Abstract
Cockayne syndrome is a multi‐systemic, autosomal recessive disease characterised by postnatal growth failure and progressive multi‐organ dysfunction. The main clinical features are severe dwarfism (<−2 SD), microcephaly (<−3 SD), psychomotor delay, sensorial loss (cataracts, pigmentary retinopathy, and deafness), and cutaneous photosensitivity. Here, 13 new cases of Cockayne syndrome are reported, which have been clinically diagnosed and confirmed using a biochemical transcription assay. The wide clinical variability, ranging from prenatal features to normal psychomotor development, is emphasised. When cardinal features are lacking, the diagnosis of Cockayne syndrome should be considered when presented with growth retardation, microcephaly, and one of the suggesting features such as enophthalmia, limb ataxia, abnormal auditory evoked responses, or increased ventricular size on cerebral imaging.
Keywords: Cockayne syndrome, DNA repair, mental retardation
Cockayne syndrome is a multi‐systemic, autosomal recessive disease (MIM 216400) characterised by postnatal growth failure and progressive multi‐organ dysfunction, first described in 1936.1 The main clinical features are severe dwarfism (<−2 SD), microcephaly (<−3 SD), psychomotor delay, sensorial loss (cataracts, pigmentary retinopathy, and deafness) and cutaneous photosensitivity. Two types have been defined:2 type I, corresponding to a less severe form (symptoms occurring after birth); and type II, corresponding to a more severe infantile form with prenatal growth failure, severe neurological dysfunction, and premature death. Almost all Cockayne syndrome cases are due to mutations of the CSA gene on chromosome 5 or mutations of the CSB gene on chromosome 10q.3,4
Here, we report on 13 new cases of Cockayne syndrome whose diagnoses have been confirmed by transcriptional assays, and emphasise the wide clinical variability ranging from prenatal features to normal psychomotor development. The observation of multi‐systemic involvement in some severe forms suggests that mitochondrial dysfunction should be considered as a differential diagnosis.
Methods
Patients
Thirteen patients followed by eight clinicians from different French genetics medical centres were included in the study. The only criterion for inclusion was a positive biochemical diagnosis by the French reference laboratory (Pr Sarasin)—that is, 24 hours after UV irradiation normal fibroblasts have recovered a normal level of RNA synthesis (RRS); the range of RRS deficiency in our patients was 60–90% of controls, associated with a normal level of unscheduled DNA synthesis (UDS) following UV irradiation (data not shown).
Cell culture
Fibroblasts were established from unexposed skin biopsy specimens as previously described,5 and routinely grown in MEM, 15%, FCS, 1% glutamine, and 1% antibiotics.
Unscheduled DNA synthesis
UDS was measured, as previously described,5 on patients' fibroblasts after UV‐C irradiation (mainly at 254 nm). The doses routinely used ranged from 0 to 20 J/m2.
Recovery of RNA synthesis
RRS was carried out on patients' fibroblasts as described by Lehmann,6 except that the measure of RNA synthesis was quantified by autoradiography in the same way as for UDS. Cells were UV‐C irradiated at 20 J/m2 and RNA synthesis was measured for one hour, 23 hours after UV irradiation.
Results
Table 1, table 2, and fig 1 summarise the clinical, radiological, and biological features of the 13 cases.
Table 1 Clinical, biological, and radiological features of patients with Cockayne syndrome.
