Abstract
Aims
To evaluate whether procalcitonin (PCT) and C reactive protein (CRP) are able to discriminate between sepsis and systemic inflammatory response syndrome (SIRS) in critically ill children.
Methods
Prospective, observational study in a paediatric intensive care unit. Kinetics of PCT and CRP were studied in patients undergoing open heart surgery with cardiopulmonary bypass (CPB) (SIRS model; group I1) and patients with confirmed bacterial sepsis (group II).
Results
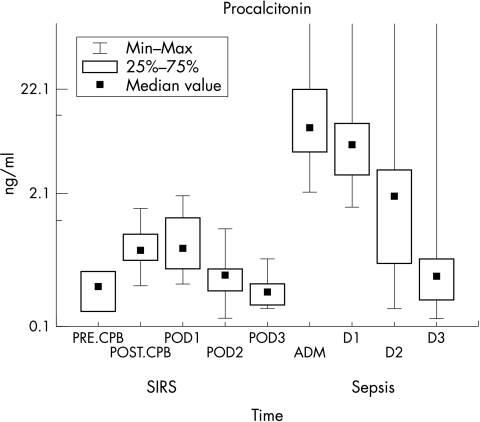
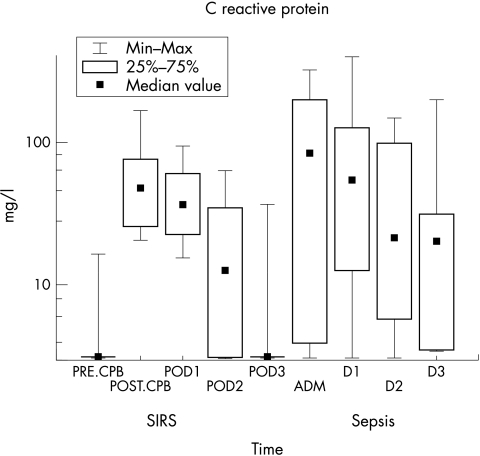
In group I, PCT median concentration was 0.24 ng/ml (reference value <2.0 ng/ml). There was an increment of PCT concentrations which peaked immediately after CPB (median 0.58 ng/ml), then decreased to 0.47 ng/ml at 24 h; 0.33 ng/ml at 48 h, and 0.22 ng/ml at 72 h. CRP median concentrations remained high on POD1 (36.6 mg/l) and POD2 (13.0 mg/l). In group II, PCT concentrations were high at admission (median 9.15 ng/ml) and subsequently decreased in 11/14 patients who progressed favourably (median 0.31 ng/ml). CRP levels were high in only 11/14 patients at admission. CRP remained high in 13/14 patients at 24 h; in 12/14 at 48 h; and in 10/14 patients at 72 h. Median values were 95.0, 50.9, 86.0, and 20.3 mg/l, respectively. The area under the ROC curve was 0.99 for PCT and 0.54 for CRP. Cut off concentrations to differentiate SIRS from sepsis were >2 ng/ml for PCT and >79 mg/l for CRP.
Conclusion
PCT is able to differentiate between SIRS and sepsis while CRP is not. Moreover, unlike CRP, PCT concentrations varied with the evolution of sepsis.
