Abstract
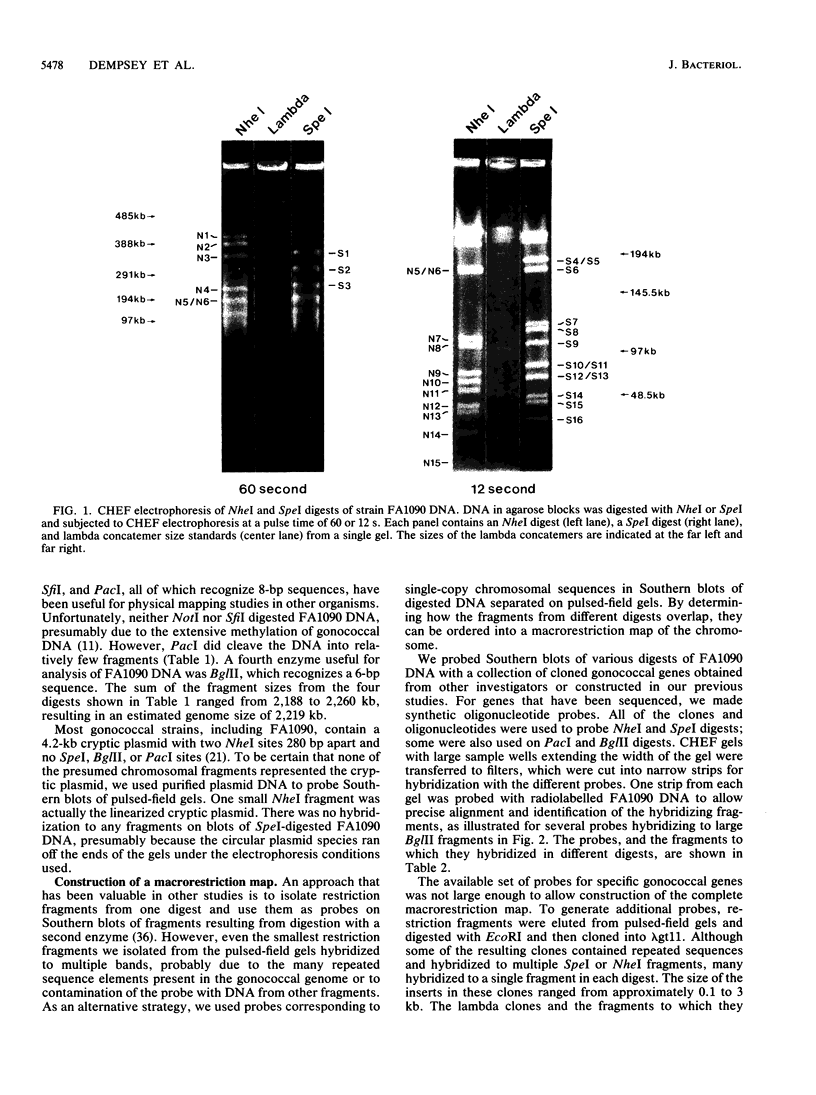
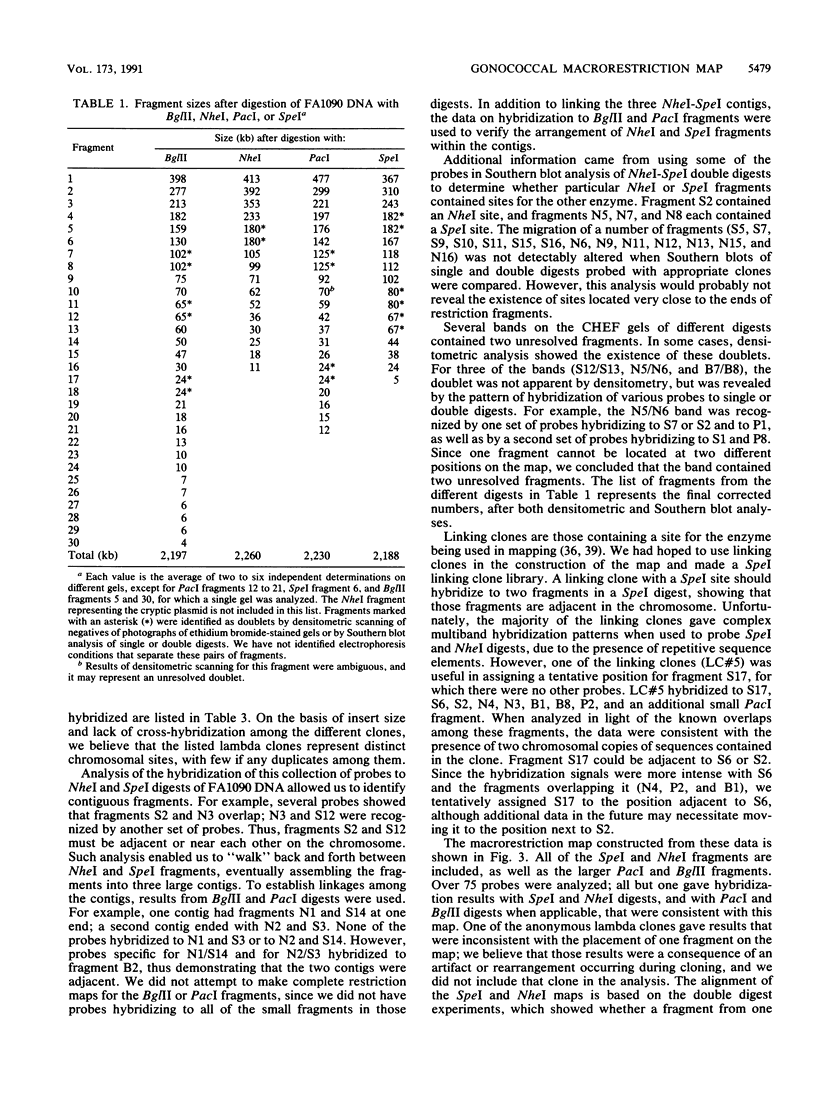
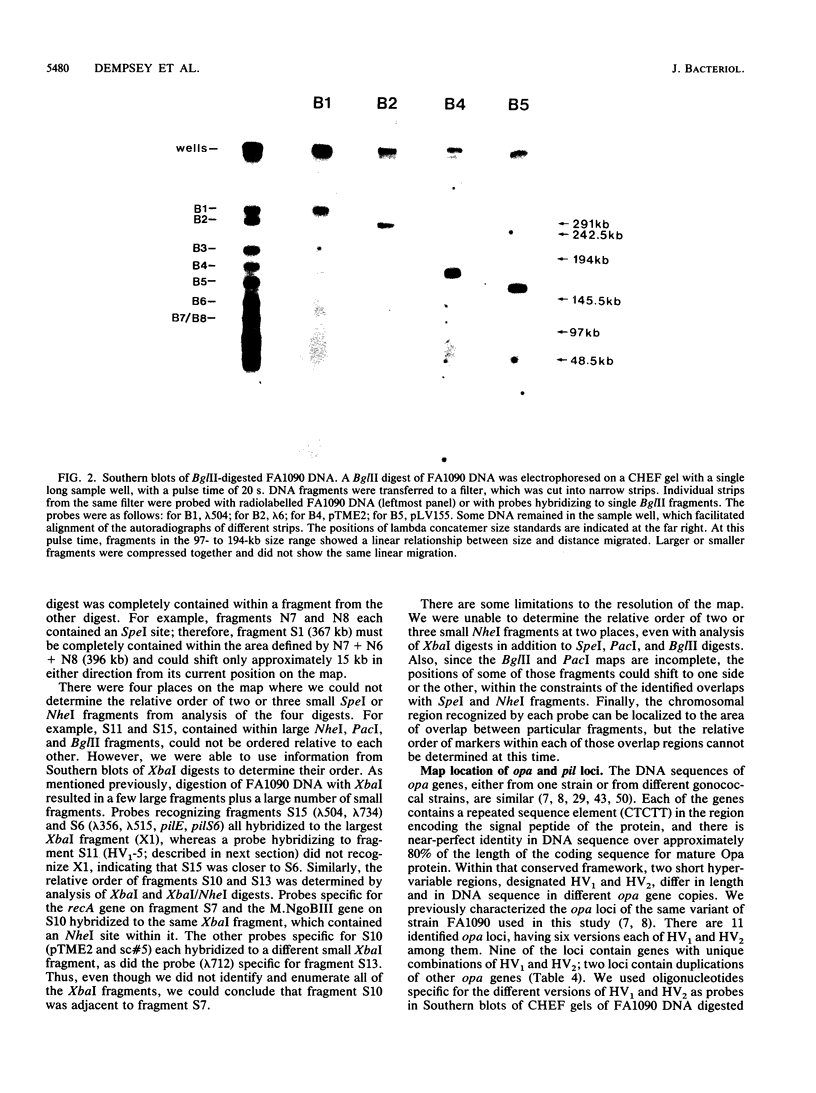
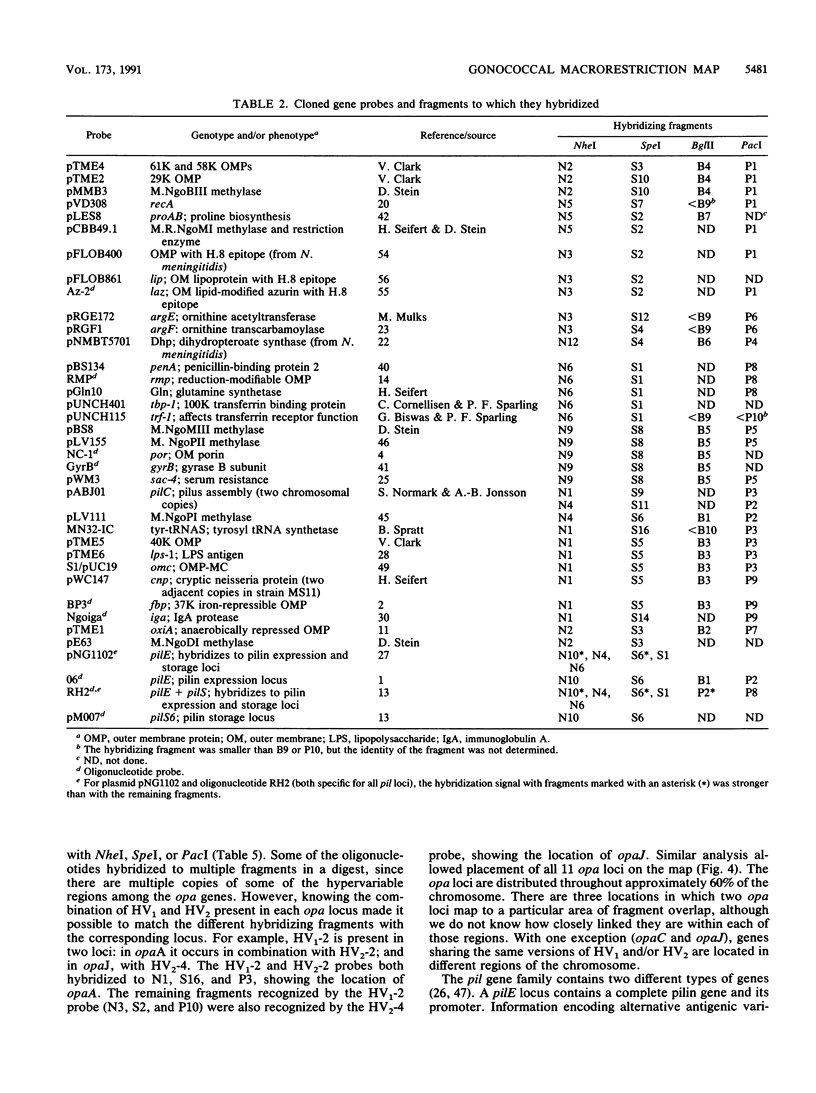
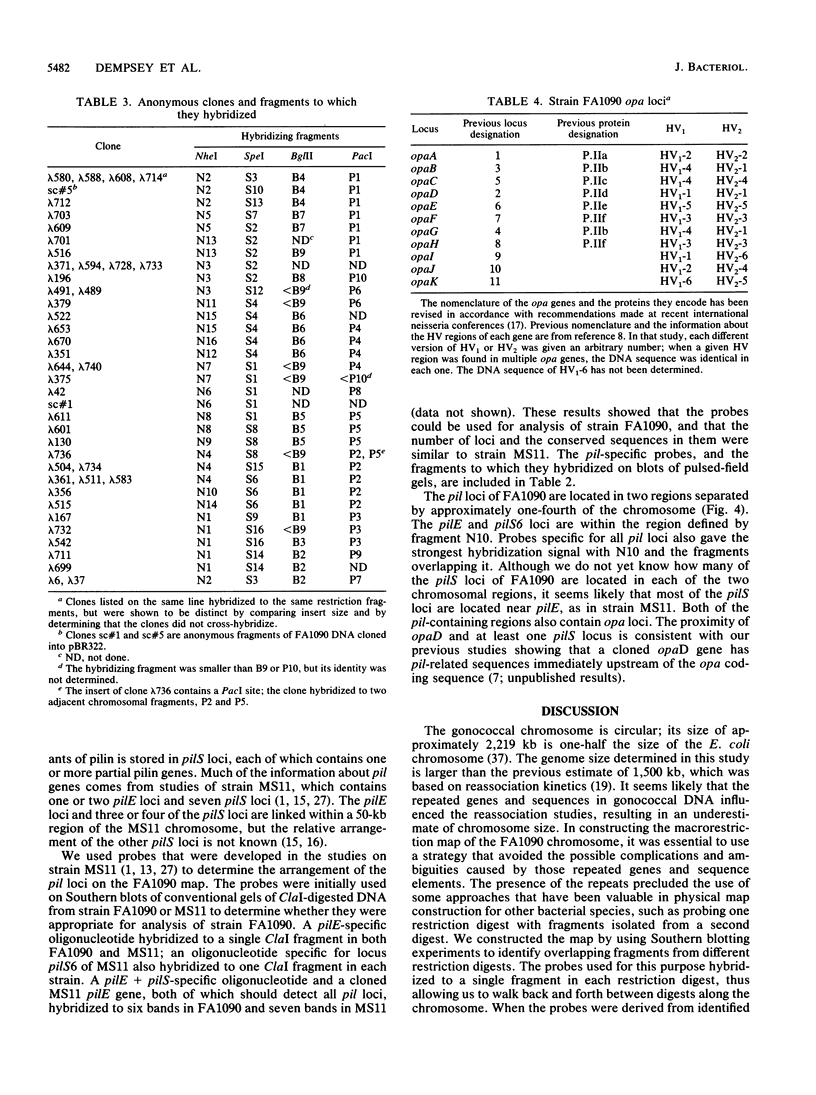
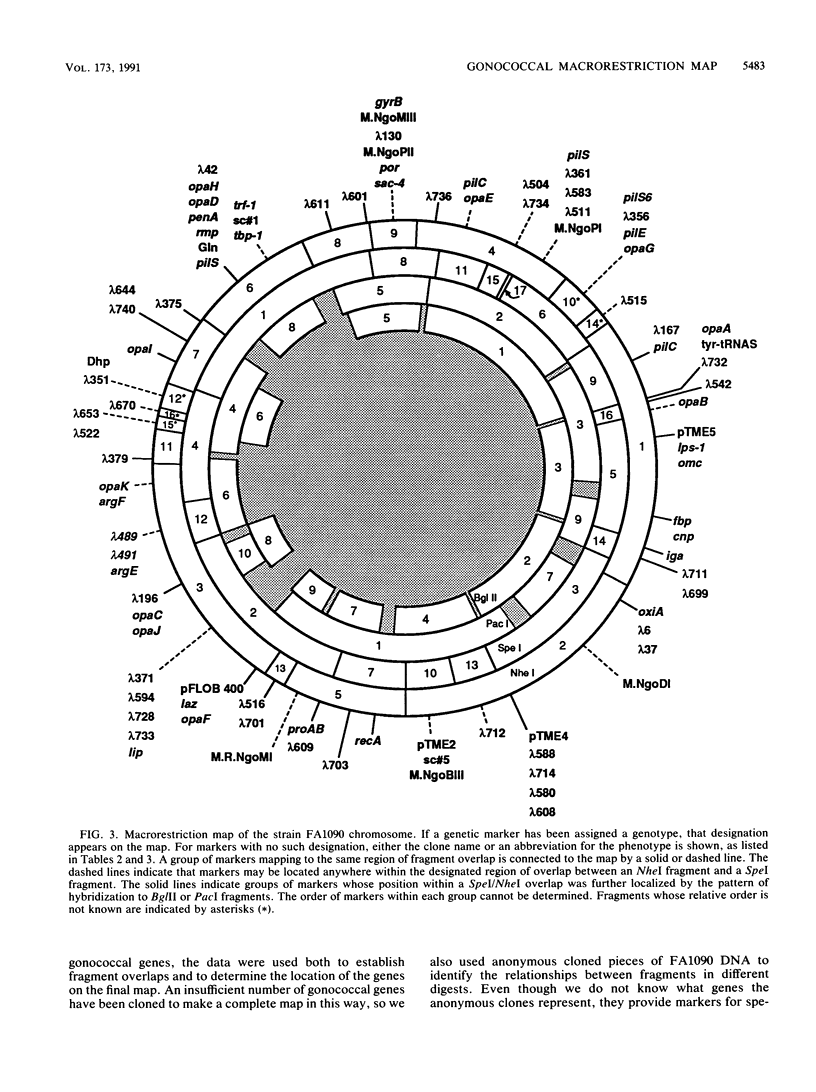
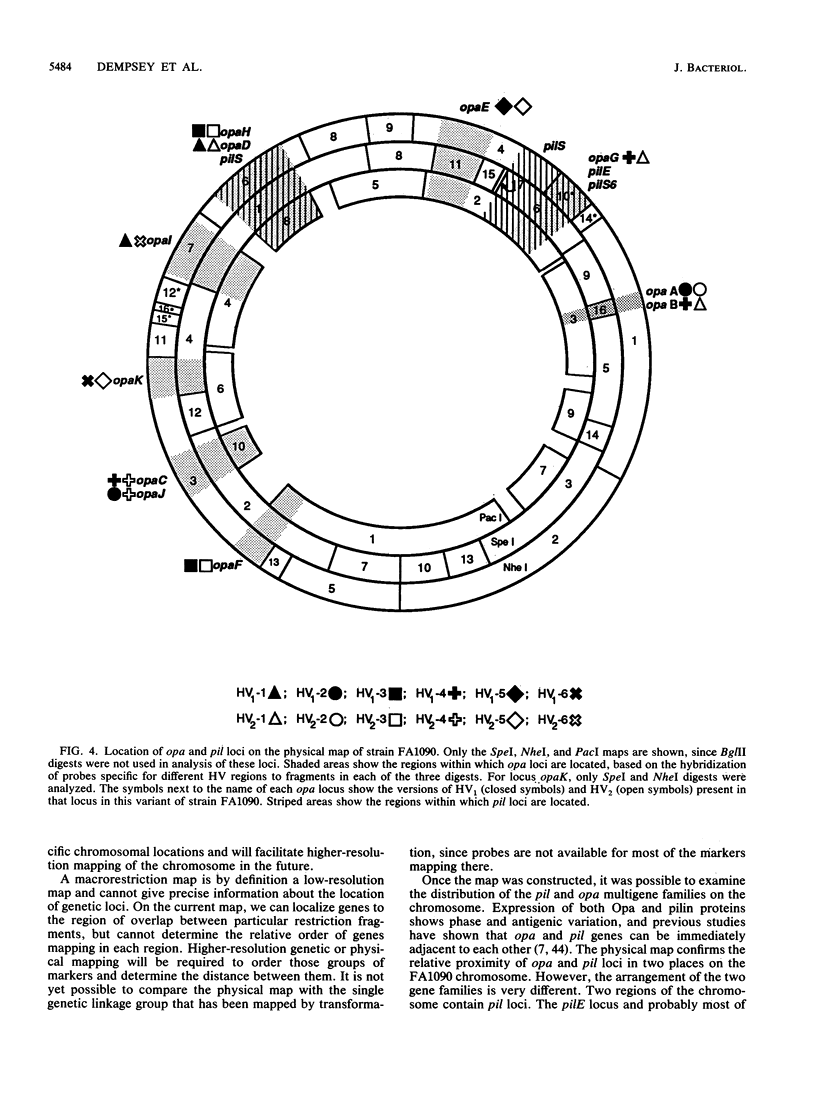
A physical map of the chromosome of Neisseria gonorrhoeae FA1090 has been constructed. Digestion of strain FA1090 DNA with NheI, SpeI, BglII, or PacI resulted in a limited number of fragments that were resolved by contour-clamped homogeneous electric field electrophoresis. The estimated genome size was 2,219 kb. To construct the map, probes corresponding to single-copy chromosomal sequences were used in Southern blots of digested DNA separated on pulsed-field gels, to determine how the fragments from different digests overlapped. Some of the probes represented identified gonococcal genes, whereas others were anonymous cloned fragments of strain FA1090 DNA. By using this approach, a macrorestriction map of the strain FA1090 chromosome was assembled, and the locations of various genetic markers on the map were determined. Once the map was completed, the repeated gene families encoding Opa and pilin proteins were mapped. The 11 opa loci of strain FA1090 were distributed over approximately 60% of the chromosome. The pil loci were more clustered and were located in two regions separated by approximately one-fourth of the chromosome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergström S., Robbins K., Koomey J. M., Swanson J. Piliation control mechanisms in Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3890–3894. doi: 10.1073/pnas.83.11.