| Family | 1 | 1 | 2 | 3 | 4 | 5 | 6 | 6 | 7 | 8 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| Clinical findings | |||||||||||||
| Sex | F | M | M | M | F | M | F | M | M | M | M | M | F |
| Origin | Caucasian | Caucasian | Caucasian | Caucasian | Indian | Caucasian | Caucasian | Caucasian | Caucasian | Turkish | Turkish | Caucasian | Caucasian |
| Consanguinity | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Age at diagnosis (y) | 6 | 3 | 5 | 3.5 | 2 | 10 | 2 | <1 | 2 | 7 | 1 | 6 | 3.5 |
| First symptoms | Birth | Birth | Birth | Birth | 32 WG | 18 mth | Birth | Birth | Birth | Birth | 32 WG | Birth | Birth |
| IUGR | IUGR | IUGR | Microcephaly | IUGR | Microcephaly | IUGR | IUGR | IUGR | IUGR | Microcephaly | Microcephaly | ||
| Telangiectasis | Telangiectasis | Cataracts | Cardiomyopathy | Flat vertebral bodies | Cataracts | Cataracts | Vermis atrophy | Cataracts | |||||
| Gastrostomy | − | − | − | − | + | − | + | + | + | − | − | − | − |
| Death (y) | No | Yes (5) | No | Yes (7) | No | No | Yes | Yes | Yes (5) | No | No | No | No |
| Causes | Liver | Cachexia | Cachexia | Cachexia | |||||||||
| Dysmorphism | |||||||||||||
| Enophthalmia | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Thin skin | + | + | + | − | − | + | − | − | + | − | |||
| Photosensitivity | − | − | − | + | + | + | + | − | + | − | − | + | − |
| Eczema | − | − | − | − | − | − | − | + | − | − | + | − | + |
| Caries | − | NA | − | NA | + | NA | NA | NA | + | NA | − | NA | |
| Bird‐like nose | − | − | − | − | − | + | − | − | + | + | + | + | − |
| Prenatal findings | |||||||||||||
| IUGR | + | + | − | − | + | − | + | + | − | + | − | ||
| Microcephaly | + | + | − | + | + | − | + | + | − | + | − | ||
| Vermis atrophy | − | − | − | − | − | − | − | − | − | + | − | ||
| Oligohydramnios | − | + | − | − | − | − | − | − | − | + | − | ||
| Cardiomyopathy | − | − | − | − | + | − | − | − | − | − | − | ||
| Postnatal findings | |||||||||||||
| Height and weight −2 SD | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Microcephaly −3 SD | + | − | + | + | + | + | + | + | + | + | + | + | + |
| Delayed psychomotor development | − | + | + | + | + | + | + | + | + | + | + | + | + |
| Limb spasticity | − | + | − | + | − | − | − | + | + | + | + | + | − |
| Ataxia and/or gait abnormality | − | − | + | + | NA | + | NA | NA | NA | NA | − | ||
| Seizures | − | + | − | − | − | − | − | − | − | − | − | − | − |
| Hemiplegia | − | + | − | − | − | − | − | − | − | − | − | − | − |
| Truncal hypotonia | − | + | − | − | + | − | + | + | + | + | + | + | + |
| Nerve conduction velocities | Abnormal | ||||||||||||
| Ophthalmological findings | |||||||||||||
| Cataracts | + | + | − | + | + | − | + | + | + | − | − | + | − |
| Optic atrophy | + | + | − | − | − | + | + | + | − | ||||
| Pigmentary retinopathy | − | − | + | + | + | + | + | + | − | ||||
| Intra‐ocular calcifications | + | + | − | − | − | − | − | − | − | − | − | ||
| Telangiectasis | + | + | − | − | − | − | − | − | − | − | − | ||
| Visual evoked responses | Abnormal | Normal | Abnormal | ||||||||||
| Electroretinogram | Abnormal | Abnormal | Normal | ||||||||||
| Otolaryngological findings | |||||||||||||
| Sensorineural deafness | − | − | + | + | + | + | + | − | − | + | + | − | − |
| Auditory evoked responses | Abnormal | Abnormal | Abnormal | Abnormal | Abnormal | Abnormal | Abnormal | Abnormal | |||||
| Visceral | |||||||||||||
| High blood pressure | − | − | − | + | − | − | − | − | − | − | − | − | − |
| Dilated cardiomyopathy | − | − | − | − | + | − | − | − | − | − | − | − | − |
| Vascular liver | − | + | − | − | − | − | − | − | − | − | − | − | − |
| Renal complications | − | − | − | + | − | − | − | − | − | − | + | − | − |
| Laboratory findings | |||||||||||||
| Anaemia | − | + | − | − | − | − | − | − | + | − | − | − | − |
| Serum aminotransferase 2N | − | + | − | + | + | − | − | − | − | − | + | + | − |
