Abstract
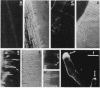
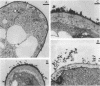
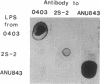
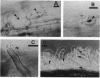
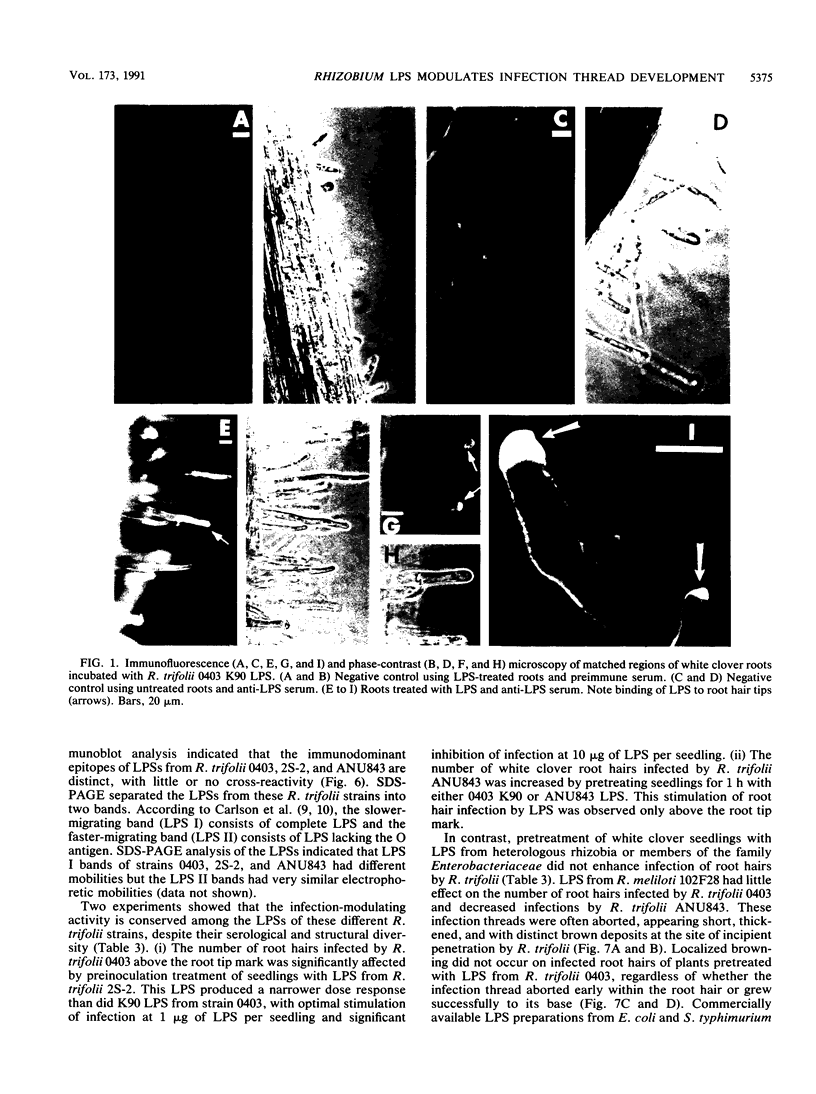
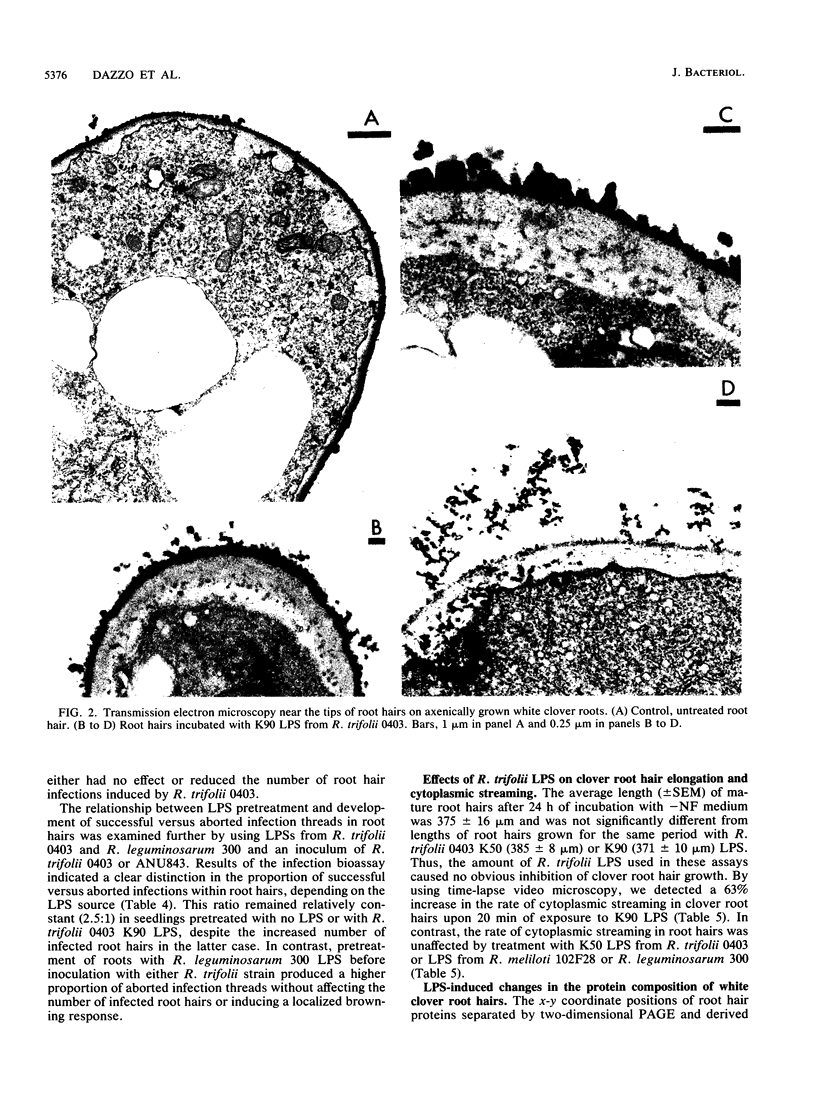
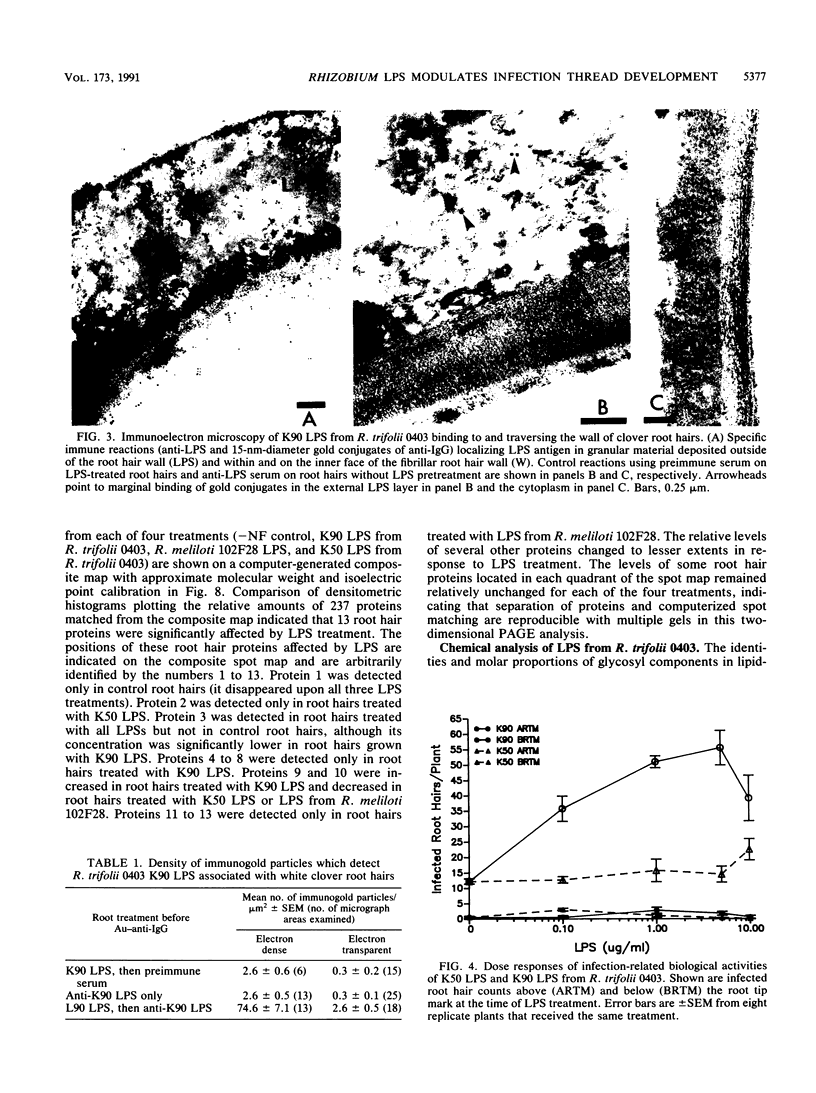
The interaction between Rhizobium lipopolysaccharide (LPS) and white clover roots was examined. The Limulus lysate assay indicated that Rhizobium leguminosarum bv. trifolii (hereafter called R. trifolii) released LPS into the external root environment of slide cultures. Immunofluorescence and immunoelectron microscopy showed that purified LPS from R. trifolii 0403 bound rapidly to root hair tips and infiltrated across the root hair wall. Infection thread formation in root hairs was promoted by preinoculation treatment of roots with R. trifolii LPS at a low dose (up to 