Keywords: procalcitonin (PCT), C reactive protein (CRP), cardiopulmonary bypass (CPB), systemic inflammatory response syndrome (SIRS), sepsis
Bacterial sepsis is a major cause of morbidity and mortality in neonates and children.2 Rapid detection of bacterial sepsis is difficult because the first signs of disease are usually non‐specific.3 Early diagnosis of severe infections and the prompt initiation of adequate antimicrobial therapy are essential for successful treatment.4
Cardiac surgery and the use of cardiopulmonary bypass (CPB) have significantly improved the prognosis of paediatric patients with congenital heart disease. However, extended blood contact with foreign surfaces, the use of hypothermia, myocardial ischaemia, reperfusion, and surgical trauma trigger activation of the immune system, the complement pathway, and release of cytokines, leading to a systemic inflammatory response immediately after CPB. During the first hours or days after surgery, it is difficult to differentiate between the normal inflammatory response and infectious complications.5,6
Laboratory parameters such as C reactive protein (CRP) and leucocyte count are often abnormally elevated after cardiac surgery albeit the absence of infection.5,7 Procalcitonin (PCT) has been recently proposed as a more specific marker of infection, being able to discriminate between systemic inflammatory responses and sepsis.8
We present results of the second part of a study conducted in critically ill patients presenting with bacterial sepsis. We aimed to compare this group of septic children with those with systemic inflammatory response syndrome (SIRS) from the first part of the study.1
Methods
Patients
This study received the approval of the Ethics Committee of the School of Medicine, University of São Paulo, Brazil. After informed consent of parents, 14 children were enrolled in this second part of the study. Group II consisted of children presenting with bacterial sepsis confirmed by either haemoculture (n = 10), cerebrospinal fluid (CSF) culture (n = 1), or urine culture (n = 3). Seven boys and seven girls were included; age ranged from 3 days to 192 months. Bacterial sepsis was defined according to the American College of Chest Physicians and Society of Critical Care Medicine guidelines, slightly modified to fit both neonatal and paediatric populations.9 All paediatric patients with bacterial sepsis received antibiotics according to the decision of the attendant physician. Patients who had received antibiotics, anti‐inflammatory drugs, or corticosteroids prior to hospitalisation were excluded. Children presenting with endocrine, liver, or renal dysfunction were also excluded because these conditions might have decreased production and clearance of acute phase proteins such as CRP.
Methods
Arterial blood samples (3 ml) were drawn in sterile vacuum tubes with no additives (Becton Dickinson) and centrifuged; serum aliquots were stored at –20°C until analysis. Patients in group II were tested at admission (before administration of antibiotics), and on the first (24 h), second (48 h), and third days (72 h) of treatment.
CRP concentrations were determined in serum samples by immunonephelometry (nephelometer‐2, Dade‐Behring, La Défense, France; reference values were <5.0 mg/l).7 Briefly, polystyrene particles coated with monoclonal antibodies specific to human CRP are aggregated when mixed with serum samples containing CRP from patients. These aggregates scatter a beam of light passed through the sample. The intensity of scattered light is proportional to the concentration of the relevant protein in the sample. The result is evaluated by comparison with a standard of known concentration.
Procalcitonin was evaluated by an immunoluminometric assay (LUMItest PCT, BRAHMS Diagnostica, GmbH, Germany; recommended reference interval for SIRS is 0.5–2.0 ng/ml). Briefly, 20 μl of serum or plasma samples are added to a tube coated with an anti‐katacalcin antibody. Samples are incubated at room temperature for 1 hour, and a second antibody anti‐calcitonin labelled with a luminescent acridine derivative is added to the reaction. Then, samples are placed in a luminometer and hydrogen peroxide and sodium hydroxide solutions are automatically injected. These substances react with the acridine derivative bound to the anti‐calcitonin antibody leading to emission of light as the acridine turns into acridone. The intensity of emitted light is directly proportional to the PCT concentration.10
Statistical analysis
PCT and CRP concentrations were presented as median (min–max). Results comparing sampling times within group II were made by means of the Wilcoxon test. Values of p < 0.01 were considered statistically significant. The Friedman test was used to evaluate statistical significance of differences between group I (SIRS) and II (sepsis). Sampling times were compared in pairs: after CPB versus admission; POD1 versus 24 h; POD2 versus 48 h; POD3 versus 72 h (POD = post‐operation day). Values of p < 0.01 were considered statistically significant.
Sensitivity, specificity, and predictive values were calculated for different concentrations of PCT and CRP by means of the receiver operator curve (ROC) for patients of group II (sepsis).
Results
Characteristics of group II patients (sepsis) are shown in table 1. The median age in group I was 1.5 months (0.1–192.0), and the median of hospital stay was 20.0 days (4.0–72.0).
