Abstract
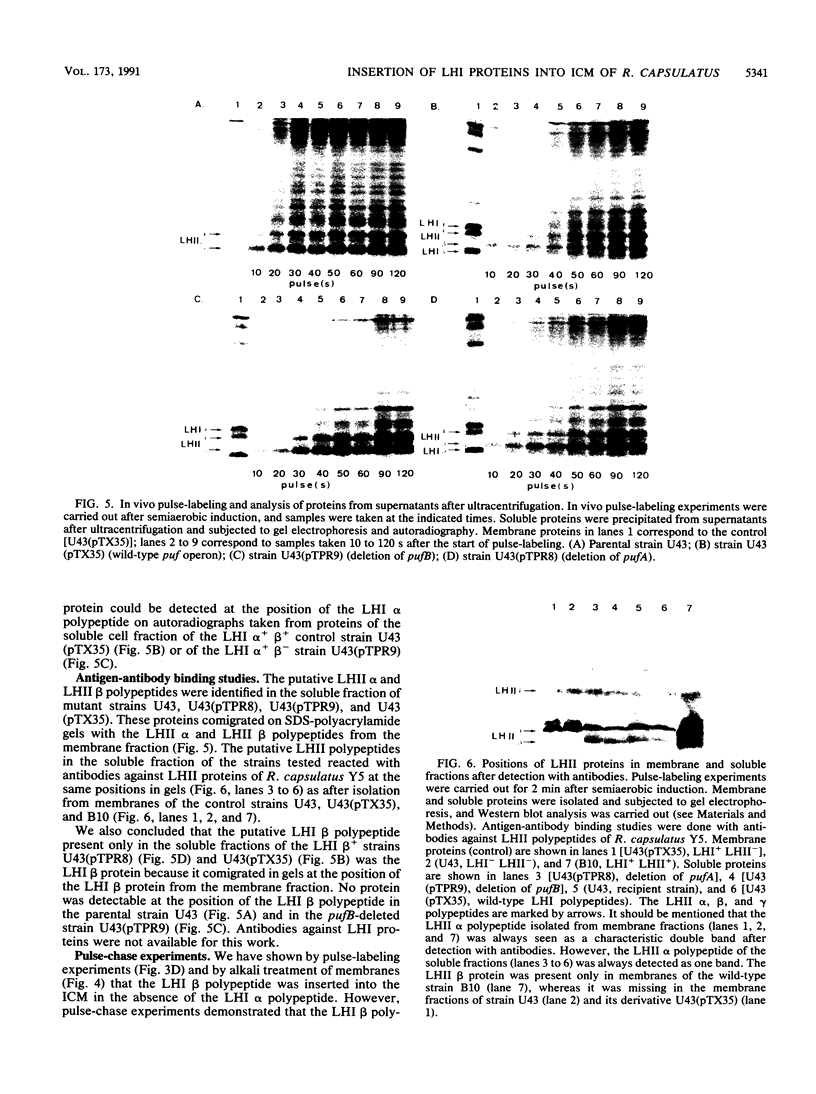
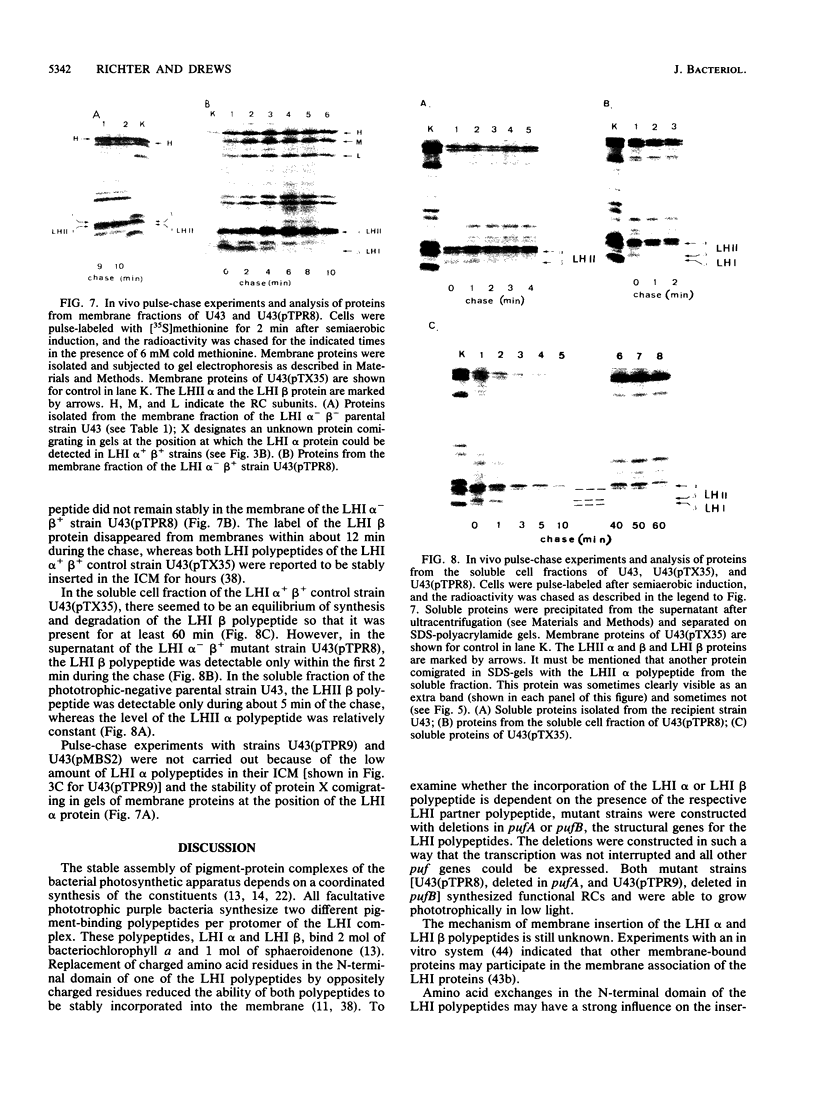
The light-harvesting complex I (LHI) of Rhodobacter capsulatus is an oligomer of basic subunits each consisting of the two different pigment-binding polypeptides LHI alpha and LHI beta, encoded by the pufA (LHI alpha) and pufB (LHI beta) genes. Pulse-labeling experiments showed that in the presence of the LHI alpha polypeptide, the LHI beta polypeptide was inserted earlier into the intracytoplasmic membrane than was the LHI alpha polypeptide. Each of the pufA and pufB genes was deleted to test whether the LHI alpha and beta polypeptides, respectively, are inserted into the intracytoplasmic membrane independently of the LHI partner polypeptide. Neither deletion mutant strain formed the LHI antenna, but a functional reaction center complex was present. Pulse-labeling experiments indicated that the LHI beta polypeptide was inserted into the intracytoplasmic membrane with the same kinetics and in the same amounts regardless of whether the LHI alpha polypeptide was present. However, the LHI beta polypeptide did not accumulate in the membrane in the absence of the LHI alpha protein but was degraded linearly within about 12 min. In contrast to the LHI beta protein, only trace amounts of the LHI alpha polypeptide were inserted into or attached to the membrane if the LHI beta polypeptide was not synthesized.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer C. E., Young D. A., Marrs B. L. Analysis of the Rhodobacter capsulatus puf operon. Location of the oxygen-regulated promoter region and the identification of an additional puf-encoded gene. J Biol Chem. 1988 Apr 5;263(10):4820–4827. [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Bowyer J. R., Hunter C. N., Ohnishi T., Niederman R. A. Photosynthetic membrane development in Rhodopseudomonas sphaeroides. Spectral and kinetic characterization of redox components of light-driven electron flow in apparent photosynthetic membrane growth initiation sites. J Biol Chem. 1985 Mar 25;260(6):3295–3304. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Clark W. G., Davidson E., Marrs B. L. Variation of levels of mRNA coding for antenna and reaction center polypeptides in Rhodopseudomonas capsulata in response to changes in oxygen concentration. J Bacteriol. 1984 Mar;157(3):945–948. doi: 10.1128/jb.157.3.945-948.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierstein R. Synthesis of pigment-binding protein in toluene-treated Rhodopseudomonas capsulata and in cell-free systems. Eur J Biochem. 1984 Feb 1;138(3):509–518. doi: 10.1111/j.1432-1033.1984.tb07945.x. [DOI] [PubMed] [Google Scholar]
