Abstract
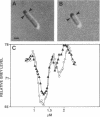
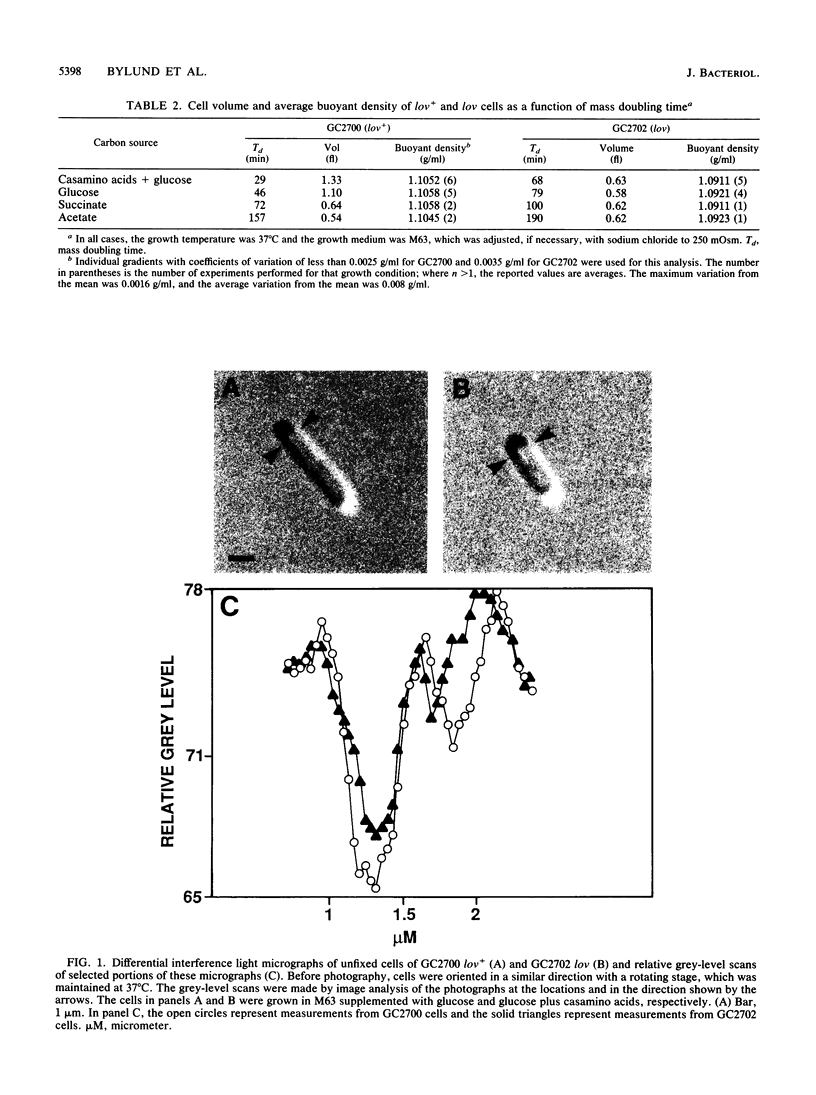
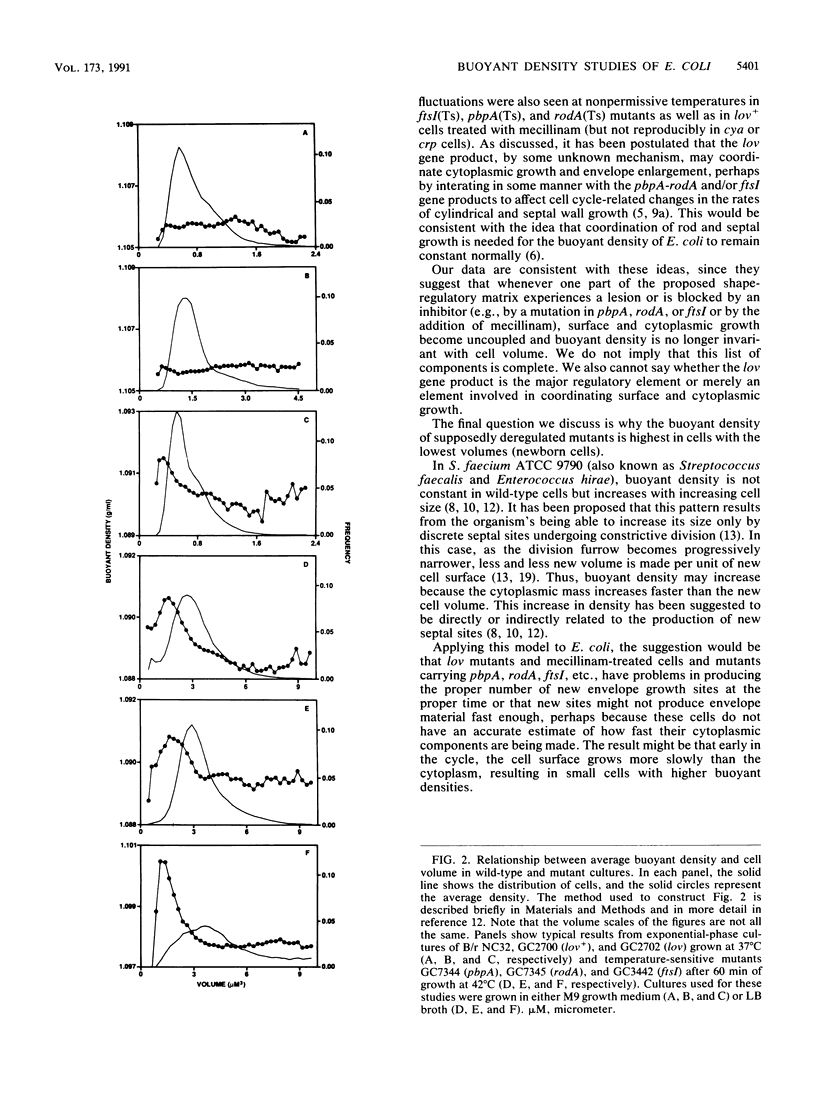
The buoyant density of wild-type Escherichia coli cells has previously been reported not to vary with growth rate and cell size or age. In the present report we confirm these findings, using Percoll gradients, and analyze the recently described lov mutant, which was selected for its resistance to mecillinam and has been suggested to be affected in the coordination between mass growth and envelope synthesis. The average buoyant density of lov mutant cells was significantly lower than that of wild-type cells. Similarly, the buoyant density of wild-type cells decreased in the presence of mecillinam. The density of the lov mutant, like that of the wild type, was invariant over a 2.8-fold range in growth rate. In this range, however, the average cell volume was also constant. Analysis of buoyant density as a function of cell volume in individual cultures revealed that smaller (newborn) lov mutant cells had higher density than larger (old) cells; however, the density of the small cells never approached that of the wild-type cells, whose density was independent of cell size (age). A pattern similar to that of lov mutant cells was observed in cells carrying the mecillinam-resistant mutations pbpA(Ts) and rodA(Ts) and the division mutation ftsI(Ts) at nonpermissive temperatures as well as in wild-type cells treated with mecillinam, but not in mecillinam-resistant crp or cya mutants.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aono R., Yamasaki M., Tamura G. High and selective resistance to mecillinam in adenylate cyclase-deficient or cyclic adenosine 3',5'-monophosphate receptor protein-deficient mutants of Escherichia coli. J Bacteriol. 1979 Feb;137(2):839–845. doi: 10.1128/jb.137.2.839-845.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin W. W., Sheu M. J., Bankston P. W., Woldringh C. L. Changes in buoyant density and cell size of Escherichia coli in response to osmotic shocks. J Bacteriol. 1988 Jan;170(1):452–455. doi: 10.1128/jb.170.1.452-455.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botsford J. L. Cyclic nucleotides in procaryotes. Microbiol Rev. 1981 Dec;45(4):620–642. doi: 10.1128/mr.45.4.620-642.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouloc P., Jaffé A., D'Ari R. Preliminary physiologic characterization and genetic analysis of a new Escherichia coli mutant, lov, resistant to mecillinam. Rev Infect Dis. 1988 Jul-Aug;10(4):905–910. doi: 10.1093/clinids/10.4.905. [DOI] [PubMed] [Google Scholar]
