Abstract
Background
Semen cryopreservation is a widely available method of maintaining fertility in male cancer patients. However this facility is not always used.
Aims
To identify the barriers to successful sperm banking in a group of adolescent and young adult patients.
Methods
Questionnaires were administered to 55 patients aged 13–21 years who had received potentially gonadotoxic therapy between 1997 and 2001 and had been offered sperm banking.
Results
Forty five questionnaires were completed; 67% of respondents were able to bank sperm. Those who had been unsuccessful were younger and described higher levels of anxiety at diagnosis and greater difficulty in talking about fertility. They also described less understanding of sperm banking at the time of diagnosis.
Conclusion
Most adolescent cancer patients who have been offered fertility preservation are able to bank sperm. Younger patients may be helped by the provision of high quality information and more open discussion of the technique.
Keywords: adolescent cancer, fertility preservation
Improvements in treatment for childhood cancer have been such that 1 in 1000 of the general population are now long term survivors.1 One consequence of this is an increased interest in and understanding of the long term side effects of treatment. A major side effect of many therapies in young men is infertility. The germinal epithelium of the testis, from which the spermatozoa develop, is very sensitive to the effects of a range of drugs, including cisplatin, procarbazine, and alkylating agents such as cyclophosphamide and chlorambucil. These agents form part of the treatment protocols for many common malignancies, including Hodgkin's and non‐Hodgkin's lymphoma, osteosarcoma, Ewing's sarcoma, and testicular germ cell tumours. When followed up at a median interval of 11.6 years after treatment for a range of childhood malignancies, 10/33 young men were azoospermic (no sperm) and 6/33 were oligospermic (<20×106 sperm per ml).2 Testicular radiation and high doses of cyclophosphamide are strongly associated with poor sperm counts.3 Treatment for Hodgkin's disease is a particular problem as regimes containing both chlorambucil and procarbazine can induce azoospermia in as many as 97% of patients.4,5,6
Cryopreservation of a semen sample obtained by masturbation is the only widely available method for the preservation of fertility in these patients. Patients as young as 13 have been shown to have normal sperm counts and no differences have been described in sperm concentrations, motility, and morphology between boys aged 14–17 and adults (>20 years).7,8 Thirty three of 115 Hodgkin's disease patients used their cryopreserved semen in the decade after finishing treatment, and 30% of these were associated with a successful pregnancy.9 Despite the availability and efficacy of semen cryopreservation it is still not offered routinely. Only 51% of male cancer patients aged 14–40 were offered sperm banking, and only 24% had successfully stored semen, despite 77% of men who were childless at the time of treatment wanting to conceive children in the future.10 Despite over 90% of oncologists feeling that sperm banking should be offered to all men at risk of infertility, 48% either never discussed it or only did so with a minority of patients.11
UK recommendations are that sperm banking should be offered to all sexually mature boys at risk of infertility,12 and almost all UK paediatric oncology centres offer it to sexually mature adolescents under the age of 18 years.13 Future fertility is a sensitive topic, and it is difficult to find the appropriate time to discuss it with an adolescent who has just been diagnosed with cancer. As a result of this a proportion of post‐pubertal patients fail to bank sperm. We conducted this study to identify what proportion of our patients were able to store semen, and what factors affected their success or failure.
Methods
Male patients, aged between 13 and 21 years at diagnosis, diagnosed between 1997 and 2001, were identified from the Manchester Children's Tumour Registry and from hospital records at Royal Manchester Children's Hospital and the Christie Hospital. Patients who had not received gonadotoxic treatment were excluded, as were patients who had died between diagnosis and the time of interview in 2001. We did not include patients diagnosed more than four years prior to the interview date as we felt that they would not reliably recall events at this interval. We identified a total of 171 patients alive in 2001 who had received potentially gonadotoxic therapy containing alkylating agents, procarbazine, cisplatin, or radiotherapy involving the testes. Those patients who had been offered sperm banking were identified by the documentation of this in their hospital notes. Ethical constraints prevented us from asking all 171 patients whether they had been offered sperm banking, and the legal restrictions of the HFEA prevented us from identifying patients with semen stored in the local assisted conception unit (ACU). We identified 55 patients where discussion of semen cryopreservation was documented in their notes. In order to discover as much as possible about these patients' experience, a simple two page questionnaire was designed to elucidate their recall of events and feelings around the time of discussion of sperm banking and their feelings at the time of administration (see ADCwebsite: http://www.archdischild.com/supplemental). Patients were asked to score their feelings about their future fertility, their diagnosis, and their understanding of sperm banking both at diagnosis and at time of interview, and the ease with which they and their families could talk about fertility, on simple 1 to 10 visual analogue scales. Patients were encouraged to describe their experience of sperm banking in as much detail as they wished. The questionnaire was administered after obtaining informed consent, either face to face at clinic visits, or by post. The study received ethical approval from Salford and Trafford and South Manchester LRECs. Comparisons between groups were made using the Wilcoxon signed ranks test.
