Abstract
Obese children have more respiratory symptoms than their normal weight peers and respiratory related pathology increases with increasing weight. Some will need specialist assessment (box 1). Obesity produces mechanical effects on respiratory system performance. Breathlessness, wheeze, and cough are not related to increased airway responsiveness and may respond more to weight loss than bronchodilator therapy. A significant number of obese children have signs and symptoms of obstructive sleep apnoea largely related to the effect of obesity on upper airway dimensions. It seems likely that unless action is taken soon,34 increasing numbers of children will experience preventable respiratory morbidity as a result of nutritional obesity.
Keywords: asthma, lung function, obesity, obstructive sleep apnoea
“The commonest form of malnutrition in the western world is obesity.” (Mervyn Deitel, Professor of Surgery and Nutritional Sciences, University of Toronto, 1989)
Obesity occurs when there is an excessive accumulation of body fat. More than half of British adults are overweight and there is evidence that the prevalence of obesity in UK children is increasing at accelerating rates.1 Compared to thin children, obese children have a twofold increased risk of being overweight adults. Obesity may have effects on both the upper and lower airway and pulmonologists are increasingly involved in the management of obese children.
The commonest and simplest method of measuring and determining obesity is body mass index (BMI) which is defined as: mass (kg)/ht (m)2. In adults a BMI >30 defines obesity, but as the normal BMI changes throughout childhood and is age and gender specific, a centile chart has to be used in children. In UK centile charts, overweight is taken as a BMI >91st centile and obesity a BMI >98th centile. Other methods of assessment include waist circumference, waist/hip ratios, skinfold thickness, abdominal fat from CT/MRI scans, and bioelectrical impedance. Dual energy x ray absorptiometry (DEXA) has also been used successfully in children for the assessment of total and regional measurement of bone mass, lean mass, and fat mass.2
Lung volumes and function in obesity
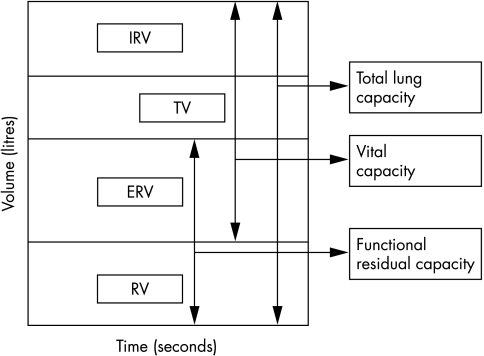
There are limited data in obese children, but obese adults have a decreased sitting expiratory reserve volume (ERV) (fig 1) and functional residual capacity (FRC) compared to normal controls; these parameters are further reduced in the supine position due to the increased gravitational effects of the large abdomen.3 Total lung capacity (TLC) and vital capacity (VC) may also be reduced; however, residual volume (RV) is usually maintained even in morbidly obese patients. RV as a fraction of TLC may be increased. Morbid obesity also affects dynamic lung volumes. An increase in body mass correlates with reduced FEV1.4,5 This decrease is proportional to changes in the forced vital capacity (FVC), so the ratio usually remains normal.6 Abnormalities in flow rates over high and low volumes have been noted in the obese and airway resistance is higher.7
Figure 1 Normal spirometry tracing. IRV, inspiratory reserve capacity; TV, tidal volume; ERV, expiratory reserve volume; RV, residual volume.
The data in children confirm reduced FRC and static lung volumes.2 Fat deposits over the chest and abdomen cause decreased chest wall and diaphragm movement. Chest wall compliance is decreased and work of breathing is increased.
Cross‐sectional population based data from 2464 children aged 9, 12, and 15 years showed that height adjusted FVC and FEV1 increased with increasing weight within each group, but decreased significantly with increasing total body fat as a percentage of weight—that is, ventilatory function decreased with increasing proportions of body fat.8 Several studies with smaller numbers of obese children have found lung function to be within predicted norms. Li et al studied 64 children with primary obesity in an effort to determine the predominant pulmonary function abnormality in this group.2 They also tried to correlate the degree of lung impairment with the degree of obesity. Ventilatory function was largely within normal limits (three children had mild airways obstruction). Unexpectedly, the commonest abnormalities they found were reduced FRC (46%) and diffusion impairment (33%). A significant negative correlation between FRC and degree of obesity was found when using DEXA scan for measurement of obesity; however this relationship did not hold true when BMI was used as the determinant.