- Drews G. Structure and functional organization of light-harvesting complexes and photochemical reaction centers in membranes of phototrophic bacteria. Microbiol Rev. 1985 Mar;49(1):59–70. doi: 10.1128/mr.49.1.59-70.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörge B., Klug G., Gad'on N., Cohen S. N., Drews G. Effects on the formation of antenna complex B870 of Rhodobacter capsulatus by exchange of charged amino acids in the N-terminal domain of the alpha and beta pigment-binding proteins. Biochemistry. 1990 Aug 21;29(33):7754–7758. doi: 10.1021/bi00485a026. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl F. U., Lecker S., Schiebel E., Hendrick J. P., Wickner W. The binding cascade of SecB to SecA to SecY/E mediates preprotein targeting to the E. coli plasma membrane. Cell. 1990 Oct 19;63(2):269–279. doi: 10.1016/0092-8674(90)90160-g. [DOI] [PubMed] [Google Scholar]
- Jackson W. J., Kiley P. J., Haith C. E., Kaplan S., Prince R. C. On the role of the light-harvesting B880 in the correct insertion of the reaction center of Rhodobacter capsulatus and Rhodobacter sphaeroides. FEBS Lett. 1987 May 4;215(1):171–174. doi: 10.1016/0014-5793(87)80135-7. [DOI] [PubMed] [Google Scholar]
- Kiley P. J., Kaplan S. Molecular genetics of photosynthetic membrane biosynthesis in Rhodobacter sphaeroides. Microbiol Rev. 1988 Mar;52(1):50–69. doi: 10.1128/mr.52.1.50-69.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug G., Cohen S. N. Effects of translation on degradation of mRNA segments transcribed from the polycistronic puf operon of Rhodobacter capsulatus. J Bacteriol. 1991 Feb;173(4):1478–1484. doi: 10.1128/jb.173.4.1478-1484.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug G., Cohen S. N. Pleiotropic effects of localized Rhodobacter capsulatus puf operon deletions on production of light-absorbing pigment-protein complexes. J Bacteriol. 1988 Dec;170(12):5814–5821. doi: 10.1128/jb.170.12.5814-5821.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug G., Drews G. Construction of a gene bank of Rhodopseudomonas capsulata using a broad host range DNA cloning system. Arch Microbiol. 1984 Nov;139(4):319–325. doi: 10.1007/BF00408373. [DOI] [PubMed] [Google Scholar]