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berish S. A., Mietzner T. A., Mayer L. W., Genco C. A., Holloway B. P., Morse S. A. Molecular cloning and characterization of the structural gene for the major iron-regulated protein expressed by Neisseria gonorrhoeae. J Exp Med. 1990 May 1;171(5):1535–1546. doi: 10.1084/jem.171.5.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonetti N. H., Sparling P. F. Molecular cloning and characterization of the structural gene for protein I, the major outer membrane protein of Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9084–9088. doi: 10.1073/pnas.84.24.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Connell T. D., Black W. J., Kawula T. H., Barritt D. S., Dempsey J. A., Kverneland K., Jr, Stephenson A., Schepart B. S., Murphy G. L., Cannon J. G. Recombination among protein II genes of Neisseria gonorrhoeae generates new coding sequences and increases structural variability in the protein II family. Mol Microbiol. 1988 Mar;2(2):227–236. doi: 10.1111/j.1365-2958.1988.tb00024.x. [DOI] [PubMed] [Google Scholar]
- Connell T. D., Shaffer D., Cannon J. G. Characterization of the repertoire of hypervariable regions in the Protein II (opa) gene family of Neisseria gonorrhoeae. Mol Microbiol. 1990 Mar;4(3):439–449. doi: 10.1111/j.1365-2958.1990.tb00610.x. [DOI] [PubMed] [Google Scholar]