Table 1 Characteristics of the 14 patients with sepsis (group II).
| Patients | Age (month) | Gender | ICU stay (days) | Culture | Outcome |
|---|---|---|---|---|---|
| 1 | 6 | F | 4 | Klebsiella pneumoniae BC | NS |
| 2 | 23 | F | 6 | Pseudomonas aeruginosa BC | NS |
| 3 | 4 | F | 24 | Pseudomonas aeruginosa BC | S |
| 4 | 192 | M | 12 | Staphylococcus aureus BC | S |
| 5 | 13 | M | 8 | Streptococcus sp BC | S |
| 6 | 16 | F | 17 | Pseudomonas aeruginosa BC | S |
| 7 | 0.5 | M | 41 | Staphylococcus aureus BC | NS |
| 8 | 0.8 | M | 48 | Proteus mirabilis UC | S |
| 9 | 0.6 | M | 9 | Escherichia coli UC | S |
| 10 | 0.1 | M | 27 | Staphylococcus epidermidis BC | S |
| 11 | 0.5 | F | 5 | Staphylococcus aureus BC | S |
| 12 | 1 | F | 25 | Escherichia coli UC | S |
| 13 | 2 | M | 33 | Neisseria meningitidis CSF | S |
| 14 | 0.1 | F | 72 | Enterobacter sp BC | S |
ICU, intensive care unit; BC, blood culture; UC, urine culture; CSF, cerebrospinal fluid; NS, non‐survivor; S, survivor.
Results for group I patients have been reported previously.1 All 14 patients progressed favourably, with no signs or symptoms of infection.
Figure 1 shows PCT concentrations in the 14 patients of group I (SIRS) and 14 patients of group II (sepsis). There was an increment of PCT concentrations in all 14 patients at admission. Median values (min–max) were: 9.15 ng/ml (2.1–607.7) at admission (adm); 6.25 ng/ml (1.5–619.9) at 24 h (D1); 3.22 ng/ml (0.1–149.1) at 48 h (D2); and 0.31 ng/ml (0.1–153.5) at 72 h (D3).
Figure 1 Plot of PCT versus time in group I (SIRS) and group II (sepsis) patients.
Figure 2 shows CRP concentrations of group I (SIRS) and group II (sepsis). CRP levels were above the reference interval (>3.5 mg/l) in only 11 of 14 patients at admission; in 13 of 14 patients at 24 h (D1); in 12 of 14 at 48 h (D2); and in 10 of 14 patients at 72 h (D3). Median values (min–max) were: 95.0 mg/l (3.1–322.0) at admission (adm); 50.9 mg/l (3.1–393.1) at 24 h (D1); 86.0 mg/l (3.1–148.7) at 48 h (D2); 20.3 mg/l (3.5–200.0) at 72 h (D3).
Figure 2 Plot of CRP versus time in group I (SIRS) and group II (sepsis) patients.
Sensitivity (SE), specificity (SP), positive predictive values (PPV), and negative predictive values (NPV) have been calculated by means of the receiver operator curve (ROC). Results are shown in table 2. The area under ROC curve was 0.99 for PCT (95% CI 0.97 to 1) and 0.54 for CRP (95% CI 0.38 to 0.72).
Table 2 Diagnostic value of PCT and CRP at various thresholds in the 14 patients of group II (sepsis).
| SE | SP | PPV | NPV | |
|---|---|---|---|---|
| PCT (ng/ml) | ||||
| 0.5 | 73 | 67 | 64 | 75 |
| 1.0 | 71 | 92 | 89 | 80 |
| 1.5 | 68 | 98 | 97 | 79 |
| 2.0 | 88 | 100 | 100 | 86 |
| 5.0 | 41 | 100 | 61 | 67 |
| CRP (mg/l) | ||||
| 5.0 | 76 | 40 | 50 | 68 |
| 10.0 | 70 | 44 | 50 | 64 |
| 30.0 | 52 | 70 | 58 | 64 |
| 50.0 | 45 | 80 | 64 | 64 |
| 100.0 | 30 | 97 | 89 | 63 |
SE, sensitivity; SP, specificity; PPV, positive predictive value; NPV, negative predictive value.