- Kuhn A., Zhu H. Y., Dalbey R. E. Efficient translocation of positively charged residues of M13 procoat protein across the membrane excludes electrophoresis as the primary force for membrane insertion. EMBO J. 1990 Aug;9(8):2385–2389. doi: 10.1002/j.1460-2075.1990.tb07413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Michel H., Epp O., Deisenhofer J. Pigment-protein interactions in the photosynthetic reaction centre from Rhodopseudomonas viridis. EMBO J. 1986 Oct;5(10):2445–2451. doi: 10.1002/j.1460-2075.1986.tb04520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennoyer J. D., Kramer H. J., van Grondelle R., Westerhuis W. H., Amesz J., Niederman R. A. Excitation energy transfer in Rhodopseudomonas sphaeroides chromatophore membranes fused with liposomes. FEBS Lett. 1985 Mar 11;182(1):145–150. doi: 10.1016/0014-5793(85)81172-8. [DOI] [PubMed] [Google Scholar]
- Richter P., Cortez N., Drews G. Possible role of the highly conserved amino acids Trp-8 and Pro-13 in the N-terminal segment of the pigment-binding polypeptide LHI alpha of Rhodobacter capsulatus. FEBS Lett. 1991 Jul 8;285(1):80–84. doi: 10.1016/0014-5793(91)80729-m. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Werner P. K., Müller M. Insertion of proteins into bacterial membranes: mechanism, characteristics, and comparisons with the eucaryotic process. Microbiol Rev. 1989 Sep;53(3):333–366. doi: 10.1128/mr.53.3.333-366.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidhauser T. J., Helinski D. R. Regions of broad-host-range plasmid RK2 involved in replication and stable maintenance in nine species of gram-negative bacteria. J Bacteriol. 1985 Oct;164(1):446–455. doi: 10.1128/jb.164.1.446-455.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J. The structure and insertion of integral proteins in membranes. Annu Rev Cell Biol. 1990;6:247–296. doi: 10.1146/annurev.cb.06.110190.001335. [DOI] [PubMed] [Google Scholar]
- Sockett R. E., Donohue T. J., Varga A. R., Kaplan S. Control of photosynthetic membrane assembly in Rhodobacter sphaeroides mediated by puhA and flanking sequences. J Bacteriol. 1989 Jan;171(1):436–446. doi: 10.1128/jb.171.1.436-446.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiehle H., Cortez N., Klug G., Drews G. A negatively charged N terminus in the alpha polypeptide inhibits formation of light-harvesting complex I in Rhodobacter capsulatus. J Bacteriol. 1990 Dec;172(12):7131–7137. doi: 10.1128/jb.172.12.7131-7137.