- Correia F. F., Inouye S., Inouye M. A 26-base-pair repetitive sequence specific for Neisseria gonorrhoeae and Neisseria meningitidis genomic DNA. J Bacteriol. 1986 Sep;167(3):1009–1015. doi: 10.1128/jb.167.3.1009-1015.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia F. F., Inouye S., Inouye M. A family of small repeated elements with some transposon-like properties in the genome of Neisseria gonorrhoeae. J Biol Chem. 1988 Sep 5;263(25):12194–12198. [PubMed] [Google Scholar]
- Gibbs C. P., Reimann B. Y., Schultz E., Kaufmann A., Haas R., Meyer T. F. Reassortment of pilin genes in Neisseria gonorrhoeae occurs by two distinct mechanisms. Nature. 1989 Apr 20;338(6217):651–652. doi: 10.1038/338651a0. [DOI] [PubMed] [Google Scholar]
- Gotschlich E. C., Seiff M., Blake M. S. The DNA sequence of the structural gene of gonococcal protein III and the flanking region containing a repetitive sequence. Homology of protein III with enterobacterial OmpA proteins. J Exp Med. 1987 Feb 1;165(2):471–482. doi: 10.1084/jem.165.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas R., Meyer T. F. The repertoire of silent pilus genes in Neisseria gonorrhoeae: evidence for gene conversion. Cell. 1986 Jan 17;44(1):107–115. doi: 10.1016/0092-8674(86)90489-7. [DOI] [PubMed] [Google Scholar]