These effects are potentially reversible, and improvement in lung volumes, function, gas exchange, and ventilation perfusion matching has been observed in morbidly obese adult patients following weight loss.4,5
Is airway anatomy different in obesity?
Fatty infiltration of muscles directly narrows the upper airway, while subcutaneous fat deposits also exert forces on regional structures, thus limiting visualisation of laryngeal structures. The paediatric airway differs from the adult airway in a few important ways. This includes a more cephalad larynx, a shorter neck, larger tongue, and more protuberant occiput. These factors in combination with large tonsils and adenoids can make airway obstruction a significant concern. Fatty deposits can obscure surface anatomy of the neck and thus hinder emergency airway management. Obesity in children should be used as an additional marker for an already potentially difficult intubation.
Box 1: When should an obese child be referred to a pulmonologist?
-
Symptoms suggestive of obstructive sleep apnoea [(a) plus one or more of (b), (c), (d), (e)]
Heavy snoring with restless nights
Witnessed obstructive apnoea
Morning headaches
Morning nausea or vomiting
Daytime sleepiness or poor performance
Symptoms suggestive of asthma not responding to standard therapy
-
Prader–Willi syndrome with
significant obesity and snoring
before starting growth hormone therapy
Asthma and obesity
Asthma is characterised by increased airway responsiveness with chronic inflammation, resulting in reversible airway obstruction. Responsiveness includes both bronchodilation in response to β2 agonists and bronchoconstriction to a stimulus, for example, exercise or methacholine challenge. Symptoms include wheeze, cough, shortness of breath, and chest tightness.
Asthma and obesity are both increasingly prevalent, so does the trend in BMI explain the trend in asthma? Are there factors which promote obesity and precipitate asthma? The critical question is whether obese individuals are more prone to having reversible airways disease, or are they more likely just to have a label of “asthma”? If they have a label of asthma, is this true reversible airways disease, or is it a non‐reversible side effect of obesity? If it is reversible, is it reversible in the short term with bronchodilators/steroids, or in the long term would weight loss result in a resolution of symptoms?
There have been numerous reports in the literature suggesting an association in adults and children between wheezing, asthma, and obesity, and a number of explanatory hypotheses have been generated.5,6,9,10,11 It has been proposed that the narrowed peripheral airways in obesity may cause increased airway responsiveness,12 as may the rapid shallow breathing pattern observed in the morbidly obese, both of which may contribute towards bronchospasm in obesity. Other proposed links with asthma include gastro‐oesophageal reflux, which is increased in obesity, a low intake of antioxidants, and the production of IL‐6 and cyclooxygenase 2 from adipose tissue, causing airway inflammation.13
Three recently published large studies have examined whether asthma is more prevalent in obese children. Schachter et al studied 5993 Caucasian Australian children aged 7–12 years,14 using pooled data from seven epidemiological studies from 1991 to 1993; the data were not collected for this purpose. Data were accepted when details of height, weight, age, skin prick responses to aeroallergens, and airway responsiveness as assessed by a standard histamine challenge were available. Symptoms, doctor diagnosis of asthma, and medication use were obtained by questionnaire. After adjustments for atopy, sex, age, smoking, and family history were made, BMI was found to be a significant risk factor for symptoms of cough and wheeze, but not for asthma or airway hyperresponsiveness in either boys or girls.
In 2004, in another large study of 5984 children from Israel, symptoms and treatment were recorded by questionnaire and spirometry was performed.15 Obesity was defined as >95th centile for BMI. Results showed that obese children recorded wheeze more than non‐obese children, asthma was diagnosed more often and inhaler use was increased among the obese, but bronchial hyperreactivity was significantly greater among non‐obese asthmatic children.