5 micrograms per plant) but inhibited at a higher dose. This biological activity of LPS was restricted to the region of the root present at the time of exposure to LPS, higher with LPS from cells in the early stationary phase than in the mid-exponential phase, incubation time dependent, incapable of reversing inhibition of infection by NO3- or NH4+, and conserved among serologically distinct LPSs from several wild-type R. trifolii strains (0403, 2S-2, and ANU843). In contrast, infections were not increased by preinoculation treatment of roots with LPSs from R. leguminosarum bv. viciae strain 300, R. meliloti 102F28, or members of the family Enterobacteriaceae. Most infection threads developed successfully in root hairs pretreated with R. trifolii LPS, whereas many infections aborted near their origins and accumulated brown deposits if pretreated with LPS from R. meliloti 102F28. LPS from R. leguminosarum 300 also caused most infection threads to abort. Other specific responses of root hairs to infection-stimulating LPS from R. trifolii included acceleration of cytoplasmic streaming and production of novel proteins. Combined gas chromatography-mass spectroscopy and proton nuclear magnetic resonance analyses indicated that biologically active LPS from R. trifolii 0403 in the early stationary phase had less fucose but more 2-O-methylfucose, quinovosamine, 3,6-dideoxy-3-(methylamino)galactose, and noncarbohydrate substituents (O-methyl, N-methyl, and acetyl groups) on glycosyl components than did inactive LPS in the mid-exponential phase. We conclude that LPS-root hair interactions trigger metabolic events that have a significant impact on successful development of infection threads in this Rhizobium-legume symbiosis.