Discussion
The present study aimed to analyse the kinetics of PCT and CRP in two different situations—SIRS and sepsis—and to determine whether these two laboratory markers might be used to discriminate between the two entities. In this second part of the study (group II), 14 paediatric patients presenting with confirmed sepsis (group II) were enrolled and compared with 14 patients of group I (SIRS).1
Following cytotoxic chemotherapy, fever might be a signal of invasive bacterial disease or a drug reaction associated with SIRS. Stryjewski et al found that PCT and interleukin‐8 could be used to detect bacterial sepsis in febrile, neutropenic children.11 Kuse et al showed that PCT allows differentiation between rejection and infection in patients presenting with fever of unknown origin (FUO) and receiving prednisolone following liver transplantation.12 Another study from Sauer et al concluded that serum PCT correlates with the severity of sepsis among deeply immunosuppressed paediatric bone marrow transplant recipients, and that it may reliably identify children at risk of developing graft versus host disease, who received prophylaxis with prednisone.13 Our study showed that corticosteroids did not affect either PCT or CRP kinetics in group I (SIRS), as we could observe an increment of PCT and CRP after CPB, and of CRP in the other sampling times. However, it is noteworthy that PCT increments found after CPB did not exceed the reference interval for SIRS (<2 ng/ml).
There are several studies in the literature evaluating the ability of PCT to diagnose infection in patients with different underlying pathologies.14,15,16 In septic children, those reports tend to indicate that PCT could be used as a laboratory marker to discriminate between SIRS and sepsis.15,17,18,19 In our study, PCT concentrations of group II patients had already increased in all 14 patients at admission (median 9.15 ng/ml), thus confirming its ability to diagnose infection. These data corroborate the study of Gendrel et al who compared the concentrations of PCT, CRP, interleukin‐6, and α‐interferon in paediatric patients, aiming at discriminating between viral and bacterial infections.20 They concluded that the best laboratory parameter was PCT (83% sensitivity and 93% specificity) when the cut off was 1.0 ng/ml. Moreover, 47% of children presenting with viral infections showed CRP concentrations above 10.0 mg/l, and 26.9% above 20.0 mg/l, corroborating the lack of specificity of CRP. In our study, PCT concentrations in septic patients were high at admission and at 24 h. However, when concentrations found at admission (median 22.12 ng/ml) were compared with those obtained at 48 h (median 3.22 ng/ml) or at 72 h (median 0.42 ng/ml), a statistically significant difference was found (p < 0.001). These data have confirmed that PCT concentrations modulate more quickly than CRP, thus indicating that it could be used to test response to antibiotic therapy.21 Moreover, in the 11 survivors of group II (sepsis), PCT concentrations returned to the SIRS interval (0.5–2.0 ng/ml) until the last time of sampling (72 h). In contrast, PCT concentrations remained above the reference interval until 72 h in the three patients that died.22,23
Taking CRP concentrations into consideration with respect to group II, CRP did not detect 3 of 14 septic patients at admission, which might postpone the introduction of antibiotics. Moreover, CRP remained high until 72 h showing that, unlike PCT, it could not be used to test response to antibiotics.17,24
Early identification of patients with insidious sepsis would allow early therapeutic intervention, what might influence patients' outcome.25 In our study, PCT was also able to discriminate between post‐CPB time (representing SIRS) and the first sampling time of septic patients (admission). Comparisons were made by means of the Friedman test (p < 0.001). When post‐CPB concentrations were compared to the 24 h sampling time, there was a statistically significant difference (p < 0.001). In contrast, CRP did not succeed in discriminating these situations tested in pairs, confirming its inability to differentiate SIRS and sepsis (p < 0.01).26
In the present study, PCT proved to be more specific than CRP, and also to have a higher positive predictive value to diagnose sepsis in comparison with CRP (table 2). Similar specificity was observed by Lopez and Enguix (94% and 100%, respectively).15,18 However, in the first study the best cut‐off for PCT was 0.59 ng/ml, while it was 8 ng/ml in the second report. In a systematic review, Simon et al reported that the diagnostic accuracy of PCT was greater than that of CRP in distinguishing between bacterial infection and SIRS among hospitalised patients.27
Conclusions
We conclude that SIRS (group I) did influence serum CRP concentrations immediately after surgery, and at 24 h, 48 h, and 72 h, while PCT concentrations remained within the predicted SIRS range (0.5–2.0 ng/ml) at all sampling times. Therefore, PCT was able to discriminate between SIRS and sepsis, while CRP was not. In the present study, unlike CRP, PCT changed with the evolution of sepsis. These data might indicate that PCT, but not CRP, could be used to test response to antibiotics.