Finally, two cross‐sectional surveys of 11–12 year olds from 1989 (n = 873) and 2000 (n = 1321) in New Zealand were used to test the association between obesity and asthma.16 Symptoms were recorded by questionnaire, airway responsiveness was assessed by standard exercise challenge, and skin prick tests for atopy were performed in the 2000 cohort only. Increases in the reported prevalence of symptoms and diagnosis of asthma were not explained by the concurrent increase in the prevalence of obesity. Despite the increase in respiratory pathology there was a fall in prevalence of positive exercise challenge between cohorts. Bronchial hyperreactivity to exercise was not more common in obese children and there was no association between BMI and atopy in the 2000 dataset.
In each of these large studies symptoms were recorded in response to questionnaires and there was a clear increase in symptoms in association with obesity. However, when objective assessments of lung function and airway responsiveness were performed, there was no correlation with obesity.
Are there differences between girls and boys?
Some studies found an association between obesity and asthma in women but not in men. In the 1994–95 Canadian Health Population Survey, the prevalence of asthma increased with increasing BMI only in females.17 In a Taiwanese study, Taiwanese teenage girls showed an association between an increase in airway responsiveness and atopy with obesity.18
The European Community Respiratory Health Survey found that the association between symptoms and obesity was similar in both men and women. Schachter et al found a higher BMI to be a risk factor for atopy, wheeze ever, and cough in girls only; no link with asthma was found.14 In contrast, Bibi et al found that symptoms were more common in obese boys.15
Wheeze and obesity: is it asthma?
“All that wheezes is not asthma.” (Chevalier Jackson, US laryngologist, 1965)
The definition of asthma would seem to be crucial. The criteria for asthma diagnosis and asthma definition vary between studies and frequently rely on self reported symptoms or physician diagnosis based solely on self reported symptoms. Review of the evidence suggests that a higher BMI in children is associated with a higher prevalence of symptoms commonly attributed to asthma such as wheeze, but not a higher prevalence of objective asthma. If the symptoms are not asthma, then what are they? Obese children are less fit and may have more symptoms of breathlessness on exertion than their peers. An increased perception of symptoms in the obese may further complicate the issue. Obese children may have more gastro‐oesophageal reflux which can cause respiratory symptoms and an increase in sleep disordered breathing.19 Paediatricians should be cautious about diagnosing asthma in an obese child on the basis of self reported symptoms alone, and should seek objective evidence from peak flow recordings, exercise tests, or laboratory measurement of airway reactivity.
Obstructive sleep apnoea
During sleep, muscle tone is decreased. There is relaxation of pharyngeal muscles, particularly during REM, sleep and hence narrowing of the airway. This can lead to obstruction, particularly in those patients with predisposing factors such as obesity or adenotonsillar hypertrophy. In obstructive sleep apnoea (OSA) there is complete or partial obstruction leading to hypoxia, arousal, and sleep disturbance. Children with OSA can have very interrupted sleep with many episodes of obstruction and wakening. This can result in daytime sleepiness, morning headaches and vomiting, poor school performance, and quality of life. Nightmares and enuresis can occur, and longstanding OSA can lead to life threatening cardiopulmonary problems such as pulmonary hypertension and cor pulmonale.
Obesity is a well known risk factor for OSA. The size of the upper airways has been shown to correlate with OSA. Obese patients with OSA have increased fat deposition in the soft palate and uvula, as well as in the neck area and pharynx, contributing to narrowing of the upper airway. A study of 30 adult patients showed that the volume of fat adjacent to upper airway correlated with the apnoea/hypopnoea index (AHI). However, Shafer et al found a correlation between AHI, BMI, and abdominal fat, but not nasopharyngeal/neck fat.20 The size and patency of the upper airway are also influenced by lung volumes, which are also reduced in obesity.
A large American study examined risk factors for sleep disordered breathing in children.21 Children aged 2–18 years (n = 273) with a family history of sleep apnoea, and neighbourhood controls (n = 126) were assessed with overnight multichannel monitoring. Overall 25 children had obstructive sleep apnoea (apnoea/hypopnoea index >10), 28% of whom were obese. The odds ratio for obesity related to OSA was 4.69 (CI 1.58–13.33) Obesity was defined as BMI >28 at any age, which given the changes in normal centiles throughout childhood, is somewhat unusual. However, analysis repeated with BMI as a continuous variable showed that for each increase of 1 kg/m2 in BMI above the mean, the risk of OSA increased by 12%.
