Abstract
Aim
To identify clinical features which predict those most at risk of co‐morbidities within an obesity clinic.
Methods
Children attending an obesity clinic had fasting glucose, insulin, and lipids measured prior to a standard oral glucose tolerance test (OGTT). History and examination established birth weight, family history of type 2 diabetes/obesity, pubertal status, and presence of acanthosis nigricans. Central and total fat mass was estimated by bio‐impedance.
Results
Of the 126 children evaluated, 10.3% (n = 13) had impaired glucose tolerance (IGT); the majority (n = 11) of these would not have been identified on fasting glucose alone. Those with IGT were more likely to have a parental history of type 2 diabetes (relative risk 3.5). IGT was not associated with acanthosis nigricans. Twenty five per cent (n = 19) of those evaluated (n = 75) had evidence of the “metabolic syndrome” (MS). HDL cholesterol and triglyceride levels were related to insulin sensitivity (HOMA‐R); HDL cholesterol was also related to birth weight SDS. We observed a trend for those with MS to have a lower birth weight SDS. The severity of obesity did not influence the likelihood of IGT or MS.
Conclusions
Significant numbers of obese children have associated co‐morbidities. Analysis of fasting blood glucose samples alone is not satisfactory to adequately evaluate glucose homoeostasis. The overall level of obesity does not predict co‐morbidities. Special attention should be given to those with parental diabetes and a history of low birth weight who are more likely to have IGT and abnormal lipid profiles respectively.
Keywords: obesity, impaired glucose tolerance, metabolic syndrome
There is now good evidence that children and adolescents with significant obesity can manifest impaired glucose tolerance,1 frank diabetes,2 or the metabolic syndrome.3,4 Furthermore, recent evidence suggests that impaired glucose tolerance can rapidly progress to overt diabetes and that this process and the worsening of insulin resistance and other features of the “metabolic syndrome” can be ameliorated by weight control.5,6 The combination of limited clinical resources for the treatment of obesity with potentially more therapeutic options becoming available for therapy in adolescence7,8 necessitates a re‐evaluation of our service provision. Greater emphasis may need to be placed on targeting those most at risk of co‐morbidities associated with their obesity rather than obesity per se. With this in mind we examined how to best identify those likely to have impaired glucose tolerance or metabolic syndrome within our obesity clinic, making them a priority for interventions as they become available. The aims of this study were therefore to characterise the metabolic status of children in our obesity clinic and identify factors that predict the clinical phenotype in terms of glucose homoeostasis and the clustering of cardiovascular risk factors that constitute the “metabolic syndrome”.
Methods
All children were referred to our obesity clinic from either general practice or hospital colleagues with the referral criteria being solely a weight >99.6th centile for age and sex. On the first visit, each had a full history, including birth history and the presence of obesity and/or diabetes in either or both parents documented. They were then examined and formally staged for pubertal development by the method of Tanner and Whitehouse. Height was measured to the nearest 0.1 cm using a Harpenden Stadiometer, while weight was measured on SECA scales to the nearest 0.1 kg. Estimation of fat mass and regional fat distribution was performed using a Tanita Bioimpedance model BC‐418MA. BMI was calculated as weight (kg)/height (m)2 and BMI standard deviation score (BMI SDS, representing increases or decreases around the 50th centile for age) was calculated using the British 1990 growth reference data supplied by the Child Growth Foundation. Obesity was defined as being present if the BMI SDS is greater than +2.37 in boys and +2.25 in girls, figures that have been derived by extrapolating the adult cut‐off of 30 kg/m2 back into childhood.9 Blood pressure was measured in the sitting position using an oscillometric method (Dinamap vital signs monitor 8100) with an appropriate sized cuff for arm diameter. Fasting insulin levels were determined using either an ELISA method (DakoCytomation; Code No. K6219) or a two‐site immunoradiometric assay.10 Lipid analysis was performed using an Olympus Diagnostics System Group assay.