| AAC in blood | Normal | Normal | Normal | Normal | Normal | Normal | |||||||
| OAC in urine | Normal | Normal | Abnormal | Normal | Abnormal | Abnormal | Abnormal | ||||||
| Lactic acidaemia | + | + | + | − | − | + | |||||||
| High lactate in CSF | − | − | + | − | |||||||||
| High ketones in blood | + | + | − | − | − | ||||||||
| Cerebral findings | |||||||||||||
| Calcifications | + | + | + | + | − | − | − | − | − | − | − | + | − |
| Hydrocephalus | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Increased ventricular size and/or cerebral atrophy | + | + | − | + | − | + | + | + | + | + | + | + | − |
| Delay in myelination | + | + | − | + | + | + | − | + | − | − | − | ||
| Abnormal cerebellum | − | − | − | + | − | + | − | + | − | + | + | − | − |
| Abnormal corpus callosum | − | − | − | − | − | + | − | − | + | − | |||
| Recovery of RNA synthesis (RRS) | |||||||||||||
| % of controls | − | 40 | 33 | 15 | 10 | 22 | 14 | 35 | 15 | 10 | − | 18 | 20 |
NA, not applicable (patient too young); [+], present; [−], absent; OAC, organic acid chromatography; AAC, amino acid chromatography; IUGR, intrauterine growth retardation; WG, weeks gestation.
Abnormal in the OAC line indicates high urinary excretion of alpha‐ketoglutarate for patient 3, lactate for patient 10, and 3‐hydroxybutyrate for patient 12.
A blank cell indicates that the information was not provided or not known.
Table 2 Summary of features of patients with Cockayne syndrome compared with the largest series described previously2,7.
| Total | Nance and Berry2 | Lehmann et al7 | |
|---|---|---|---|
| Sex | 9M/4F | 75M/60F (+ 5 unknown) | |
| First symptoms at birth | 12/13 | ||
| First symptoms like IUGR and/or microcephaly | 12/13 | ||
| Gastrostomy | 4/13 | ||
| Dysmorphism | |||
| Enophthalmia | 13/13 | 17/20 | |
| Thin skin | 6/13 | ||
| Photosensitivity | 6/13 | 67/92 (73%) | 24/25 |
| Eczema | 3/13 | ||
| Caries | 2/5 | 43/50 (86%) | 9/14 |
| Bird‐like nose | 5/13 | ||
| Prenatal findings | |||
| IUGR | 6/11 | ||
| Microcephaly | 7/11 | ||
| Vermis atrophy | 1/11 | ||
| Oligoamnios | 2/11 | ||
| Cardiomyopathy | 1/11 | ||
| Postnatal findings | |||
| Height and weight −2 SD | 13/13 | almost 100% | 29/29 |
| Microcephaly −3 SD | 12/13 | almost 100% | 29/29 |
| Delayed psychomotor development | 12/13 | 50/131 (38%) | 29/29 |
| Limb spasticity | 7/13 | 55/131 (42%) | |
| Limb ataxia and/or gait abnormality | 3/6 | 81/131 (62%) | 25/27 |
| Seizures | 1/13 | 11/131 (8%) | |
| Hemiplegia | 1/13 | 1/131 (<5%) | |
| Truncal hypotonia | 9/13 | ||
| Abnormal nerve conduction velocities | 1/1 | ||
| Ophthalmological findings | |||
| Cataracts | 8/13 | 46/128 (36%) | 6/24 |
| Optic atrophy | 5/9 | 43/128 (34%) | |
| Pigmentary retinopathy | 6/9 | 70/128 (55%) | 13/24 |
| Intraocular calcifications | 2/11 | ||
| Telangiectasis | 2/11 | ||
| Abnormal visual evoked responses | 2/3 | ||
| Abnormal electroretinogram | 2/3 | ||
| Otolaryngological findings | |||
| Sensorineural deafness | 7/13 | 47/78 (60%) | 16/27 |
| Abnormal auditory evoked responses | 8/8 | ||
| Visceral | |||
| High blood pressure | 1/13 | ||
| Dilated cardiomyopathy | 1/13 | ||
| Vascular liver | 1/13 | ||
| Renal complications | 2/13 | about 10% | |
| Laboratory findings | |||
| Anaemia | 2/13 | ||
| Serum aminotransferase 2N | 5/13 | ||
| Abnormal AAC in blood | 0/6 | ||
| Abnormal OAC in urine | 4/7 | ||
| Lactic acidaemia | 4/6 | ||
| High lactate in CSF | 1/4 | ||
| High ketones in blood | 2/5 | ||
| Cerebral findings | |||
| Calcifications | 5/13 | ||
| Hydrocephalus | 0/13 | 2/45 | |
| Increase ventricular size and/or cerebral atrophy | 10/13 | 30/45 (66%) | |
| Delay in myelination | 6/13 | ||
| Abnormal cerebellum | 5/13 | ||
| Abnormal corpus callosum | 2/10 |
The table shows the numbers [+] with the indicated features; percentages with the features are indicated in parentheses.
