Abstract
Background
Mortality from SIDS has declined since the recommendation that infants are not placed prone to sleep. SIDS mortality is higher in infants born preterm than those born at term.
Aim
To determine if risk factors for SIDS are any different for preterm and term infants.
Methods
Mortality data over time were used to determine whether the reduction in SIDS mortality rates had occurred equally in term and preterm infants. Data from two New Zealand studies (a case‐control study and a case‐cohort study) were used to determine if any differences existed in risk factors for SIDS between term and preterm infants before and after the SIDS prevention campaign.
Results
SIDS mortality appears to have decreased by similar proportions in term and preterm infants. Risk factors for SIDS were similar in preterm and term infants, except for parity where there was a significant interaction. Increasing parity was a risk factor for SIDS in term infants but not preterm infants.
Conclusion
SIDS rates have decreased at comparable rates in term and preterm infants, but preterm birth still remains a risk factor for SIDS. The magnitude of the odds ratios associated with modifiable risk factors were similar for both groups. There may however be a difference in risk associated with parity between term and preterm infants. The messages for risk factors for SIDS are applicable to mothers of preterm as well as term infants.
Keywords: SIDS, preterm, risk factors, parity
Preterm birth is well recognised as a risk factor for sudden infant death syndrome (SIDS), with the risk of SIDS increasing as gestation decreases.1,2 We have reported an increased risk of SIDS associated with preterm infants both before2 and after3 the change in prevalence of prone sleeping position and the subsequent reduction in SIDS. The preterm birth rate has been increasing in the developed world over the last 20 years.4,5,6 Thus the proportion of SIDS deaths associated with preterm births may have increased.
Oyen et al have reported that the risk from prone and side sleep position are increased among infants born preterm compared to term infants.7 No other study has examined whether or not risk factors for SIDS are the same for preterm infants. The aim of this study is to examine whether the risk factors for SIDS and their magnitude are the same for term and preterm infants.
Methods
Three sources of data were used for these analyses. The first was national mortality data, which were collated from yearly reports of fetal and infant deaths published by the Ministry of Health.8 Data reported here are for the time period 1986 to 2000, as prior to 1986 data are not available by gestational age category. Preterm birth for the national mortality data was defined as less than 38 weeks completed gestational age as the information in most of the reports was coded categorically (<30, 30–34, 35–37, 38–39, 40+, and unknown).
The second data source was the New Zealand Cot Death Study, which has been described in detail previously.2,9 In brief it was a nationwide case‐control study from November 1987 to October 1990, examining risk factors for SIDS. Most of the data collected preceded the SIDS Prevention Campaign.10 There were 716 post‐neonatal deaths, of which 485 were classified as SIDS. Obstetric records were examined in 465 (95.9%), and 393 (81.0%) parents were interviewed. They were compared with 1800 randomly sampled control subjects, who were representative of all births in the study regions during the same time period. Obstetric records were examined in 1762 (97.9%), and 1592 (88.4%) parents were interviewed. The parents of control subjects were interviewed about infant care practices on a nominated date, which was randomly allocated so that the age distribution of the controls was the same as that expected for the cases. Data collected included information on sociodemographic variables (region, marital status season, socioeconomic status, ethnicity, age mother left school), antenatal factors (attendance at antenatal care, antenatal classes), obstetric history (age of mother at first pregnancy, age of mother at pregnancy, parity, caffeine intake in last trimester, alcohol during pregnancy, urinary tract infection, multiple birth), infant factors (infant gender, infant age, birth weight, admission to the neonatal unit, apnoea), and childcare practices (sleep position, maternal smoking, breast feeding at discharge from hospital, sharing a bed with an adult, dummy use).
The third source of data was a prospective case‐cohort study that took place for all live births in New Zealand between October 1991 and September 1993 (approximately 118 000 births). This was after the launch of the SIDS Prevention Campaign in New Zealand. The data came from all SIDS cases in this time period and a random sample of all other infants. In the two year cohort there were 232 post‐neonatal SIDS deaths and a total of 1200 controls sampled. Data were available for 127 cases (54.7%) and 922 controls (76.8%). Data were recorded by nurses at two points in time, at the first contact (approximately 3 weeks of age) and at 2 months of age. The full methods and variables collected have been published previously.3 The low response rate among the cases and differences between the responding and non‐responding cases means caution is required to extrapolate the results in general; however the prospective nature of the data collection meant that differential selection bias was not present and the results are valid within the sample.