Results
Forty five of 55 questionnaires were completed. The mean age of the cohort at the time of diagnosis was 17.1 years (12–21), and the mean age at the time of completing the questionnaire was 19.2 years (15–24). The average interval between diagnosis and interview was 2.1 years (0–5). Twenty five patients were offered sperm banking within a week of their diagnosis, and a further 13 between 1 and 4 weeks after diagnosis. For the others discussion took place at intervals up to 6 months. For 32 of 45 patients a member of the medical staff had discussed sperm banking, and for 9 of 45 a member of nursing staff had discussed this. Eighty one per cent of patients with whom sperm banking was discussed by medical staff were successful, while 67% of those with whom sperm banking was discussed by nursing staff were successful; this difference was not significant. Both parents were present at this discussion for 13 patients, just the mother for 12, just the father for 7, and for 8 patients no‐one else was present. Thirty of 45 patients (67%) who responded were successful in producing a semen sample that was suitable for cryopreservation. The range of diagnoses in this group are shown in table 1; as expected, Hodgkin's disease and osteosarcoma predominate.
Table 1 The distribution of diagnoses in the patient cohort.
| Diagnosis | Gonadotoxic therapy | No. patients |
|---|---|---|
| Hodgkin's disease | Procarbazine, chlorambucil | 12 |
| Osteosarcoma | Doxorubicin, cisplatin | 10 |
| Testicular tumours | Cisplatin | 9 |
| Non‐Hodgkin's lymphoma | Cyclophosphamide | 3 |
| Other | Ifosfamide, melphalan, TBI | 11 |
TBI, total body irradiation.
Fifteen of 45 patients were unable to bank sperm. Of these patients, three were too ill at the time of diagnosis to complete the process, and one was pre‐pubertal. These four patients were unsuccessful for reasons beyond their control and as we were interested in the potentially reversible causes of failure to sperm bank we have excluded these patients from this comparison. Two patients were able to produce a sperm sample which was azoospermic and therefore not suitable for cryopreservation; as they were able to produce a semen sample these two patients have been included in the successful group for the purposes of this comparison. Both of these azoospermic patients were 21 years at diagnosis, had testicular germ cell tumours, and went for sperm banking before any therapy was given. We thus compared a group of 32 patients who were successful in producing a semen sample with a group of 9 patients who were unsuccessful.
Age
Patients who were unable to produce a sperm sample were significantly younger than those who were successful. The average age at diagnosis for the unsuccessful group was 15.3 years (range 14–18 years) compared to 17.8 years for the successful group (range 12–21 years) (p < 0.05). We also evaluated the 10 patients who did not return the questionnaire. The mean age of these patients was 17.7 years (15–20); Hodgkin's disease and osteosarcoma were the predominant diagnoses. Four of these 10 patients were unsuccessful in sperm banking, and three of these four declined sperm banking.
Concern about future fertility
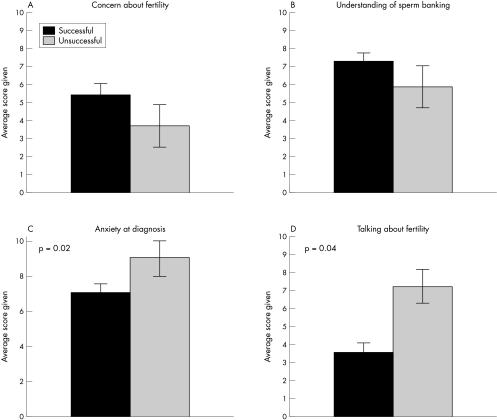
Patients were asked to rate their concern about their future fertility at the time of diagnosis and at the time of interview. Average concern was significantly greater at time of interview than at time of diagnosis. Individual patients' comments were typically focused on the diagnosis rather than fertility: “When I was first diagnosed I was concerned only about the cancer, nothing else bothered me”, “I am now concerned about getting better and will cross fertility when and if it becomes an issue”. When we compared the successful and unsuccessful groups we found that the concern about future fertility was higher in the successful group, although this difference did not reach significance (fig 1A).
Figure 1 Comparisons between patients who were unable to produce a semen sample (n = 9) and those who were successful (n = 32). (A) Patient recalled average concern about fertility at the time of diagnosis. (B) Patient recalled average understanding of sperm banking at the time of diagnosis. (C) Reported levels of anxiety at the time of diagnosis. (D) Reported difficulty in talking about fertility.