Full text
PDF





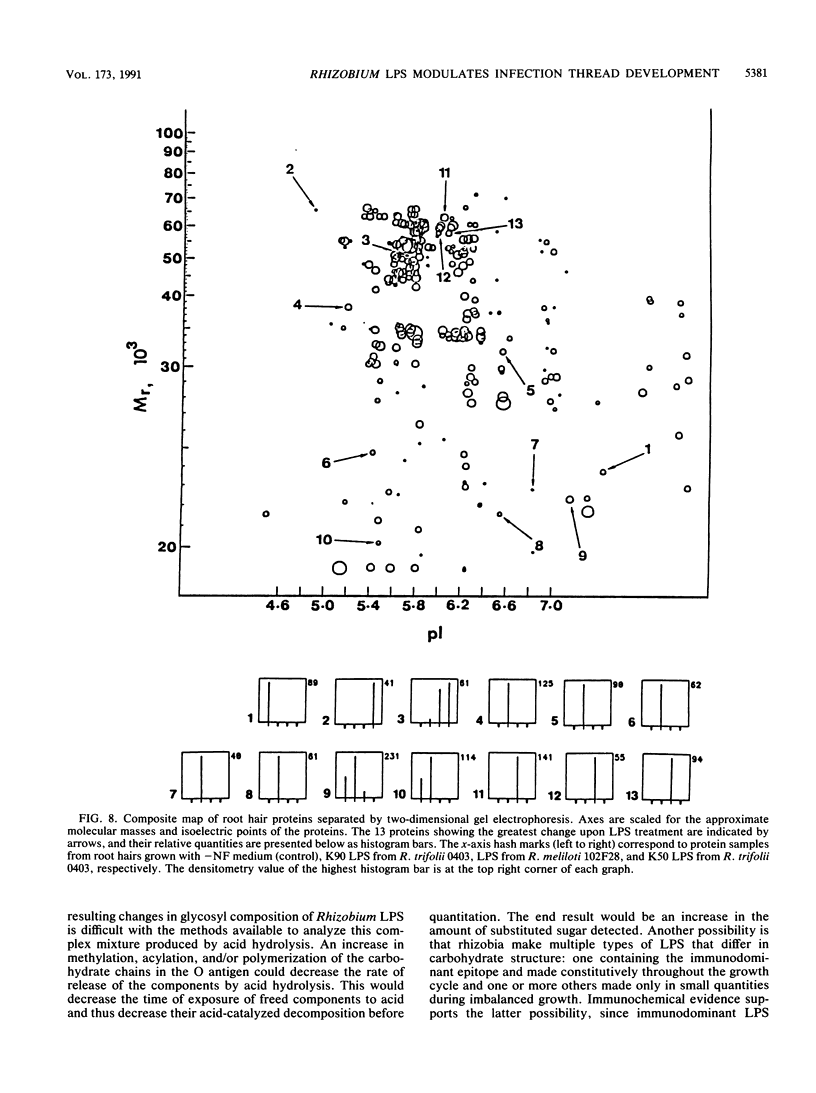
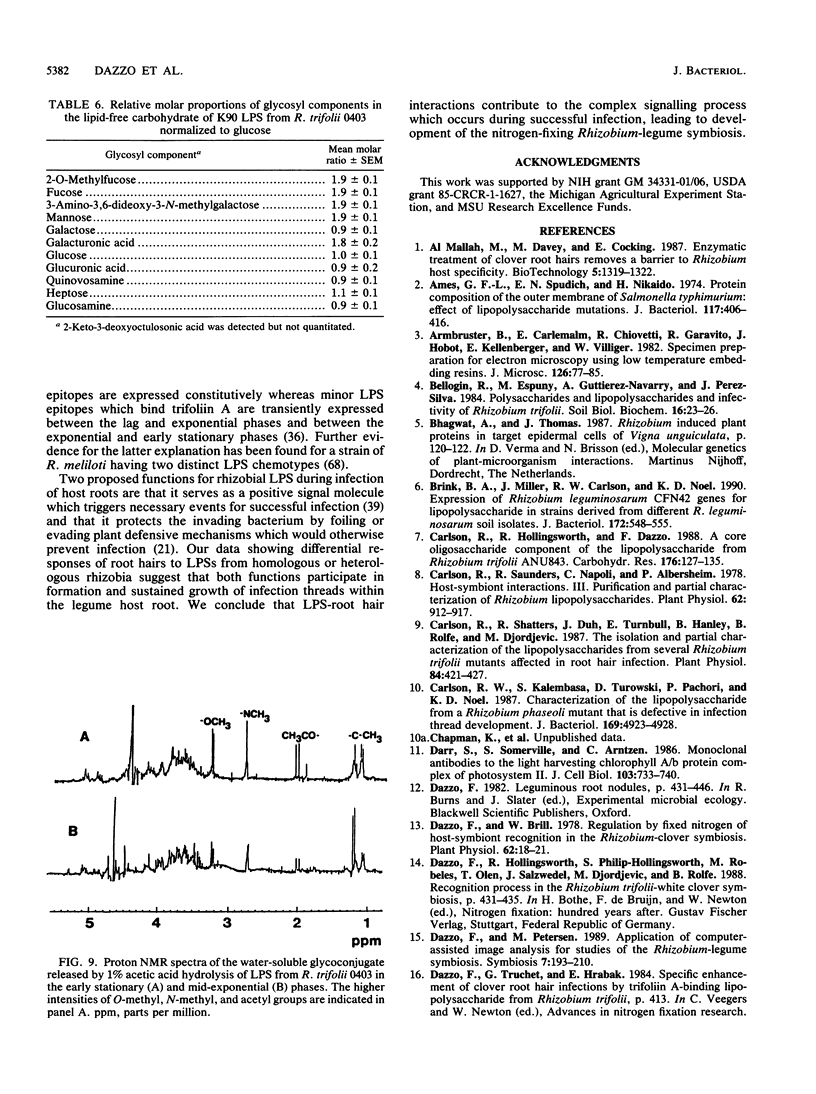


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster B. L., Carlemalm E., Chiovetti R., Garavito R. M., Hobot J. A., Kellenberger E., Villiger W. Specimen preparation for electron microscopy using low temperature embedding resins. J Microsc. 1982 Apr;126(Pt 1):77–85. doi: 10.1111/j.1365-2818.1982.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Brink B. A., Miller J., Carlson R. W., Noel K. D. Expression of Rhizobium leguminosarum CFN42 genes for lipopolysaccharide in strains derived from different R. leguminosarum soil isolates. J Bacteriol. 1990 Feb;172(2):548–555. doi: 10.1128/jb.172.2.548-555.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W., Hollingsworth R. L., Dazzo F. B. A core oligosaccharide component from the lipopolysaccharide of Rhizobium trifolii ANU843. Carbohydr Res. 1988 May 1;176(1):127–135. doi: 10.1016/0008-6215(88)84064-3. [DOI] [PubMed] [Google Scholar]
- Carlson R. W., Kalembasa S., Turowski D., Pachori P., Noel K. D. Characterization of the lipopolysaccharide from a Rhizobium phaseoli mutant that is defective in infection thread development. J Bacteriol. 1987 Nov;169(11):4923–4928. doi: 10.1128/jb.169.11.4923-4928.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W., Sanders R. E., Napoli C., Albersheim P. Host-Symbiont Interactions: III. Purification and Partial Characterization of Rhizobium Lipopolysaccharides. Plant Physiol. 1978 Dec;62(6):912–917. doi: 10.1104/pp.62.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W., Shatters R., Duh J. L., Turnbull E., Hanley B., Rolfe B. G., Djordjevic M. A. The Isolation and Partial Characterization of the Lipopolysaccharides from Several Rhizobium trifolii Mutants Affected in Root Hair Infection. Plant Physiol. 1987 Jun;84(2):421–427. doi: 10.1104/pp.84.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darr S. C., Somerville S. C., Arntzen C. J. Monoclonal antibodies to the light-harvesting chlorophyll a/b protein complex of photosystem II. J Cell Biol. 1986 Sep;103(3):733–740. doi: 10.1083/jcb.103.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Brill W. J. Bacterial polysaccharide which binds Rhizobium trifolii to clover root hairs. J Bacteriol. 1979 Mar;137(3):1362–1373. doi: 10.1128/jb.137.3.1362-1373.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Brill W. J. Regulation by fixed nitrogen of host-symbiont recognition in the Rhizobium-clover symbiosis. Plant Physiol. 1978 Jul;62(1):18–21. doi: 10.1104/pp.62.