For analysis of the data from the case‐control and case‐cohort studies, preterm birth was defined as less than 37 weeks completed gestation. This differs from the Ministry of Health data but does not affect the analyses regarding risk factors.
The studies had approval from each of the local ethics committees.
Statistical methods
Logistic regression was used to estimate odds ratios, both univariately and after adjusting for possible confounders. Analysis was carried out in SAS.
For multivariate analysis of gestational age the multivariate models were reduced to include only variables available for both the case‐control and case‐cohort studies to make them comparable. Variables included were infant gender, ethnicity, maternal age at pregnancy, marital status, age mother left school, parity, birth weight, sleep position, breast feeding at discharge, maternal smoking, bed sharing, and gestational age.
Variables in the final multivariate model for the case‐control study were reduced to include only those that remained significant and variables of particular interest in terms of comparisons between the preterm and term groups. Potential confounders remaining in the final multivariate model included sociodemographic (region, marital status, season), antenatal (timing of antenatal care), obstetric (maternal age, parity, smoking, caffeine, alcohol, urinary tract infection, multiple pregnancy), infant (infant age, gender, birth weight, admission to neonatal unit, apnoea), and childcare practices (sleep position, breast feeding, bed sharing, dummy use). Overall effects of independent variables were calculated using χ2 statistics estimated from the logistic regressions. Interactions were tested by carrying out logistic regression on the combined groups; interaction terms were constructed between an indicator variable for preterm status and the variables of interest. Interaction terms were added to both univariate and multivariate logistic regressions. A significant interaction implies that the odds ratios were different (p < 0.05) between the two groups.
Differences in age distributions were tested using t tests.
Results
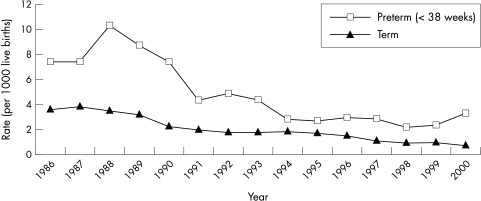
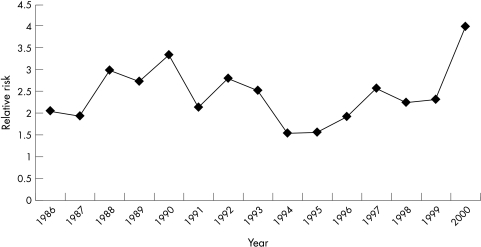
SIDS mortality in New Zealand reached a peak in 1988 at a rate of 4.4 per 1000 live births and declined steeply in 1991. Figure 1 shows the SIDS mortality rates in those born preterm and those born at term. Although there is some fluctuation in the ratio of preterm and term SIDS rates, there was no statistically significant overall trend in this ratio, suggesting that the effect of the prevention programme on preterm SIDS has been the same as that for the term infants (fig 2).
Figure 1 Rate of term and preterm SIDS by registration year.
Figure 2 Relative risk of preterm to term SIDS rates.
The only major change that has occurred between the case‐control study and the case‐cohort study is that of sleep position. In New Zealand during the case‐control study (1987–90) the prevalence of prone sleeping was 33% in the general infant population. In the cohort study (1991–93) the proportion of infants sleeping prone at the initial visit (mean = 2.6 weeks) was 0.7% and at 2 months was 3.0%.
Data from both the case‐control and case‐cohort studies showed that the shorter the gestational age the higher the risk of SIDS (table 1).
Table 1 Risk of SIDS by gestational age.