- Hill S. A., Morrison S. G., Swanson J. The role of direct oligonucleotide repeats in gonococcal pilin gene variation. Mol Microbiol. 1990 Aug;4(8):1341–1352. doi: 10.1111/j.1365-2958.1990.tb00713.x. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. T. Estimate of the genome size of various microorganisms. J Bacteriol. 1969 Jun;98(3):1400–1401. doi: 10.1128/jb.98.3.1400-1401.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koomey J. M., Falkow S. Cloning of the recA gene of Neisseria gonorrhoeae and construction of gonococcal recA mutants. J Bacteriol. 1987 Feb;169(2):790–795. doi: 10.1128/jb.169.2.790-795.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korch C., Hagblom P., Ohman H., Göransson M., Normark S. Cryptic plasmid of Neisseria gonorrhoeae: complete nucleotide sequence and genetic organization. J Bacteriol. 1985 Aug;163(2):430–438. doi: 10.1128/jb.163.2.430-438.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen B. E., Rådström P., Jenkins A., Ask E., Facinelli B., Sköld O. Cloning and characterization of a DNA fragment that confers sulfonamide resistance in a serogroup B, serotype 15 strain of Neisseria meningitidis. Antimicrob Agents Chemother. 1990 Nov;34(11):2277–2279. doi: 10.1128/aac.34.11.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. R., Cooperider J. W., Mulks M. H. Sequence of the argF gene encoding ornithine transcarbamoylase from Neisseria gonorrhoeae. Gene. 1990 Sep 28;94(1):139–140. doi: 10.1016/0378-1119(90)90482-7. [DOI] [PubMed] [Google Scholar]