Among obese children with witnessed apnoeas and loud snoring, the prevalence of OSA is high.22 In a small study of 22 obese children, 46% had abnormal polysomnograms and there was a positive correlation between degree of obesity and apnoea index.23
Management of OSA in the obese child
Weight loss is the most effective long term treatment, but the least likely to take place.
Adenotonsillar hypertrophy is associated with OSA in both obese and non‐obese patients. Even moderate or normal sized tonsillar/adenoid tissue may have effects on a compromised airway; tonsillectomy and/or adenoidectomy is therefore indicated (but may not solve the problem).
Continuous positive airways pressure (CPAP) delivered overnight by nasal or, occasionally, full face mask, is highly effective provided there is adequate compliance. Occasionally bi‐level support (BiPAP) is needed, with a higher pressure delivered during inspiration on the background of a lower continuous pressure.
The obese child who snores loudly, has restless nights, and has obstructive apnoea, or morning sluggishness, morning headaches, nausea, or vomiting should be referred for sleep study.
Obesity hypoventilation syndrome
This is also known as Pickwickian syndrome, named after the fat character Joe in The Pickwick Papers by Charles Dickens. Obesity hypoventilation syndrome (OHS) is defined as a combination of obesity and awake arterial hypercapnia (PaCO2 >45 mm Hg) in the absence of other causes of hypoventilation. Patients may present with hypersomnolence, fatigue, or morning headaches as with OSA, but have chronic daytime hypercapnia and hypoxaemia associated with polycythaemia; later pulmonary hypertension and right ventricular failure develop. At night they have sleep hypoventilation, with or without obstructive sleep apnoea‐hypopnoea events.24 They have a decreased response to hypercapnia and hypoxia awake and asleep, and compared to the norm, their nocturnal minute ventilation falls significantly.25
There are isolated case reports in children,26 but in the older literature this syndrome is combined/confused with Prader–Willi syndrome. However, from the adult literature it is clear that this is a separate disorder associated with gross obesity; in one study, almost half the patients with a BMI >50 had chronic daytime hypoventilation.27 As children with nutritional obesity become more common, this disorder may be increasingly seen in the paediatric age range.
Although the cause of the hypoventilation is uncertain, it may be a combination of mechanical loading of the respiratory system secondary to extreme obesity causing sleep disordered breathing, with chronic hypoxia and hypercapnia leading to blunting of chemoreceptor responsiveness in susceptible individuals. However, there has been recent interest in the role of leptin in control of ventilation. The leptin deficient mouse develops obesity‐hypoventilation syndrome, but in adults with OHS, leptin levels are high, leading to speculation of central leptin resistance.28
Treatment with non‐invasive ventilation at night can restore the chemoresponsiveness, suggesting that this is a secondary rather than primary neurological control dysfunction. Loss of weight can reverse daytime hypercapnia, and improve blood gases and lung volumes.29
Syndromes and obesity
Prader–Willi syndrome
This is a genetic disorder; 70% of patients have a deletion of the long arm of chromosome 15 at q11q13 where the paternally derived chromosome has been deleted. Obesity due to excessive intake and inactivity tend to occur as the neonatal hypotonia improves.
These children have a variety of abnormalities of breathing, including OSA and sleep related alveolar hypoventilation. Abnormal ventilatory response to hypercapnia is probably secondary to obesity; however, ventilatory response to hypoxia is abnormal in both obese and non‐obese patients with Prader–Willi syndrome, as is peripheral chemoreceptor responsiveness.30 The abnormal responses to hypoxia and hypercapnia can be exacerbated by obesity, and children with Prader–Willi syndrome do not arouse normally to the hypoxia caused by airway obstruction These patients may also have a primary abnormality of the circadian rhythm of REM sleep and often suffer from excessive daytime sleepiness.31
Children with Prader–Willi syndrome are therefore at risk from a variety of abnormalities of breathing during sleep, including obstructive sleep apnoea and sleep related alveolar hypoventilation. The fact that they may not arouse normally to the hypoxia caused by airway obstruction puts them at higher risk of OSA morbidity, including sudden death. Children with Prader–Willi syndrome who are obese and have symptoms of OSA, or who are being considered for growth hormone treatment (associated with increased risk of sudden death), should be referred for sleep polysomnography.