| Gestational age | Cases | Controls | Univariate odds ratio (95%CI) | Multivariate* odds ratio (95%CI) |
|---|---|---|---|---|
| Case‐control study (1987–90) | ||||
| <37 | 88 (19.0) | 83 (4.7) | 6.04 (3.71 to 9.82) | 2.75 (1.41 to 5.38) |
| 37–38 | 87 (18.8) | 252 (14.4) | 2.27 (1.58 to 3.26) | 1.52 (0.94 to 2.46) |
| 39–40 | 227 (49.0) | 1016 (58.0) | 1.47 (1.08 to 1.99) | 1.24 (0.85 to 1.81) |
| 41+ | 61 (13.2) | 401 (22.9) | 1.00 | 1.00 |
| Case‐cohort study (1991–93) | ||||
| <37 | 21 (17.2) | 53 (6.0) | 3.57 (1.81 to 7.05) | 6.57 (1.23 to 35.17) |
| 37–38 | 16 (13.1) | 117 (13.1) | 1.19 (0.59 to 2.36) | 1.86 (0.41 to 8.38) |
| 39–40 | 64 (52.5) | 540 (60.5) | 1.03 (0.61 to 1.73) | 1.69 (0.53 to 37.16) |
| 41+ | 21 (17.2) | 182 (20.4) | 1.00 | 1.00 |
*Multivariate model controls for infant gender, ethnicity, maternal age at pregnancy, marital status, age mother left school, parity, birth weight, sleep position, breast feeding at discharge, maternal smoking, and bed sharing.
Regrouping this data to define the risk for preterm infants (<37 weeks completed gestation) compared with term infants (37+ weeks gestation) found an odds ratio (OR) in the case‐control study of 4.72 (95% CI 3.43 to 6.50). This odds ratio reduced in multivariate analysis to 2.11 (95% CI 1.19 to 3.74). In the case‐cohort study the univariate risk of SIDS among those born <37 weeks gestation was OR = 3.42 (95% CI 1.98 to 5.92), and after adjustment was OR = 4.08 (95% CI 1.09 to 15.32).
Table 2 shows the univariate and multivariate odds ratios for SIDS risk factors for preterm and term infants in the case‐control study. Only the four modifiable (prone sleep position, maternal smoking, not breast feeding, bed sharing) risk factors and those variables that showed a significant interaction between preterm and term infants are shown. All the modifiable risk factors were significant risk factors for both term and preterm infants, and none showed a significant interaction with preterm status. At the univariate level there was a significant interaction with infant age, parity, and multiple births. Young infant age was a risk factor for SIDS for term infants but not for preterm infants. Preterm infants showed no significant increase in risk for any parity; however for term infants the risk of SIDS increased with increasing parity. Multiple birth was also a SIDS risk for term but not for preterm infants.
Table 2 The univariate and multivariate odds ratios for SIDS risk factors for preterm and term infants in the case‐control study.
| Preterm | Term | Univariate interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Univariate | Multivariate* | Cases | Controls | Univariate OR | Multivariate* | ||
| n | n | OR (95% CI) | OR (95% CI) | n | n | (95% CI) | OR (95% CI) | ||
| Parity | 0.01 | ||||||||
| 0 | 30 | 24 | 1.00 | 1.00 | 64 | 514 | 1.00 | 1.00 | |
| 1 | 16 | 24 | 0.53 (0.23 to 1.22) | 0.52 (0.11 to 2.39) | 108 | 483 | 1.80 (1.29 to 2.51) | 3.29 (2.02 to 5.39) | |
| 2+ | 42 | 35 | 0.96 (0.48 to 1.93) | 0.63 (0.12 to 3.29) | 203 | 671 | 2.43 (1.79 to 3.29) | 4.56 (2.76 to 7.52) | |
| Smoking | 0.44 | ||||||||
| No | 17 | 47 | 1.00 | 1.00 | 113 | 1028 | 1.00 | 1.00 | |
| Yes | 53 | 28 | 5.23 (2.55 to 10.74) | 6.64 (1.74 to 25.37) | 203 | 477 | 3.87 (3.00 to 4.99) | 1.79 (1.28 to 2.52) | |
| Multiple pregnancy | 0.04 | ||||||||
| Yes | 14 | 14 | 1.09 (0.48 to 2.49) | 12 | 17 | 3.46 (1.63 to 7.31) | |||
| No | 56 | 61 | 1.00 | 1.00 | 304 | 1489 | 1.00 | 1.00 | |
| Infant age | 0.04 | ||||||||
| <13 | 22 | 30 | 0.65 (0.29 to 1.43) | 0.57 (0.13 to 2.43) | 175 | 693 | 1.91 (1.39 to 2.63) | 1.84 (1.24 to 2.71) | |