Understanding of sperm banking
The average understanding of sperm banking at diagnosis was high with an average score of 7.2 out of 10, although this was significantly greater at the time of interview. When the two groups were compared, the understanding of sperm banking was lower in the unsuccessful group, although again this difference did not reach significance (fig 1B).
Anxiety
Reported anxiety levels at the time of diagnosis were significantly greater than at the time of interview. Fifty eight per cent of patients felt that their levels of anxiety had affected their ability to think about fertility. When the two groups were compared, reported anxiety levels were significantly greater in the unsuccessful group (p = 0.02) (fig 1C).
Difficulties in discussing fertility
Those who were able to produce a semen sample reported significantly less difficulty in discussing fertility than those who were unsuccessful (p = 0.04) (fig 1D). There was no difference between the reported difficulties in discussing fertility as individuals and the reported difficulty in talking with their families.
One of our aims in this study was to identify potentially reversible barriers to successful sperm banking. Most patients felt that the facilities provided were adequate (although these consisted of a small plain room and old pornographic magazines), and that the staff were helpful and supportive. Most patients went without their parents and those who were accompanied expressed embarrassment at this. Most patients had a positive or at least neutral view of the experience. However a small group of patients had very negative feelings including finding it “incredibly humiliating and stressful” and “personally humiliating and emotionally draining”. Many patients felt that more detailed information would help others in the future.
Discussion
In order to give patients the opportunity of future fertility it is important that sperm banking is offered routinely to all post‐pubertal males. This is the first study of sperm banking in an adolescent and young adult population. We show that most patients who are given the opportunity are able to sperm bank. These facilities are available in 20 of 22 UK paediatric oncology centres, with 85% of centres reporting use of semen cryopreservation.13 If this procedure is to become routine, then two hurdles must be overcome. Firstly sperm banking must be discussed with all sexually mature patients who are to undergo treatment that may be gonadotoxic, and secondly we must understand the reasons why some patients are unable to store semen even when it is offered to them.
In our study we were only able to identify 55 of 171 patients with a record of discussion of sperm banking. There may have been other patients with whom this was discussed but not documented, but it is also possible that a significant number of patients received no information about fertility preservation. There are many potential reasons for this lack of discussion. There may have been doubts about the gonadotoxicity of the treatment, the patient may have been judged to be unable to produce ejaculate, or there may have simply been a reluctance to discuss a potentially embarrassing subject. A recent study showed that only 10% of oncologists offered sperm banking routinely to all men at risk of infertility, and the commonest reason for failure to bank was failure to offer the opportunity.10 We feel it is better to involve the patient in a full discussion about fertility preservation and for them to have the opportunity to consider these issues rather than to make decisions on their behalf.
In our cohort of patients who were offered semen cryopreservation, two thirds were successful in producing a sample, although this was not always suitable for cryopreservation. The remaining third were significantly younger, reported more anxiety at the time of diagnosis, and had more difficulty in talking about fertility. The majority of patients felt that their levels of anxiety had prevented them from thinking clearly about fertility preservation, and this proportion was higher in the unsuccessful group (71% versus 53%). These patients did not have particularly strong feelings about the facilities available at the ACU; most were positive or neutral about the helpfulness of the staff and the room and material provided. Interestingly eight of the nine patients who were unable to produce a semen sample did not get to the ACU; one recurring theme was that most patients would have preferred not to travel to the ACU for sperm banking, wanting either to do this at the oncology unit or at home. Alternative strategies to masturbation have been used to obtain semen samples, although most are not widely available. Rectal electrostimulation, epididymal aspiration, and testicular biopsy can all obtain viable spermatozoa.14 There may also be justification for the cryopreservation of oligospermic samples as advances in assisted fertilisation techniques, such as intracytoplasmic sperm injection, may allow successful fertilisation in the future.