Treatment in children with Prader–Willi syndrome is difficult as they struggle to lose weight. Adenotonsillectomy may help in a few, but the majority with significant OSA need to be considered for CPAP or bi‐level ventilatory support. The well known behavioural problems in children with Prader–Willi syndrome can make this challenging. A reduced life expectancy appears to relate to complications of morbid obesity.
Down's syndrome
Patients with Down's syndrome are at increased risk for airway obstruction secondary to problems of micrognathia, hypotonia, macroglossia, and midfacial hypoplasia, along with obesity. Higher body mass index has been shown to be significantly associated with a higher apnoea index and a lower SaO2 level in Down's syndrome.32 However, in a large prospective study of 108 children with Down's syndrome where there was a prevalence of sleep disordered breathing in 54.6%, with a significantly higher prevalence in boys (64.7%), only tonsillar hyperplasia was significantly associated with sleep disordered breathing.33 First line treatment is adenotonsillectomy.
Footnotes
Competing interests: none
References
- 1.Stamatakis E, Primatesta P, Chinn S.et al Overweight and obesity trends from 1974 to 2003 in English children: what is the role of socioeconomic factors? Arch Dis Child 200590999–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li A M, Chan D, Wong E.et al The effects of obesity on pulmonary function. Arch Dis Child 200388361–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koenig S M. Pulmonary complications of obesity. Am J Med Sci 2001321249–279. [DOI] [PubMed] [Google Scholar]
- 4.Carey I M, Cook D G, Strachan D P. The effects of adiposity and weight change on forced expiratory volume decline in a longitudinal study of adults. Int J Obes Relat Metab Disord 199923979–985. [DOI] [PubMed] [Google Scholar]
- 5.Camargo C A, Jr, Weiss S T, Zhang S.et al Prospective study of body mass index, weight change, and risk of adult‐onset asthma in women. Arch Intern Med 19991592582–2588. [DOI] [PubMed] [Google Scholar]
- 6.Guerra S, Sherrill D L, Bobadilla A.et al The relation of body mass index to asthma, chronic bronchitis, and emphysema. Chest 20021221256–1263. [DOI] [PubMed] [Google Scholar]
- 7.Zerah F, Harf A, Perlemuter L.et al Effects of obesity on respiratory resistance. Chest 19931031470–1476. [DOI] [PubMed] [Google Scholar]
- 8.Lazarus R, Colditz G, Berkey C S.et al Effects of body fat on ventilatory function in children and adolescents: cross‐sectional findings from a random population sample of school children. Pediatr Pulmonol 199724187–194. [DOI] [PubMed] [Google Scholar]
- 9.Gennuso J, Epstein L H, Paluch R A.et al The relationship between asthma and obesity in urban minority children and adolescents. Arch Pediatr Adolesc Med 19981521197–1200. [DOI] [PubMed] [Google Scholar]
- 10.Hancox R J, Milne B J, Poulton R.et al Sex differences in the relation between body mass index and asthma and atopy in a birth cohort. Am J Respir Crit Care Med 2005171440–445. [DOI] [PubMed] [Google Scholar]
- 11.Schachter L M, Salome C M, Peat J K.et al Obesity is a risk for asthma and wheeze but not airway hyperresponsiveness. Thorax 2001564–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubinstein I, Zamel N, DuBarry L.et al Airflow limitation in morbidly obese, nonsmoking men. Ann Intern Med 1990112828–832. [DOI] [PubMed] [Google Scholar]
- 13.Jubber A S. Respiratory complications of obesity. Int J Clin Pract 200458573–580. [DOI] [PubMed] [Google Scholar]
- 14.Schachter L M, Peat J K, Salome C M. Asthma and atopy in overweight children. Thorax 2003581031–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bibi H, Shoseyov D, Feigenbaum D.et al The relationship between asthma and obesity in children: is it real or a case of over diagnosis? J Asthma 200441403–410. [DOI] [PubMed] [Google Scholar]