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Truchet G. L., Sherwood J. E., Hrabak E. M., Abe M., Pankratz S. H. Specific phases of root hair attachment in the Rhizobium trifolii-clover symbiosis. Appl Environ Microbiol. 1984 Dec;48(6):1140–1150. doi: 10.1128/aem.48.6.1140-1150.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Yanke W. E., Brill W. J. Trifolin: a Rhizobium recognition protein from white clover. Biochim Biophys Acta. 1978 Mar 20;539(3):276–286. doi: 10.1016/0304-4165(78)90032-6. [DOI] [PubMed] [Google Scholar]
- Djordjevic S. P., Ridge R. W., Chen H. C., Redmond J. W., Batley M., Rolfe B. G. Induction of pathogenic-like responses in the legume Macroptilium atropurpureum by a transposon-induced mutant of the fast-growing, broad-host-range Rhizobium strain NGR234. J Bacteriol. 1988 Apr;170(4):1848–1857. doi: 10.1128/jb.170.4.1848-1857.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervin S. E., Hubbell D. H. Root Hair Deformations Associated with Fractionated Extracts from Rhizobium trifolii. Appl Environ Microbiol. 1985 Jan;49(1):61–68. doi: 10.1128/aem.49.1.61-68.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHRAEUS G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J Gen Microbiol. 1957 Apr;16(2):374–381. doi: 10.1099/00221287-16-2-374. [DOI] [PubMed] [Google Scholar]
- Gharyal P. K., Ho S. C., Wang J. L., Schindler M. O-antigen from Bradyrhizobium japonicum lipopolysaccharide inhibits intercellular (symplast) communication between soybean (Glycine max) cells. J Biol Chem. 1989 Jul 25;264(21):12119–12121. [PubMed] [Google Scholar]
- Graham T. L., Sequeira L., Huang T. S. Bacterial lipopolysaccharides as inducers of disease resistance in tobacco. Appl Environ Microbiol. 1977 Oct;34(4):424–432. doi: 10.1128/aem.34.4.424-432.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth R. I., Abe M., Sherwood J. E., Dazzo F. B. Bacteriophage-induced acidic heteropolysaccharide lyases that convert the acidic heteropolysaccharides of Rhizobium trifolii into oligosaccharide units. J Bacteriol. 1984 Nov;160(2):510–516. doi: 10.1128/jb.160.2.510-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth R. I., Carlson R. W. 27-Hydroxyoctacosanoic acid is a major structural fatty acyl component of the lipopolysaccharide of Rhizobium trifolii ANU 843. J Biol Chem. 1989 Jun 5;264(16):9300–9303. [PubMed] [Google Scholar]
- Hollingsworth R. I., Hrabak E. M., Dazzo F. B. Synthesis of 3,6-dideoxy-3-(methylamino)hexoses for g.l.c.-m.s. identification of Rhizobium lipopolysaccharide components. Carbohydr Res. 1986 Oct 15;154:103–113. doi: 10.1016/s0008-6215(00)90026-0. [DOI] [PubMed] [Google Scholar]