The initial discussion about sperm banking was held promptly with the majority of this cohort, and for most patients it was conducted by their oncologist. There was variation in the people present at this discussion, but for 32 of 45 patients either one or both parents were present. We did not find that patients were particularly concerned about their parents being present at this discussion although this has been previously suggested,11 but they were unhappy with their parents accompanying them to the ACU. Many patients commented that they felt the process would have been easier had there been more information available, particularly about the practical issues of sperm banking. Previous work has suggested that nurses are not comfortable when discussing sexuality with this group of patients, but suggested that with appropriate training they could provide support by addressing these practicalities.15
What is already known on this topic
Sperm banking is the only widely available method of fertility preservation
Access to this facility is widespread in the UK, but the use of this facility by adolescent cancer patients is not known, nor have potential problems with its use been evaluated
In conclusion, the majority of adolescent cancer patients are able to store viable semen when offered the opportunity. Those who failed to bank sperm were younger, had greater levels of anxiety at diagnosis, and more difficulty in talking about fertility. This study supports the recommendation that semen cryopreservation should be offered as a routine procedure to all sexually mature adolescents at risk of fertility impairment. In addition, we feel that the provision of high quality information, sperm banking at the oncology unit rather than at the ACU, and specific training for medical and nursing staff in dealing with these vulnerable patients may increase the success rate still further to allow all patients to have a chance of fertility preservation. However much we improve these services it is important not to forget that it is unlikely that this experience can ever be easy:
“Conceiving a child was supposed to be wreathed in hope, not this sad, solitary procedure. I had no choice; I closed my eyes and did what I had to do.”16
What this study adds
This is the first study of the experience of sperm banking in this age group of patients. Most patients who had been documented as having been offered sperm banking were successful. Failure to sperm bank was associated with younger age
Sperm banking should be discussed as a routine part of the care of this group of patients
The questionnaire is available on the ADCwebsite: http://www.archdischild.com/supplemental
Copyright ©2006 BMJ Publishing Group & Royal College of Paediatrics and Child Health
Supplementary Material
Acknowledgements
The authors would like to thank all consultants at Royal Manchester Children's and Christie Hospitals who gave permission for their patients to be included in this study.
Footnotes
Competing interests: none
The questionnaire is available on the ADCwebsite: http://www.archdischild.com/supplemental
References
- 1.Hawkins M M, Stevens M C. The long term survivors. Br Med Bull 199652898–923. [DOI] [PubMed] [Google Scholar]
- 2.Thomson A B, Campbell A J, Irvine D S.et al Semen quality and spermatozoal DNA integrity in survivors of childhood cancer: a case‐control study. Lancet 2002360361–367. [DOI] [PubMed] [Google Scholar]
- 3.Relander T, Cavallin‐Stahl E, Garwicz S.et al Gonadal and sexual function in men treated for childhood cancer.Med Pediatr Oncol 20003552–63. [DOI] [PubMed] [Google Scholar]
- 4.Viviani S, Santoro A, Ragni G.et al Gonadal toxicity after combination chemotherapy for Hodgkin's disease. Comparative results of MOPP vs ABVD. Eur J Cancer Clin Oncol 198521601–605. [DOI] [PubMed] [Google Scholar]
- 5.Clark S T, Radford J A, Crowther D.et al Gonadal function following chemotherapy for Hodgkin's disease: a comparative study of MVPP and a seven‐drug regime. J Clin Oncol 199513134–139. [DOI] [PubMed] [Google Scholar]
- 6.Ortin T T, Shostak C A, Donaldson S S. Gonadal status and reproductive function following treatment for Hodgkin's disease in childhood: the Stanford experience. Int J Radiat Oncol Biol Phys 199019873–880. [DOI] [PubMed] [Google Scholar]
- 7.Muller J, Sonksen J, Sommer P.et al Cryopreservation of semen from pubertal boys with cancer. Med Pediatr Oncol 200034191–194. [DOI] [PubMed] [Google Scholar]
- 8.Kliesch S, Behre H M, Jurgens H.et al Cryopreservation of semen from adolescent patients with malignancies. Med Pediatr Oncol 19962620–27. [DOI] [PubMed] [Google Scholar]
- 9.Blackhall F H, Atkinson A D, Maaya M B.et al Semen cryopreservation, utilisation and reproductive outcome in men treated for Hodgkin's disease. Br J Cancer 200287381–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schover L R, Brey K, Lichtin A.et al Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J Clin Oncol 2002201880–1889. [DOI] [PubMed] [Google Scholar]
- 11.Schover L R, Brey K, Lichtin A.et al Oncologists' attitudes and practices regarding banking sperm before cancer treatment. J Clin Oncol 2002201890–1897. [DOI] [PubMed] [Google Scholar]
- 12.Wallace W H B, Thomson A B. Preservation of fertility in children treated for cancer. Arch Dis Child 200388493–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glaser A W, Phelan L, Crawshaw M.et al Fertility preservation in adolescent males with cancer in the United Kingdom: a survey of practice. Arch Dis Child 200489736–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grundy R, Gosden R G, Hewitt M.et al Fertility preservation for children treated with cancer: scientific advances and research dilemmas. Arch Dis Child 200184355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinn B, Kelly D. Sperm banking and fertility concerns: enhancing practice and the support available to men with cancer. Eur J Oncol Nursing 2000455–58. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong L, Jenkins S.It's not about the bike: my journey back to life. New York: Penguin Putnam, 2000
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.