- McClelland M., Jones R., Patel Y., Nelson M. Restriction endonucleases for pulsed field mapping of bacterial genomes. Nucleic Acids Res. 1987 Aug 11;15(15):5985–6005. doi: 10.1093/nar/15.15.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShan W. M., Williams R. P., Hull R. A. A recombinant molecule from a disseminating strain of Neisseria gonorrhoeae that confers serum bactericidal resistance. Infect Immun. 1987 Dec;55(12):3017–3022. doi: 10.1128/iai.55.12.3017-3022.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. F., Gibbs C. P., Haas R. Variation and control of protein expression in Neisseria. Annu Rev Microbiol. 1990;44:451–477. doi: 10.1146/annurev.mi.44.100190.002315. [DOI] [PubMed] [Google Scholar]
- Meyer T. F., Mlawer N., So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982 Aug;30(1):45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- Palermo D. A., Evans T. M., Clark V. L. Expression of a cloned lipopolysaccharide antigen from Neisseria gonorrhoeae on the surface of Escherichia coli K-12. Infect Immun. 1987 Nov;55(11):2844–2849. doi: 10.1128/iai.55.11.2844-2849.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L., Brooks G. F., Falkow S. Expression of gonococcal protein II in Escherichia coli by translational fusion. Mol Microbiol. 1989 May;3(5):663–671. doi: 10.1111/j.1365-2958.1989.tb00214.x. [DOI] [PubMed] [Google Scholar]
- Pohlner J., Halter R., Beyreuther K., Meyer T. F. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. 1987 Jan 29-Feb 4Nature. 325(6103):458–462. doi: 10.1038/325458a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Scocca J. J. The role of transformation in the variability of the Neisseria gonorrhoeae cell surface. Mol Microbiol. 1990 Mar;4(3):321–327. doi: 10.1111/j.1365-2958.1990.tb00599.x. [DOI] [PubMed] [Google Scholar]