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadros M. H., Suter F., Seydewitz H. H., Witt I., Zuber H., Drews G. Isolation and complete amino-acid sequence of the small polypeptide from light-harvesting pigment-protein complex I (B870) of Rhodopseudomonas capsulata. Eur J Biochem. 1984 Jan 2;138(1):209–212. doi: 10.1111/j.1432-1033.1984.tb07902.x. [DOI] [PubMed] [Google Scholar]
- Tai S. P., Kaplan S. Intracellular localization of phospholipid transfer activity in Rhodopseudomonas sphaeroides and a possible role in membrane biogenesis. J Bacteriol. 1985 Oct;164(1):181–186. doi: 10.1128/jb.164.1.181-186.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troschel D., Müller M. Development of a cell-free system to study the membrane assembly of photosynthetic proteins of Rhodobacter capsulatus. J Cell Biol. 1990 Jul;111(1):87–94. doi: 10.1083/jcb.111.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yeates T. O., Komiya H., Rees D. C., Allen J. P., Feher G. Structure of the reaction center from Rhodobacter sphaeroides R-26: membrane-protein interactions. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6438–6442. doi: 10.1073/pnas.84.18.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youvan D. C., Bylina E. J., Alberti M., Begusch H., Hearst J. E. Nucleotide and deduced polypeptide sequences of the photosynthetic reaction-center, B870 antenna, and flanking polypeptides from R. capsulata. Cell. 1984 Jul;37(3):949–957. doi: 10.1016/0092-8674(84)90429-x. [DOI] [PubMed] [Google Scholar]
- Youvan D. C., Ismail S., Bylina E. J. Chromosomal deletion and plasmid complementation of the photosynthetic reaction center and light-harvesting genes from Rhodopseudomonas capsulata. Gene. 1985;38(1-3):19–30. doi: 10.1016/0378-1119(85)90199-4. [DOI] [PubMed] [Google Scholar]
- Zhu Y. S., Hearst J. E. Regulation of expression of genes for light-harvesting antenna proteins LH-I and LH-II; reaction center polypeptides RC-L, RC-M, and RC-H; and enzymes of bacteriochlorophyll and carotenoid biosynthesis in Rhodobacter capsulatus by light and oxygen. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7613–7617. doi: 10.1073/pnas.83.20.7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Transcending the impenetrable: how proteins come to terms with membranes. Biochim Biophys Acta. 1988 Jun 9;947(2):307–333. doi: 10.1016/0304-4157(88)90013-5. [DOI] [PubMed] [Google Scholar]