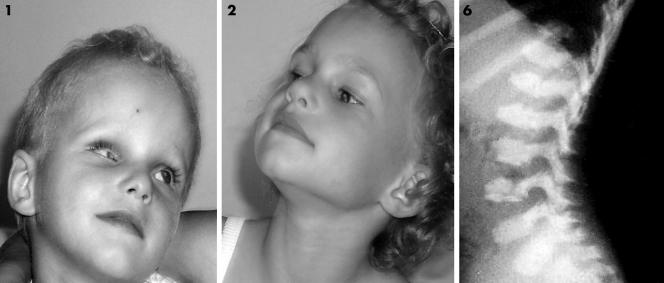
Figure 1 Patients with Cockayne syndrome. The only constant dysmorphic feature is enophthalmia. For patient 6, note the flat vertebral bodies which were one of the presenting symptoms. Consent was obtained for publication of this figure.
The series included nine males and four females. There were three familial cases with two patients (1–2, 7–8, and 10–11) in offspring of each family (1, 6, and 8). Average age of diagnosis was below 5 years old, ranging from 1 year (two recurrent cases in offspring) to 10 years. Five died between 4 and 7 years of age (patients 2, 4, 7, 8, and 9); eight are still alive.
Intrauterine growth retardation (IUGR) and microcephaly were noted in six cases. In addition, vermis atrophy (patient 11) and cardiomyopathy (patient 4) were occasionally detected prenatally.
Among postnatal manifestations, enophthalmia and growth failure were noted in all cases. Photosensitivity, defined as abnormal reaction to sun exposure (as papulovesicular eruption, rash with oedema, or atrophic scars) was observed in half of the patients (6/13). Among ophthalmological findings, cataracts, optic atrophy, and pigmentary retinopathy were found respectively in 8/13, 5/9, and 6/9. Sensorineural deafness was detected in 7/13 cases.
Death was due to cachexia in at least three patients, although gastrostomy was performed. Patient 2 died of bleeding oesophageal varices due to portal hypertension not related to viral or mitochondrial dysfunction.
The presenting symptoms were sometimes atypical, including cardiomyopathy (patient 4), telangiectasia and intraocular calcifications responsible for blindness in two siblings (patients 1 and 2), and flat vertebral bodies in another case (fig 1; patient 6).
Finally, unusual features were observed in a few cases: cardiomyopathy, vascular liver, telangiectasia, and flat vertebral bodies (fig 1).
Among the laboratory findings, anaemia was detected in two patients (2 and 9), partly due to cachexia for patient 9. Mild serum aminotransferase elevation (2N) was noted in 5/13 patients without any other hepatic dysfunction. Lactic acidaemia, high lactate levels in cerebrospinal fluid, or abnormal excretion of organic acids detected by urinary chromatography (in patients 1, 2, 3, 5, and 12) prompted us to test the mitochondrial respiratory chain. Enzyme assays performed on muscle and/or skin fibroblasts were normal.
Discussion
We report here the clinical manifestations of 13 new cases of Cockayne syndrome confirmed by biochemical assays, and compared this series to previous reports—that is, 140 cases of Cockayne syndrome reviewed but not all confirmed by transcription test assessment,2 and 29 patients showing the characteristic defect of CS cells.7
As previously described, growth failure (height and weight <−2 SD), microcephaly (<−3 SD), and variable degree of psychomotor retardation appear to be consistently observed. Child 1 is the only patient in the series with no neurodevelopmental delay, but is only 6 years old.