What is already known on this topic
PCT has been proposed as an early marker of sepsis
PCT has been suggested to be more sensitive and specific than CRP
What this study adds
PCT is an early marker of sepsis; it is PCT is more sensitive and specific than CRP
PCT might be used to monitor infection
Abbreviations
CPB - cardiopulmonary bypass
CRP - C reactive protein
PCT - procalcitonin
POD - post‐operation day
SIRS - systemic inflammatory response syndrome
Footnotes
Funding: this research was supported by Brahms Diagnostica GmbH, represented by Analyse and later by Fanem laboratories, in Brazil
Competing interests: none declared
References
- 1.Arkader R, Troster E J, Abellan D M.et al Procalcitonin and C‐reactive protein kinetics in postoperative pediatric cardiac surgical patients. J Cardiothorac Vasc Anesth 200418160–165. [DOI] [PubMed] [Google Scholar]
- 2.Carcillo J A, Cunnion R E. Septic shock. Crit Care Clin 199713553–574. [DOI] [PubMed] [Google Scholar]
- 3.Volante E, Moretti S, Pisani F.et al Early diagnosis of bacterial infection in the neonate. J Matern Fetal Neonatal Med 200416(suppl 2)13–16. [DOI] [PubMed] [Google Scholar]
- 4.Carlet J. Rapid diagnostic methods in the detection of sepsis. Infect Dis Clin North Am 199913483–494. [DOI] [PubMed] [Google Scholar]
- 5.Wan S, LeClerc J L, Vincent J L. Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest 1997112676–692. [DOI] [PubMed] [Google Scholar]
- 6.Chew M S, Brandslund I, Brix‐Christensen V.et al Tissue injury and the inflammatory response to pediatric cardiac surgery with cardiopulmonary bypass: a descriptive study. Anesthesiology 200194745–753. [DOI] [PubMed] [Google Scholar]
- 7.Boralessa H, de Beer F C, Manchie A.et al C‐reactive protein in patients undergoing cardiac surgery. Anaesthesia 19864111–15. [DOI] [PubMed] [Google Scholar]
- 8.ED. Thomson A P, Hart C A. Procalcitonin as a marker of sepsis. Int J Antimicrob Agents 2002201–9. [DOI] [PubMed] [Google Scholar]
- 9.Carcillo J A. Pediatric septic shock and multiple organ failure. Crit Care Clin 200319413–440. [DOI] [PubMed] [Google Scholar]
- 10.Reinhart K, Karzai W, Meisner M. Procalcitonin as a marker of the systemic inflammatory response to infection. Intensive Care Med 2000261193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stryjewski G, Nylen E S, Bell M J.et al Interleukin‐6, interleukin‐8, and a rapid and sensitive assay for calcitonin precursors for the determination of bacterial sepsis in febrile neutropenic children. Pediatr Crit Care Med 20056129–135. [DOI] [PubMed] [Google Scholar]
- 12.ER. Langefeld I, Jaeger K.et al Procalcitonin—a new diagnostic tool in complication following liver transplantation. Intensive Care Med 200026s187–s192. [DOI] [PubMed] [Google Scholar]
- 13.Sauer M, Tiede K, Fuchs D.et al Procalcitonin, C‐reactive protein, and endotoxin after bone marrow transplantation: identification of children at high risk of morbidity and mortality from sepsis. Bone Marrow Transplantation 2003311137–1142. [DOI] [PubMed] [Google Scholar]