- Bouloc P., Jaffé A., D'Ari R. The Escherichia coli lov gene product connects peptidoglycan synthesis, ribosomes and growth rate. EMBO J. 1989 Jan;8(1):317–323. doi: 10.1002/j.1460-2075.1989.tb03379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. Rate and topography of cell wall synthesis during the division cycle of Salmonella typhimurium. J Bacteriol. 1988 Jan;170(1):422–430. doi: 10.1128/jb.170.1.422-430.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ari R., Jaffé A., Bouloc P., Robin A. Cyclic AMP and cell division in Escherichia coli. J Bacteriol. 1988 Jan;170(1):65–70. doi: 10.1128/jb.170.1.65-70.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker D. T., Higgins M. L. Cell cycle changes in the buoyant density of exponential-phase cells of Streptococcus faecium. J Bacteriol. 1987 Mar;169(3):1200–1204. doi: 10.1128/jb.169.3.1200-1204.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser D., Higgins M. Buoyant density, growth rate, and the cell cycle in Streptococcus faecium. J Bacteriol. 1989 Feb;171(2):669–673. doi: 10.1128/jb.171.2.669-673.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart A., Edwards C. Buoyant density fluctuations during the cell cycle of Bacillus subtilis. Arch Microbiol. 1987 Feb;147(1):68–72. doi: 10.1007/BF00492907. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Haines M., Whalen M., Glaser D., Bylund J. Relationship between changes in buoyant density and formation of new sites of cell wall growth in cultures of streptococci (Enterococcus hirae ATCC 9790) undergoing a nutritional shift-up. J Bacteriol. 1990 Aug;172(8):4415–4419. doi: 10.1128/jb.172.8.4415-4419.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Model for cell wall growth of Streptococcus faecalis. J Bacteriol. 1970 Feb;101(2):643–648. doi: 10.1128/jb.101.2.643-648.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S., Niki H., Ogura T., Ichinose C., Mori H., Ezaki B., Jaffé A. Chromosome partitioning in Escherichia coli: novel mutants producing anucleate cells. J Bacteriol. 1989 Mar;171(3):1496–1505. doi: 10.1128/jb.171.3.1496-1505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S., Ogura T., Niki H., Ichinose C., Mori H. Positioning of replicated chromosomes in Escherichia coli. J Bacteriol. 1990 Jan;172(1):31–39. doi: 10.1128/jb.172.1.31-39.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino F., Park W., Tomioka S., Tamaki S., Takase I., Kunugita K., Matsuzawa H., Asoh S., Ohta T., Spratt B. G. Peptidoglycan synthetic activities in membranes of Escherichia coli caused by overproduction of penicillin-binding protein 2 and rodA protein. J Biol Chem. 1986 May 25;261(15):7024–7031. [PubMed] [Google Scholar]