A measure of insulin sensitivity: homoeostatic model assessment insulin resistance (HOMA‐R) was derived from the equation:11
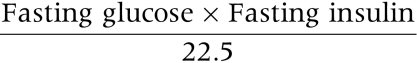 |
Split 32/33 pro‐insulin was measured as part of the genetics of simple obesity study (GOOS) in Cambridge. Sampling for the GOOS study had local ethics committee approval and informed consent was obtained from the families.
The definition of the metabolic syndrome in adults has previously been unclear due to differences between the World Health Organisation definition and that described by the Adult Treatment Panel III guidelines.12 This has recently been clarified by the International Diabetes Federation who have issued a universal and up‐to‐date classification, describing the metabolic syndrome as being present if there is central obesity (using sex and ethnic specific waist circumference cut‐off points) along with any two of the following; raised triglycerides, reduced HDL cholesterol, raised blood pressure, or raised fasting plasma glucose/previously diagnosed type 2 diabetes. Further details regarding this definition can be found at www.idf.org. However, at present, there are no established criteria for diagnosing the metabolic syndrome in childhood.13 We therefore chose to define the metabolic syndrome as being present if cases had a BMI SDS greater than 2.25 for females and 2.37 for males9 and two of the following: a systolic blood pressure greater than the 95th centile for age (using established reference ranges for children obtained from 28 043 children in six North‐West European countries14), plasma triglycerides >1.2 mmol/l for those under 14 years and >1.7 mmol/l above 14 years, HDL <0.9 mmol/l for males and <1.0 mmol/l for females, and/or impaired glucose tolerance on OGTT. This was because, firstly, waist circumference cut‐off points are less universally accepted than BMI SDS for the definition of obesity in childhood,15,16 and secondly, as fasting blood glucose appears to poorly predict those with abnormal glucose metabolism,17 we decided to use IGT on an OGTT to identify individuals with this condition.
Standard statistical tests were used throughout; group comparisons were made using two‐sample Student's t tests (to compare means) and continuity corrected χ2 tests (to compare proportions). Pearson's correlation coefficients were calculated to study the relation between pairs of continuous variables. A series of backward stepwise multiple linear regression analyses were used to look at the independent effects of several variables on serum HDL and triglyceride.
Insulin, HOMA‐R, split pro‐insulin 32/33, and triglyceride were each logarithmically transformed prior to analysis to remove skewness and render the data Gaussian. No satisfactory transformation could be found for blood glucose, and non‐parametric analyses were used for this variable.
Results
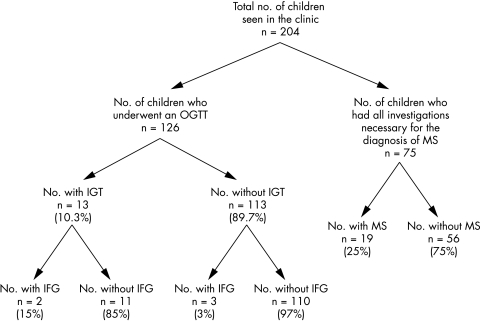
From 1998 onwards, 204 children and adolescents (55% female) have been seen with significant obesity in our clinic, with a mean age of 11.2 years (range 1.7–18 years), mean BMI of 33 (range 21.6–54), and mean BMI SDS of +3.52 (+2.3 to +6.33). The majority (n = 195) were Caucasian, with the remaining nine being either Black‐African (n = 2) or South Asian (n = 7).
Of those, 126 had a standardised oral glucose tolerance test (1.75 g/kg to maximum of 75 g). This test was introduced into the clinic in 2001 in an attempt to better characterise glucose metabolism within this population. Thirteen of these patients (10.3%) had impaired glucose tolerance and none had clinical diabetes. Of the patients with IGT, only 15% (n = 2) would have been picked up as having abnormal glucose homoeostasis by a measurement of fasting glucose alone (impaired fasting glucose). While the number of non‐Caucasian children was small, there was not a significantly higher prevalence of IGT within this group (1 of 6 (17%) non‐Caucasian children, compared to 12 of 120 Caucasian white children (10%); p = 0.6).
