Abstract
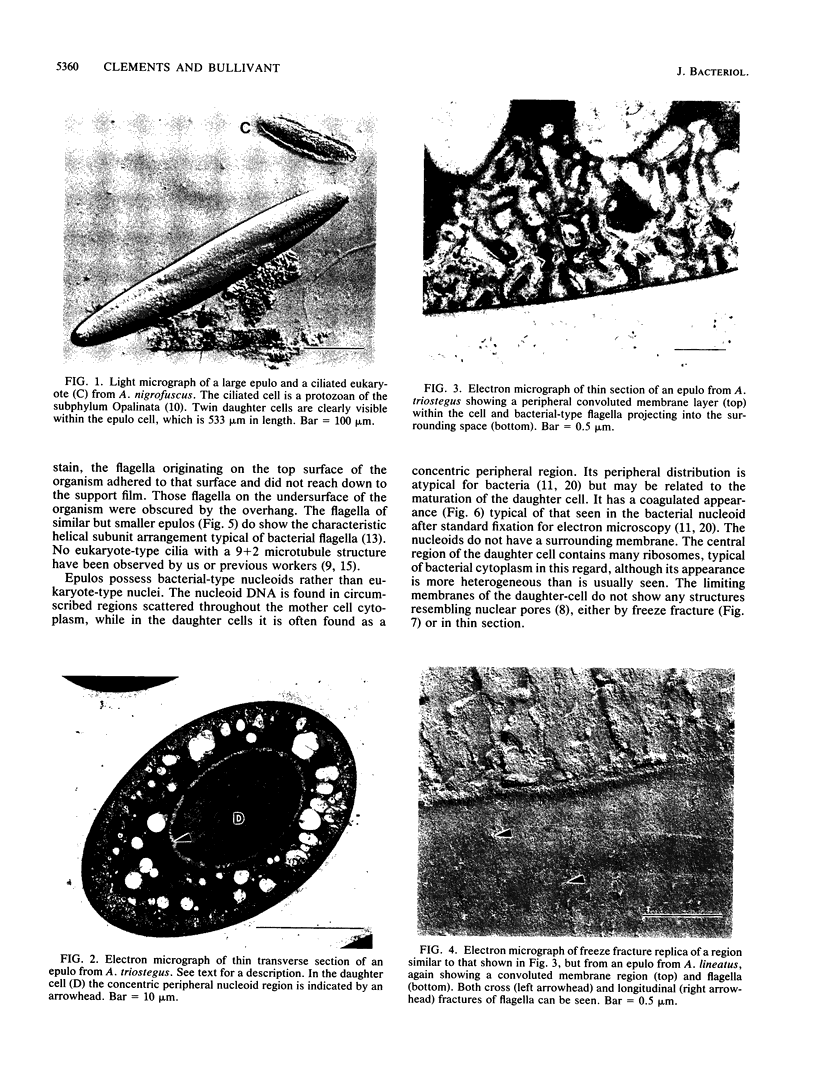
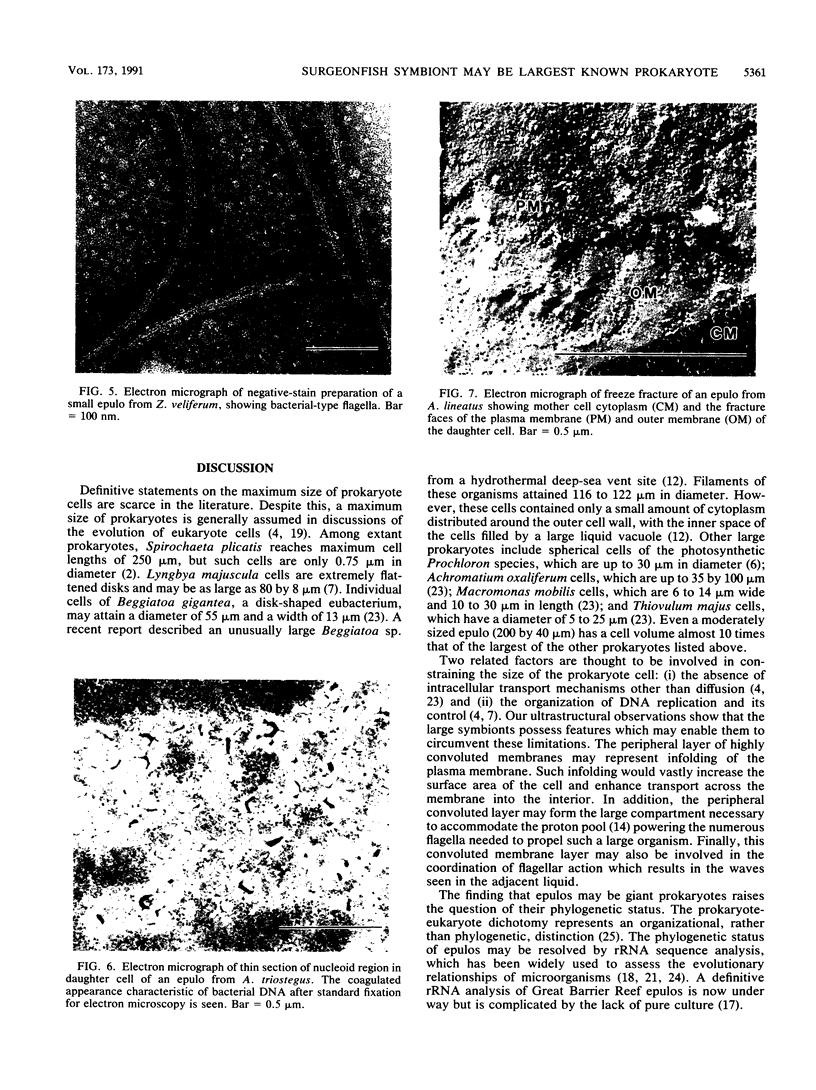
Symbionts first reported from the gut of a Red Sea surgeonfish, Acanthurus nigrofuscus (family Acanthuridae), were subsequently described as Epulopiscium fishelsoni. The taxonomic position of this very large (up to 576 microns in length) microorganism has previously been designated in the literature as either uncertain or eukaryotic. We suggest that similar symbionts from Great Barrier Reef surgeonfish may be prokaryotes, which together with E. fishelsoni from the Red Sea may represent the largest known forms of this cell type. Features identifying the symbionts as prokaryotes include the presence of bacterial-type flagella and a bacterial nucleoid and the absence of a nucleus or any other membrane-bound organelle.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A unique symbiosis in the gut of tropical herbivorous surgeonfish (acanthuridae: teleostei) from the red sea. Science. 1985 Jul 5;229(4708):49–51. doi: 10.1126/science.229.4708.49. [DOI] [PubMed] [Google Scholar]
- Blakemore R. P., Canale-Parola E. Morphological and ecological characteristics of Spirochaeta plicatilis. Arch Mikrobiol. 1973;89(4):273–289. doi: 10.1007/BF00408895. [DOI] [PubMed] [Google Scholar]