- 14.Harbarth S, Holeckova K, Froidevaux C.et al Diagnostic value of procalcitonin, interleukin‐6, and interleukin‐8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med 2001164396–402. [DOI] [PubMed] [Google Scholar]
- 15.Enguix A, Rey C, Concha A.et al Comparison of procalcitonin with C‐reactive protein and serum amyloid for the early diagnosis of bacterial sepsis in critically ill neonates and children. Intensive Care Med 200127211–215. [DOI] [PubMed] [Google Scholar]
- 16.Tugrul S, Esen F, Celebi S.et al Reliability of procalcitonin as a severity marker in critically ill patients with inflammatory response. Anaesth Intensive Care 200230747–754. [DOI] [PubMed] [Google Scholar]
- 17.Casado‐Flores J, Blanco‐Quiros A, Asensio J.et al Serum procalcitonin in children with suspected sepsis: a comparison with C‐reactive protein and neutrophil count. Pediatr Crit Care Med 20034190–195. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez Lopez A, Luaces Cubells C, Garcia Garcia J J.et al Procalcitonin in pediatric emergency departments for the early diagnosis of invasive bacterial infections in febrile infants: results of a multicenter study and utility of a rapid qualitative test for this marker. Pediatr Infect Dis J 200322895–903. [DOI] [PubMed] [Google Scholar]
- 19.Fleischhack G, Kambeck I, Cipic D.et al Procalcitonin in paediatric cancer patients: its diagnostic relevance is superior to that of C‐reactive protein, interleukin 6, interleukin 8, soluble interleukin 2 receptor and soluble tumour necrosis factor receptor II. Br J Haematol 20001111093–1102. [DOI] [PubMed] [Google Scholar]
- 20.Gendrel D, Raymond J, Assicot M.et al [Procalcitonin, C‐reactive protein and interleukin 6 in bacterial and viral meningitis in children]. Presse Med 1998271135–1139. [PubMed] [Google Scholar]
- 21.Brunkhorst F M, Heinz U, Forycki Z F. Kinetics of procalcitonin in iatrogenic sepsis. Intensive Care Med 199824888–889. [DOI] [PubMed] [Google Scholar]
- 22.Han Y Y, Doughty L A, Kofos D.et al Procalcitonin is persistently increased among children with poor outcome from bacterial sepsis. Pediatr Crit Care Med 2003421–25. [DOI] [PubMed] [Google Scholar]
- 23.Hatherill M, Tibby S M, Turner C.et al Procalcitonin and cytokine levels: relationship to organ failure and mortality in pediatric septic shock. Crit Care Med 2000282591–2594. [DOI] [PubMed] [Google Scholar]
- 24.ED. Newland P, Riordan F A.et al Procalcitonin as a diagnostic marker of meningococcal disease in children presenting with fever and a rash. Arch Dis Child 200286282–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rangel‐Frausto M S, Pittet D.et al The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA 1995273117–123. [PubMed] [Google Scholar]
- 26.Aouifi A, Piriou V, Bastien O.et al Usefulness of procalcitonin for diagnosis of infection in cardiac surgical patients. Crit Care Med 2000283171–3176. [DOI] [PubMed] [Google Scholar]
- 27.Simon L, Gauvin F, Devendra K A.et al Serum procalcitonin and C‐reactive protein levels as markers of bacterial infection: a systematic review and meta‐analysis. Clin Infect Dis 200439206–217. [DOI] [PubMed] [Google Scholar]