| 13–20 | 23 | 23 | 0.88 (0.39 to 1.99) | 1.33 (0.26 to 6.73) | 83 | 375 | 1.67 (1.16 to 2.40) | 1.80 (1.16 to 2.78) | |
| 20+ | 25 | 22 | 1.00 | 1.00 | 58 | 438 | 1.00 | 1.00 | |
| Sleep position | 0.89 | ||||||||
| Prone | 44 | 22 | 4.00 (2.00 to 8.02) | 10.97 (3.01 to 39.95) | 203 | 496 | 3.80 (2.94 to 4.92) | 4.43 (3.23 to 6.09) | |
| Non‐prone | 26 | 52 | 1.00 | 1.00 | 108 | 1003 | 1.00 | 1.00 | |
| Breast feeding | 0.78 | ||||||||
| Yes | 40 | 53 | 1.00 | 1.00 | 273 | 1416 | 1.00 | 1.00 | |
| No | 47 | 28 | 2.22 (1.19 to 4.15) | 10.05 (2.34 to 43.22) | 98 | 252 | 2.02 (1.55 to 2.63) | 1.81 (1.25 to 2.62) | |
| Bed sharing | 0.25 | ||||||||
| No | 50 | 69 | 1.00 | 1.00 | 243 | 1341 | 1.00 | 1.00 | |
| Yes | 20 | 6 | 4.60 (1.72 to 12.28) | 17.17 (2.22 to 132.62) | 71 | 157 | 2.50 (1.83 to 3.41) | 1.92 (1.25 to 2.95) | |
Only the four modifiable (prone sleep position, maternal smoking, not breast feeding, bed sharing) risk factors and those variables that showed a significant interaction between preterm and term infants are shown.
*Multivariate model controls for model included region, marital status, season, timing of antenatal care, maternal age, parity, smoking, caffeine, alcohol, urinary tract infection, multiple pregnancy, infant age, gender, birth weight, admission to neonatal unit, apnoea, sleep position, breast feeding, bed sharing, and dummy use.
In multivariate analysis containing the variables listed in the methods and the three interaction terms that were found to be significant at the univariate level, the only interaction term that remained significant was that of parity (p < 0.01).
Table 3 shows the combined effect of preterm birth and prone sleeping position. Even though the risk for infants born preterm and sleeping prone is 18.57 (95% CI 10.73 to 31.16) times that of infants born at term and sleeping non‐prone, there is no interaction (χ2 = 0.02, p = 0.89) and this magnitude of risk is what one would anticipate from the combined effect of preterm birth and prone sleeping position.
Table 3 Numbers and odds ratios for sleep position and preterm combinations in the case‐control study.
| Cases | Controls | Univariate | Multivariate* | ||
|---|---|---|---|---|---|
| n | n | OR | OR | ||
| Term | Non‐prone | 108 | 1003 | 1.00 | 1.00 |
| Term | Prone | 203 | 496 | 3.80 (2.94 to 4.92) | 4.91 (3.57 to 7.74) |
| Preterm | Non‐prone | 26 | 52 | 4.64 (2.79 to 7.74) | 2.44 (1.30 to 4.97) |
| Preterm | Prone | 44 | 22 | 18.57 (10.73 to 31.16) | 17.40 (8.15 to 37.16) |
*Multivariate model controls for model included region, marital status, season, timing of antenatal care, maternal age, parity, smoking, caffeine, alcohol, urinary tract infection, multiple pregnancy, infant age, gender, birth weight, admission to neonatal unit, apnoea, sleep position, breast feeding, bed sharing, and dummy use.
The number of preterm infants in the case‐cohort study was small (n = 74; 21 SIDS, 53 controls) which limited the power to detect interactions. However, no obvious differences were noted in the magnitude of the odds ratios between the preterm and term groups. Furthermore, the odds ratios for the term group were similar to those from the case‐control study. In contrast to the case‐control study in the case‐cohort study the preterm group also showed an increasing risk of SIDS with increased parity (though not statistically significant) at the univariate level (table 4). In this study we also found no preterm infants sleeping prone at an initial visit; one infant that later died was the only preterm infant that was sleeping prone at 2 months. At this time 3.9% of term infants were still sleeping prone.