- Bullivant S., Ames A., 3rd A simple freeze-fracture replication method for electron microscopy. J Cell Biol. 1966 Jun;29(3):435–447. doi: 10.1083/jcb.29.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPWOOD D. A., GLAUERT A. M. The fine structure of Streptomyces coelicolor. II. The nuclear material. J Biophys Biochem Cytol. 1960 Sep;8:267–278. doi: 10.1083/jcb.8.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWY J., HANSON J. ELECTRON MICROSCOPE STUDIES OF BACTERIAL FLAGELLA. J Mol Biol. 1965 Feb;11:293–313. doi: 10.1016/s0022-2836(65)80059-6. [DOI] [PubMed] [Google Scholar]
- Manson M. D., Tedesco P., Berg H. C., Harold F. M., Van der Drift C. A protonmotive force drives bacterial flagella. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3060–3064. doi: 10.1073/pnas.74.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace N. R., Olsen G. J., Woese C. R. Ribosomal RNA phylogeny and the primary lines of evolutionary descent. Cell. 1986 May 9;45(3):325–326. doi: 10.1016/0092-8674(86)90315-6. [DOI] [PubMed] [Google Scholar]
- SCHREIL W. H. STUDIES ON THE FIXATION OF ARTIFICIAL AND BACTERIAL DNA PLASMS FOR THE ELECTRON MICROSCOPY OF THIN SECTIONS. J Cell Biol. 1964 Jul;22:1–20. doi: 10.1083/jcb.22.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopf J. W., Oehler D. Z. How old are the eukaryotes? Science. 1976 Jul 2;193(4247):47–49. doi: 10.1126/science.193.4247.47. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Fox G. E. The concept of cellular evolution. J Mol Evol. 1977 Sep 20;10(1):1–6. doi: 10.1007/BF01796132. [DOI] [PubMed] [Google Scholar]