- Jaffé A., Chabbert Y. A., Derlot E. Selection and characterization of beta-lactam-resistant Escherichia coli K-12 mutants. Antimicrob Agents Chemother. 1983 Apr;23(4):622–625. doi: 10.1128/aac.23.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R., Haga J. Y., Pardee A. B. Inhibition of an early event in the cell division cycle of Escherichia coli by FL1060, an amidinopenicillanic acid. J Bacteriol. 1975 Jun;122(3):1283–1292. doi: 10.1128/jb.122.3.1283-1292.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L., Higgins M. L. Control of wall band splitting in Streptococcus faecalis. J Gen Microbiol. 1984 Apr;130(4):735–745. doi: 10.1099/00221287-130-4-735. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Higgins M. L., Doyle R. J. Surface tension-like forces determine bacterial shapes: Streptococcus faecium. J Gen Microbiol. 1981 Mar;123(1):151–161. doi: 10.1099/00221287-123-1-151. [DOI] [PubMed] [Google Scholar]
- Kubitschek H. E., Baldwin W. W., Graetzer R. Buoyant density constancy during the cell cycle of Escherichia coli. J Bacteriol. 1983 Sep;155(3):1027–1032. doi: 10.1128/jb.155.3.1027-1032.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitschek H. E., Baldwin W. W., Schroeter S. J., Graetzer R. Independence of buoyant cell density and growth rate in Escherichia coli. J Bacteriol. 1984 Apr;158(1):296–299. doi: 10.1128/jb.158.1.296-299.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitschek H. E. Buoyant density variation during the cell cycle in microorganisms. Crit Rev Microbiol. 1987;14(1):73–97. doi: 10.3109/10408418709104436. [DOI] [PubMed] [Google Scholar]
- Ogura T., Bouloc P., Niki H., D'Ari R., Hiraga S., Jaffé A. Penicillin-binding protein 2 is essential in wild-type Escherichia coli but not in lov or cya mutants. J Bacteriol. 1989 Jun;171(6):3025–3030. doi: 10.1128/jb.171.6.3025-3030.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Boyd A., Stoker N. Defective and plaque-forming lambda transducing bacteriophage carrying penicillin-binding protein-cell shape genes: genetic and physical mapping and identification of gene products from the lip-dacA-rodA-pbpA-leuS region of the Escherichia coli chromosome. J Bacteriol. 1980 Aug;143(2):569–581. doi: 10.1128/jb.143.2.569-581.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Pardee A. B. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975 Apr 10;254(5500):516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. The mechanism of action of mecillinam. J Antimicrob Chemother. 1977 Jul;3 (Suppl B):13–19. doi: 10.1093/jac/3.suppl_b.13. [DOI] [PubMed] [Google Scholar]