Insulin measurements were available for 104 of the 126. Those with IGT had significantly higher fasting insulin levels than the remainder (geometric mean (range): 30.7 (15–59) v 15.5 (2–109) mIU/l; Student's t test based on log transformed values, p < 0.001) and HOMA‐R assessments (geometric mean (range): 6.76 (3.07–11.8) v 3.14 (0.37–18.89); p < 0.001). Fasting blood sugars were raised but the difference was not statistically significant (median (range): 4.9 (4.3–5.7) v 4.6 (3.1–6.6) mmol/l; Mann‐Whitney U‐test, p = 0.053). Acanthosis nigricans was present in 12.7% of cases (26 of 204) and was not associated with IGT (p > 0.999), nor did it relate to fasting insulin levels or insulin resistance estimated by HOMAR in the larger cohort (data not shown). However, a parental history of diabetes was associated with an increased prevalence of IGT; 5 of the 19 (26.3%) with a family history had IGT compared to 8 of the remaining 106 (7.5%) (relative risk 3.5, 95% CI 1.3 to 9.5; p = 0.039).
As there have been reports linking birth weight with obesity and abnormal glucose tolerance, we examined birth weight SDS in cases with IGT and found no significant difference to the rest of the clinic population. The mean SDS was −0.27, with a standard deviation 1.43. The distribution was close to the 1990 “standard” (a Normal distribution with mean 0 and SD 1), although more children than expected were in the extreme tails; for example, 11.1% were below the 2.5th centile and 5% were above the 97.5th.
Seventy five children had all of the necessary investigations to determine the presence/absence of the metabolic syndrome. Nineteen (25%) of the children were found to fulfil the criteria for diagnosis (table 1).
Table 1 Factors used for classification of the metabolic syndrome.
| Males (n = 30) | Females (n = 45) | |
|---|---|---|
| BMI SDS | ||
| >2.37 (male) or >2.25 (female) | 30 | 45 |
| Impaired glucose tolerance on OGTT | 1 | 4 |
| HDL (mmol/l) | ||
| ⩽0.9 (male) or ⩽1.0 (female) | 7 | 17 |
| Triglyceride (mmol/l) | ||
| >1.2 if age <14 y or >1.7 if age ⩾14 y | 11 | 18 |
| Systolic BP | ||
| >95th centile | 12 | 12 |
| ⩾3 of the above factors | 6 | 13 |
Those with the metabolic syndrome had reduced insulin sensitivity compared to those without: fasted insulin (geometric mean (range): 27.6 (9–109) v 13.4 (2–53) mIU/l; p = 0.001) and HOMA‐R (geometric mean (range): 5.66 (1.96–18.89) v 2.84 (0.44–11.31) mIU/l; p = 0.001), and had evidence of pancreatic dysfunction by analysis of the split pro‐insulin 32/33 (geometric mean (range): 27.1 (11–76) v 14.2 (4–50) pmol/l; p = 0.026 but based on a smaller data set, n = 37). If those with both metabolic syndrome and impaired glucose tolerance were specifically excluded from the analysis, the split 32/33 pro‐insulin, fasting insulin, and HOMA‐R levels in the others with metabolic syndrome remained significantly elevated compared to those without it. Metabolic syndrome was not significantly related to the severity of obesity (BMI SDS), total percentage body fat, central fat, acanthosis nigricans, age, sex, pubertal status, and family history of diabetes or obesity (data not shown). Although the number of non‐Caucasian children in this group was small (4 of 75), the prevalence of the metabolic syndrome was the same in both the Caucasian and non‐Caucasian groups (18 of 71 Caucasian children (25%) and 1 of 4 non‐Caucasian children (25%)). The mean birth weight SDS was slightly lower in the group with metabolic syndrome but the difference was not statistically significant (mean −0.64 (SD 1.54) v +0.12 (SD 1.46); mean difference 0.76 (95% CI −0.06 to 1.78; p = 0.068).