Table 4 Odds ratio for parity for preterm and term infants in the case‐cohort study.
| Parity | Preterm | Term | ||||
|---|---|---|---|---|---|---|
| Cases | Controls | Univariate OR | Cases | Controls | Univariate OR | |
| n | n | (95% CI) | n | n | (95% CI) | |
| 0 | 4 | 17 | 1.00 | 14 | 251 | 1.00 |
| 1 | 4 | 14 | 1.21 (0.26 to 5.76) | 22 | 220 | 1.79 (0.90 to 3.59) |
| 2+ | 13 | 19 | 2.91 (0.79 to 10.65) | 58 | 337 | 3.09 (1.68 to 5.66) |
The preterm SIDS infants in the case‐control study were on average older at death than the term SIDS infants (18.1 v 14.4 weeks, p < 0.01) but in terms of post‐conceptional age they are 2.3 weeks younger (11.7 v 14.0 weeks, p = 0.05). The case‐cohort study showed similar results with preterm SIDS older at death than the term SIDS (18.8 v 15.9 weeks) but younger in terms of post‐conceptional age (12.2 v 15.8 weeks). However, these differences were not statistically significant.
In summary, the only risk factor that differed between the term and preterm was parity, which was found only to be a risk among the term infants in the case‐control study.
Discussion
Preterm birth continues to be a risk factor for SIDS, and the multivariate odds ratio for preterm birth from the retrospective case‐control study prior to the prevention campaign in 1991 and the prospective case‐cohort study after the prevention campaign are similar. Over the 15 years of national data the relative risk for SIDS associated with preterm birth did not change. This contrasts with the study of Alm and colleagues11 that showed the risk of SIDS with preterm birth increased in the period of falling SIDS incidence.
There has been a decrease in SIDS rates throughout the world, much of which has been attributed to the substantial decrease in the number of infants sleeping in the prone position.12 We have shown a significant decrease in SIDS rates among both term and preterm infants, indicating that prone sleep position is a causal risk factor for both preterm and term infants.
The major change that has taken place over time in relation to infant care practices and SIDS has been the reduction of the proportion of infants sleeping prone.12,13 The prevalence of infants sleeping prone was 3.9% in term infants and there were no preterm infants sleeping prone, which suggests that there may have been a greater awareness of the risk of the prone sleep position among mothers of preterm infants.
The point estimates of odds ratios we have reported here for the four main modifiable risk factors2 (prone sleep position, smoking, bed sharing, not breast feeding) for the preterm infants are similar to those for the term infants. Although the odds ratio for prone sleeping position in infants born preterm is high (OR = 18.57) compared with term infants sleeping non‐prone; this magnitude is what would be expected from the combination of two major risk factors for SIDS. This finding is similar to that from the Nordic SIDS Epidemiological Study. Although they reported a significant additive interaction between prone sleeping position and preterm birth, the odds ratios presented would indicate that there was no multiplicative interaction.7
We found an interaction between infant age at death and preterm status and the risk of SIDS at the univariate level; however this was not significant in multivariate analysis. Malloy et al have reported that the post‐conceptional age of peak vulnerability for SIDS may be 4–6 weeks later for preterm infants compared to term infants.14 It has been hypothesised that differences in post‐conceptional age between preterm and term SIDS are related to maturity and prone sleep position. However a prospective survey on very preterm and very low birth weight infants in the Netherlands found that postnatal age at death in these infants did not differ significantly from age at death in other SIDS infants.15 The preterm SIDS infants in our case‐control study are on average older at death than the term SIDS infants (18.1 v 14.4 weeks), but in terms of post‐conceptional age they are slightly younger (11.7 v 14.0 weeks).
The strongest interaction we found at the univariate level and the only one that remained statistically significant at the multivariate level was that associated with parity. The results showed an increasing risk of SIDS with increased parity among the term infants similar to the commonly reported association between parity and SIDS overall.2,16 There was however no increase in risk associated with parity among the preterm infants. We were unable to show a similar interaction in the case‐cohort study.
Over the last two decades there has been an increasing rate of preterm birth, which we have shown to be increasing at a greater rate in the higher socioeconomic groups.6 Also there has been a change in the demography of women giving birth, with average maternal age increasing and parity decreasing over this same time period. This may provide a reason for the changed risk with parity and preterm status.
What is already known on this topic
SIDS mortality is higher in infants born preterm than those born at term, both before and after the SIDS prevention campaign which recommended that infants should not be placed prone to sleep
In summary, the only factor that showed any interaction in multivariate model was parity, and that may be a chance finding. This study indicates that the risk factors for SIDS are similar in both preterm and term infants. The implication is that messages for SIDS prevention are equally applicable to mothers of preterm as well as term infants.
