbiol. 1990 Apr;56(4):1128–1134. doi: 10.1128/aem.56.4.1128-1134.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Iordanescu S., Projan S. J., Kornblum J., Edelman I. pT181 plasmid replication is regulated by a countertranscript-driven transcriptional attenuator. Cell. 1989 Oct 20;59(2):395–404. doi: 10.1016/0092-8674(89)90300-0. [DOI] [PubMed] [Google Scholar]
- Novick R. P. Staphylococcal plasmids and their replication. Annu Rev Microbiol. 1989;43:537–565. doi: 10.1146/annurev.mi.43.100189.002541. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurter W., Geiser M., Mathé D. Efficient transformation of Bacillus thuringiensis and B. cereus via electroporation: transformation of acrystalliferous strains with a cloned delta-endotoxin gene. Mol Gen Genet. 1989 Jul;218(1):177–181. doi: 10.1007/BF00330581. [DOI] [PubMed] [Google Scholar]
- Swinfield T. J., Oultram J. D., Thompson D. E., Brehm J. K., Minton N. P. Physical characterisation of the replication region of the Streptococcus faecalis plasmid pAM beta 1. Gene. 1990 Mar 1;87(1):79–90. [PubMed] [Google Scholar]
- Von Tersch M. A., Robbins H. L. Efficient cloning in Bacillus megaterium: comparison to Bacillus subtilis and Escherichia coli cloning hosts. FEMS Microbiol Lett. 1990 Aug;58(3):305–309. doi: 10.1111/j.1574-6968.1990.tb13994.x. [DOI] [PubMed] [Google Scholar]
- Von Tersch M. A., Robbins H. L., Jany C. S., Johnson T. B. Insecticidal toxins from Bacillus thuringiensis subsp. kenyae: gene cloning and characterization and comparison with B. thuringiensis subsp. kurstaki CryIA(c) toxins. Appl Environ Microbiol. 1991 Feb;57(2):349–358. doi: 10.1128/aem.57.2.349-358.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]