- Segal E., Hagblom P., Seifert H. S., So M. Antigenic variation of gonococcal pilus involves assembly of separated silent gene segments. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2177–2181. doi: 10.1073/pnas.83.7.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Condemine G. New approaches for physical mapping of small genomes. J Bacteriol. 1990 Mar;172(3):1167–1172. doi: 10.1128/jb.172.3.1167-1172.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Econome J. G., Schutt A., Klco S., Cantor C. R. A physical map of the Escherichia coli K12 genome. Science. 1987 Jun 12;236(4807):1448–1453. doi: 10.1126/science.3296194. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Hybrid penicillin-binding proteins in penicillin-resistant strains of Neisseria gonorrhoeae. Nature. 1988 Mar 10;332(6160):173–176. doi: 10.1038/332173a0. [DOI] [PubMed] [Google Scholar]
- Stein D. C., Danaher R. J., Cook T. M. Characterization of a gyrB mutation responsible for low-level nalidixic acid resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1991 Apr;35(4):622–626. doi: 10.1128/aac.35.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D. C., Silver L. E., Clark V. L., Young F. E. Cloning genes for proline biosynthesis from Neisseria gonorrhoeae: identification by interspecific complementation of Escherichia coli mutants. J Bacteriol. 1984 May;158(2):696–700. doi: 10.1128/jb.158.2.696-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A., Brown M., Nickel P., Meyer T. F. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell. 1986 Oct 10;47(1):61–71. doi: 10.1016/0092-8674(86)90366-1. [DOI] [PubMed] [Google Scholar]
- Stern A., Nickel P., Meyer T. F., So M. Opacity determinants of Neisseria gonorrhoeae: gene expression and chromosomal linkage to the gonococcal pilus gene. Cell. 1984 Jun;37(2):447–456. doi: 10.1016/0092-8674(84)90375-1. [DOI] [PubMed] [Google Scholar]
- Sullivan K. M., Saunders J. R. Nucleotide sequence and genetic organization of the NgoPII restriction-modification system of Neisseria gonorrhoeae. Mol Gen Genet. 1989 Apr;216(2-3):380–387. doi: 10.1007/BF00334379. [DOI] [PubMed] [Google Scholar]
- Sullivan K. M., Saunders J. R. Sequence analysis of the NgoPII methyltransferase gene from Neisseria gonorrhoeae P9: homologies with other enzymes recognizing the sequence 5'-GGCC-3'. Nucleic Acids Res. 1988 May 25;16(10):4369–4387. doi: 10.1093/nar/16.10.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Robbins K., Barrera O., Koomey J. M. Gene conversion variations generate structurally distinct pilin polypeptides in Neisseria gonorrhoeae. J Exp Med. 1987 Apr 1;165(4):1016–1025. doi: 10.1084/jem.165.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai W. M., Larsen S. H., Wilde C. E., 3rd Cloning and DNA sequence of the omc gene encoding the outer membrane protein-macromolecular complex from Neisseria gonorrhoeae. Infect Immun. 