- 16.Wickens K, Barry D, Friezema A.et al Obesity and asthma in 11–12 year old New Zealand children in 1989 and 2000. Thorax 2005607–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Dales R, Tang M.et al Obesity may increase the incidence of asthma in women but not in men: longitudinal observations from the Canadian National Population Health Surveys. Am J Epidemiol 2002155191–197. [DOI] [PubMed] [Google Scholar]
- 18.Huang S L, Shiao G, Chou P. Association between body mass index and allergy in teenage girls in Taiwan. Clin Exp Allergy 199929323–329. [DOI] [PubMed] [Google Scholar]
- 19.Sulit L G, Storfer‐Isser A, Rosen C L.et al Associations of obesity, sleep‐disordered breathing, and wheezing in children. Am J Respir Crit Care Med 2005171659–664. [DOI] [PubMed] [Google Scholar]
- 20.Schafer H, Pauleit D, Sudhop T.et al Body fat distribution, serum leptin, and cardiovascular risk factors in men with obstructive sleep apnea. Chest 2002122829–839. [DOI] [PubMed] [Google Scholar]
- 21.Redline S, Tishler P V, Schluchter M.et al Risk factors for sleep‐disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med 19991591527–1532. [DOI] [PubMed] [Google Scholar]
- 22.Silvestri J M, Weese‐Mayer D E, Bass M T.et al Polysomnography in obese children with a history of sleep‐associated breathing disorders. Pediatr Pulmonol 199316124–129. [DOI] [PubMed] [Google Scholar]
- 23.Marcus C L, Curtis S, Koerner C B.et al Evaluation of pulmonary function and polysomnography in obese children and adolescents. Pediatr Pulmonol 199621176–183. [DOI] [PubMed] [Google Scholar]
- 24.Olson A L, Zwillich C. The obesity hypoventilation syndrome. Am J Med 2005118948–956. [DOI] [PubMed] [Google Scholar]
- 25.Zwillich C W, Sutton F D, Pierson D J.et al Decreased hypoxic ventilatory drive in the obesity‐hypoventilation syndrome. Am J Med 197559343–348. [DOI] [PubMed] [Google Scholar]
- 26.Bourne R A, Maltby C C, Donaldson J D. Obese hypoventilation syndrome of early childhood requiring ventilatory support. Int J Pediatr Otorhinolaryngol 19881661–68. [DOI] [PubMed] [Google Scholar]
- 27.Nowbar S, Burkart K M, Gonzales R.et al Obesity‐associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med 20041161–7. [DOI] [PubMed] [Google Scholar]
- 28.O'Donnell C P, Schaub C D, Haines A S.et al Leptin prevents respiratory depression in obesity. Am J Respir Crit Care Med 19991591477–1484. [DOI] [PubMed] [Google Scholar]
- 29.Rapoport D M, Garay S M, Epstein H.et al Hypercapnia in the obstructive sleep apnea syndrome. A reevaluation of the “Pickwickian syndrome”. Chest 198689627–635. [DOI] [PubMed] [Google Scholar]
- 30.Arens R, Gozal D, Omlin K J.et al Hypoxic and hypercapnic ventilatory responses in Prader–Willi syndrome. J Appl Physiol 1994772224–2230. [DOI] [PubMed] [Google Scholar]
- 31.Nixon G M, Brouillette R T. Sleep and breathing in Prader–Willi syndrome. Pediatr Pulmonol 200234209–217. [DOI] [PubMed] [Google Scholar]
- 32.Dyken M E, Lin‐Dyken D C, Poulton S.et al Prospective polysomnographic analysis of obstructive sleep apnea in Down syndrome. Arch Pediatr Adolesc Med 2003157655–660. [DOI] [PubMed] [Google Scholar]
- 33.de Miguel‐Diez J, Villa‐Asensi J R, Alvarez‐Sala J L. Prevalence of sleep‐disordered breathing in children with Down syndrome: polygraphic findings in 108 children. Sleep 2003261006–1009. [DOI] [PubMed] [Google Scholar]
- 34.Rudolf M C, Hochberg Z, Speiser P. Perspectives on the development of an international consensus on childhood obesity. Arch Dis Child 200590994–996. [DOI] [PMC free article] [PubMed] [Google Scholar]