- Hrabak E. M., Urbano M. R., Dazzo F. B. Growth-phase-dependent immunodeterminants of Rhizobium trifolii lipopolysaccharide which bind trifoliin A, a white clover lectin. J Bacteriol. 1981 Nov;148(2):697–711. doi: 10.1128/jb.148.2.697-711.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro E. E., Vanderwel D., Kusser W. Control of lipopolysaccharide biosynthesis and release by Escherichia coli and Salmonella typhimurium. J Bacteriol. 1986 Oct;168(1):328–333. doi: 10.1128/jb.168.1.328-333.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijne J. W., Smit G., Díaz C. L., Lugtenberg B. J. Lectin-enhanced accumulation of manganese-limited Rhizobium leguminosarum cells on pea root hair tips. J Bacteriol. 1988 Jul;170(7):2994–3000. doi: 10.1128/jb.170.7.2994-3000.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Hubbell D. H. Infection thread formation as a basis of nodulation specificity in Rhizobium--strawberry clover associations. Can J Microbiol. 1969 Oct;15(10):1133–1136. doi: 10.1139/m69-206. [DOI] [PubMed] [Google Scholar]
- Maier R. J., Brill W. J. Involvement of Rhizobium japonicum O antigen in soybean nodulation. J Bacteriol. 1978 Mar;133(3):1295–1299. doi: 10.1128/jb.133.3.1295-1299.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maupin P., Pollard T. D. Improved preservation and staining of HeLa cell actin filaments, clathrin-coated membranes, and other cytoplasmic structures by tannic acid-glutaraldehyde-saponin fixation. J Cell Biol. 1983 Jan;96(1):51–62. doi: 10.1083/jcb.96.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel K. D., Vandenbosch K. A., Kulpaca B. Mutations in Rhizobium phaseoli that lead to arrested development of infection threads. J Bacteriol. 1986 Dec;168(3):1392–1401. doi: 10.1128/jb.168.3.1392-1401.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priefer U. B. Genes involved in lipopolysaccharide production and symbiosis are clustered on the chromosome of Rhizobium leguminosarum biovar viciae VF39. J Bacteriol. 1989 Nov;171(11):6161–6168. doi: 10.1128/jb.171.11.6161-6168.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnoky P., Petrovics G., Kereszt A., Grosskopf E., Ha D. T., Bánfalvi Z., Kondorosi A. Rhizobium meliloti lipopolysaccharide and exopolysaccharide can have the same function in the plant-bacterium interaction. J Bacteriol. 1990 Sep;172(9):5450–5458. doi: 10.1128/jb.172.9.5450-5458.