1989 Sep;57(9):2653–2659. doi: 10.1128/iai.57.9.2653-2659.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil M. D., McClelland M. Enzymatic cleavage of a bacterial genome at a 10-base-pair recognition site. Proc Natl Acad Sci U S A. 1989 Jan;86(1):51–55. doi: 10.1073/pnas.86.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. E., Clark V. L. Genetic loci and linkage associations in Neisseria gonorrhoeae and Neisseria meningitidis. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S92–103. doi: 10.1128/cmr.2.suppl.s92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods J. P., Dempsey J. F., Kawula T. H., Barritt D. S., Cannon J. G. Characterization of the neisserial lipid-modified azurin bearing the H.8 epitope. Mol Microbiol. 1989 May;3(5):583–591. doi: 10.1111/j.1365-2958.1989.tb00205.x. [DOI] [PubMed] [Google Scholar]
- Woods J. P., Spinola S. M., Strobel S. M., Cannon J. G. Conserved lipoprotein H.8 of pathogenic Neisseria consists entirely of pentapeptide repeats. Mol Microbiol. 1989 Jan;3(1):43–48. doi: 10.1111/j.1365-2958.1989.tb00102.x. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ley P. Three copies of a single protein II-encoding sequence in the genome of Neisseria gonorrhoeae JS3: evidence for gene conversion and gene duplication. Mol Microbiol. 1988 Nov;2(6):797–806. doi: 10.1111/j.1365-2958.1988.tb00091.x. [DOI] [PubMed] [Google Scholar]