The only consistent dysmorphic feature is profound enophthalmia or sunken eyes, due to the lack of subcutaneous orbital fat.2,8,9 However, this feature is not present in the early stages as shown by fig 1; it becomes more obvious with time. In contrast, features usually considered as mandatory for Cockayne syndrome diagnosis were not consistently observed in our series; these included thin skin and/or photosensitivity, cataracts and pigmentary retinopathy, sensorineural deafness, and intracranial calcifications with ventricular dilatation on cerebral imaging.
Finally, the presenting symptoms were quite unusual in our series, leading to consideration of other diagnoses. In patients 1 and 2, bilateral telangiectasia and intraocular calcifications were present in the neonatal period, leading to blindness; the initial diagnosis was COATS disease (MIM 300216). Conjunctival telangiectasia is usually described in xeroderma pigmentosum which may share others common features with Cockayne syndrome.10 Intrauterine growth retardation and microcephaly suggested a mitochondrial defect as the primary diagnosis in six patients, especially when associated with ataxia, dilated cardiomyopathy, pigmentary retinopathy, and/or cerebral calcifications. Patient 6 was first referred for skeletal dysplasia with flat vertebral bodies. Interestingly, a similar case has been described,9 suggesting that spine anomalies are possible manifestations of Cockayne syndrome.
Based on our series, we emphasise the wide clinical presentations in Cockayne syndrome. In addition, some degree of phenotypic variability has also been observed within the same family.11 We conclude that the diagnosis of Cockayne syndrome is not always easy in the first months of life when cardinal features are not present; it should be considered when dealing with growth retardation and microcephaly.
Footnotes
Fundings: this work was supported by the GIS–Institut des Maladies Rares
Competing interests: none declared
Consent was obtained for publication of the figure
References
- 1.Cockayne E A. Dwarfism with retinal atrophy and deafness. Arch Dis Child 1936111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nance M A, Berry S A. Cockayne syndrome: review of 140 cases. Am J Med Genet 19924268–84. [DOI] [PubMed] [Google Scholar]
- 3.Stefanini M, Fawcett H, Botta E.et al Genetic analysis of twenty‐two patients with Cockayne syndrome. Hum Genet 199697418–423. [DOI] [PubMed] [Google Scholar]
- 4.Cleaver J E, Thompson L H, Richardson A S.et al A summary of mutations in the UV‐sensitive disorders: xeroderma pigmentosum, Cockayne syndrome, and trichothiodystrophy. Hum Mut 1999149–22. [DOI] [PubMed] [Google Scholar]
- 5.Sarasin A, Blanchet‐Bardon C, Renault G.et al Prenatal diagnosis in a subset of trichothiodystrophy patients defective in DNA repair. Br J Dermatol 1992127485–491. [DOI] [PubMed] [Google Scholar]
- 6.Lehmann A R. Prenatal diagnosis of Cockayne's syndrome. Lancet 1985325486–488. [DOI] [PubMed] [Google Scholar]
- 7.Lehmann A R, Thompson A F, Harcourt S A.et al Cockayne's syndrome: correlation of clinical features with cellular sensitivity of RNA synthesis to UV irradiation. J Med Genet 199330679–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dabbagh O, Swaiman K F. Cockayne syndrome: MRI correlates of hypomyelinisation. Pediatr Neurol 19884113–116. [DOI] [PubMed] [Google Scholar]
- 9.Cirillo Silengo M, Franceschini P, Bianco R.et al Distinctive skeletal dysplasia in Cockayne syndrome. Pediatr Radiol 198616264–266. [DOI] [PubMed] [Google Scholar]
- 10.Dollfus H, Porto F, Caussade P.et al Ocular manifestations in the inherited DNA repair disorders. Survey Ophthalmol 200348107–122. [DOI] [PubMed] [Google Scholar]
- 11.Mahmoud A A, Yousef GM Al‐Hifzi I.et al Cockayne syndrome in three sisters with varying clinical presentation. Am J Med Genet 200211181–85. [DOI] [PubMed] [Google Scholar]