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvanesarajah V., Schell F. M., Gerhold D., Stacey G. Cell surface polysaccharides from Bradyrhizobium japonicum and a nonnodulating mutant. J Bacteriol. 1987 Jan;169(1):137–141. doi: 10.1128/jb.169.1.137-141.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russa R., Urbanik T., Lorkiewicz Z. Characterization of the lipopolysaccharide from the nod mutant of Rhizobium trifolii. Acta Microbiol Pol. 1983;32(1):25–30. [PubMed] [Google Scholar]
- Sen K., Nikaido H. Lipopolysaccharide structure required for in vitro trimerization of Escherichia coli OmpF porin. J Bacteriol. 1991 Jan;173(2):926–928. doi: 10.1128/jb.173.2.926-928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit G., Kijne J. W., Lugtenberg B. J. Roles of flagella, lipopolysaccharide, and a Ca2+-dependent cell surface protein in attachment of Rhizobium leguminosarum biovar viciae to pea root hair tips. J Bacteriol. 1989 Jan;171(1):569–572. doi: 10.1128/jb.171.1.569-572.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang V. C., Peralta J. M., Simons A. R. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 1983;92:377–391. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]
- Whatley M. H., Bodwin J. S., Lippincott B. B., Lippincott J. A. Role of Agrobacterium cell envelope lipopolysaccharide in infection site attachment. Infect Immun. 1976 Apr;13(4):1080–1083. doi: 10.1128/iai.13.4.1080-1083.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. N., Hollingsworth R. I., Klein S., Signer E. R. The symbiotic defect of Rhizobium meliloti exopolysaccharide mutants is suppressed by lpsZ+, a gene involved in lipopolysaccharide biosynthesis. J Bacteriol. 1990 May;172(5):2622–2632. doi: 10.1128/jb.172.5.2622-2632.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert J. S., Albersheim P. Host-symbiont interactions. I. The lectins of legumes interact with the o-antigen-containing lipopolysaccharides of their symbiont Rhizobia. Biochem Biophys Res Commun. 1976 Jun 7;70(3):729–737. doi: 10.1016/0006-291x(76)90653-7. [DOI] [PubMed] [Google Scholar]
- de Maagd R. A., Rao A. S., Mulders I. H., Goosen-de Roo L., van Loosdrecht M. C., Wijffelman C. A., Lugtenberg B. J. Isolation and characterization of mutants of Rhizobium leguminosarum bv. viciae 248 with altered lipopolysaccharides: possible role of surface charge or hydrophobicity in bacterial release from the infection thread. J Bacteriol. 1989 Feb;171(2):1143–1150. doi: 10.1128/jb.171.2.1143-1150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]