What this study adds
SIDS mortality has decreased by similar proportions in term and preterm infants, but preterm birth still remains a risk factor for SIDS. Risk factors for SIDS were similar in preterm and term infants, except for parity
The messages for SIDS prevention are equally applicable to mothers of preterm as well as term infants
Acknowledgements
The other members of the New Zealand Cot Death Study group were David M O Becroft, Rodney P K Ford, Barry J Taylor, Robert Scragg, Alistair Stewart, Sheila Williams, Ian B Hassall, Elizabeth Allen, David Barry, and Alison Roberts. We sincerely thank the parents and families who participate in this study. We also thank the research interviewers and pathologists in the study regions. Mrs C Everard coordinated the study.
Footnotes
Funding: The study was supported by the Health Research Council of New Zealand and the Hawke's Bay Medical Research Foundation. Dr Thompson and Professor Mitchell are supported by the Child Health Research Foundation.
Competing interests: none
References
- 1.Bergman A B, Ray C G, Pomeroy M A.et al Studies of the sudden infant death syndrome in King County, Washington. 3. Epidemiology. Pediatrics 197249860–870. [PubMed] [Google Scholar]
- 2.Mitchell E A, Taylor B J, Ford R P.et al Four modifiable and other major risk factors for cot death: the New Zealand study. J Paediatr Child Health 199228(suppl 1)S3–S8. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell E A, Tuohy P G, Brunt J M.et al Risk factors for sudden infant death syndrome following the prevention campaign in New Zealand: a prospective study. Pediatrics 1997100835–840. [DOI] [PubMed] [Google Scholar]
- 4.Joseph K S, Kramer M S, Marcoux S.et al Determinants of preterm birth rates in Canada from 1981 through 1983 and from 1992 through 1994. N Engl J Med 19983391434–1439. [DOI] [PubMed] [Google Scholar]
- 5.Ventura S J, Martin J A, Curtin S C.et al Births: final data for 1998. National Vital Statistics Reports 2000481–100. [PubMed] [Google Scholar]
- 6.Craig E D, Thompson J M D, Mitchell E A. Socioeconomic status and preterm birth: New Zealand trends, 1980 to 1999. Arch Dis Child Fetal Neonatal Ed 200286F142–F146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oyen N, Markestad T, Skaerven R.et al Combined effects of sleeping position and prenatal risk factors in sudden infant death syndrome: the Nordic Epidemiological SIDS Study. Pediatrics 1997100613–621. [DOI] [PubMed] [Google Scholar]
- 8.New Zealand Health Information Service Fetal and infant deaths 1997. Wellington: Ministry of Health, 200090
- 9.Mitchell E A, Scragg R, Stewart A W.et al Results from the first year of the New Zealand cot death study. N Z Med J 199110471–76. [PubMed] [Google Scholar]
- 10.Mitchell E A, Aley P, Eastwood J. The national cot death prevention programme in New Zealand. Aust J Public Health 199216158–161. [DOI] [PubMed] [Google Scholar]
- 11.Alm B, Norvenius S G, Wennergren G.et al Changes in the epidemiology of sudden infant death syndrome in Sweden 1973–1996. Arch Dis Child 20018424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell E A, Brunt J M, Everard C. Reduction in mortality from sudden infant death syndrome in New Zealand: 1986–92. Arch Dis Child 199470291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponsonby A L, Dwyer T, Cochrane J. Population trends in sudden infant death syndrome. Semin Perinatol 200226296–305. [DOI] [PubMed] [Google Scholar]
- 14.Malloy M H, Hoffman H J. Prematurity, sudden infant death syndrome, and age of death. Pediatrics 199596464–471. [PubMed] [Google Scholar]
- 15.Wierenga H, Brand R, Geudeke T.et al Prenatal risk factors for cot death in very preterm and small for gestational age infants. Early Hum Dev 19902315–26. [DOI] [PubMed] [Google Scholar]
- 16.Leach C E, Blair P S, Fleming P J.et al Epidemiology of SIDS and explained sudden infant deaths. CESDI SUDI Research Group. Pediatrics 1999104e43. [DOI] [PubMed] [Google Scholar]