We then investigated relationships between individual components of the metabolic syndrome, namely the lipids and systolic blood pressure, with potential predictor variables of age, sex, pubertal status, current BMI SDS, birth weight SDS, and the log transformed HOMAR, firstly using simple univariate analyses and then a series of backward linear regression analyses. On univariate analysis (not shown), HDL was found to be significantly negatively correlated with both age and HOMAR and positively correlated with birth weight SDS. None of the other variables, in particular gender, were significantly related to HDL. Regression analysis (see table 2) confirmed these relationships although age no longer reached statistical significance. Log triglyceride was positively correlated with both age and HOMAR on univariate analysis but age was not significant in the multiple regression (table 2). Systolic blood pressure was related solely to age (r = 0.334; p = 0.004). Results are summarised in fig 1.
Table 2 Results of multiple linear regression analyses to predict HDL and log transformed triglyceride.
| Predictors | HDL (mmol/l)* | Log10(triglyceride)† | ||
|---|---|---|---|---|
| Coeff. (SE) | p value | Coeff. (SE) | p value | |
| Constant | 1.463 (0.102) | −0.212 (0.084) | ||
| Log10(HOMAR) | −0.241 (0.074) | 0.002 | 0.227 (0.065) | 0.001 |
| Age (y) | −0.014 (0.008) | 0.093 | 0.013 (0.007) | 0.068 |
| Birth weight SDS | 0.047 (0.018) | 0.010 | ||
*57 cases with complete information; multiple R = 0.563.
†65 cases with complete information; multiple R = 0.499.
Figure 1 Breakdown of morbidity in obesity clinic.
Discussion
This report again emphasises the morbidity associated with obesity in our childhood population. Over 10% of the children and adolescents we have seen in the clinic have impaired glucose tolerance, a precursor to the development of diabetes, and a defect in glucose homoeostasis that may carry its own inherent risks.18 The level is less than that reported in multi‐racial clinics from the USA where a level of around 25% has been reported.19,20 However, it is higher than that reported from some studies in Europe21,22 while being close to that found in others,23 and very similar to that described by Viner and colleagues4 (11%) from another UK clinic. This study also emphasises the importance of characterising obese children by the use of a formal oral glucose tolerance test rather than simple fasting glucose levels that detect few of the patients who actually have abnormal glucose metabolism and who are at greatest risk of type 2 diabetes. Within a clinical setting it is interesting to note that acanthosis nigricans, a brown velvety rash often seen under the arms, on the knuckles, and around the neck, was not a good predictor of either hyper‐insulinaemia or impaired glucose tolerance in our cohort. Some have reported that this overt and easily recognisable clinical sign is a good predictor of IGT and hyper‐insulinaemia,24,25 but these reports were in Japanese and Native American groups, while others have doubted its true predictive value.26 It seems that the weight of evidence appears to be against the predictive nature of this manifestation, at least in children of Caucasian origin. Importantly, a factor that does seem to have some value as a discriminator of risk is a parent with type 2 diabetes and this has been described before but has not been universally acknowledged.22,27,28 The well documented relationship between birth weight (both large and small for gestational age) in terms of risk for metabolic syndrome and diabetes led us to explore whether these groups were over‐represented in our clinic population or in terms of risk for IGT or metabolic syndrome. The actual distribution and mean birth weight SDS of patients referred with obesity to the clinic was very similar to that of the normal population, while those with IGT had similar weight Z‐scores to those without. Interestingly we did see a trend for those with metabolic syndrome to be smaller than those without (mean BMI SDS −0.64 (SD 1.54) v 0.12 (SD 1.46)), but this did not reach statistical significance (p = 0.068). However, we were able to show that there was a clear association between low birth weight and low HDL cholesterol, a finding that has been documented before in the literature and seems to be one of the links between low birth weight and increased cardiovascular morbidity.29
What is already known on this topic
Childhood obesity is associated with increased co‐morbidities such as impaired glucose tolerance and the “metabolic syndrome”
A family history of diabetes may be important in the development of abnormal glucose homoeostasis
Twenty five per cent of those studied in our cohort had evidence of the metabolic syndrome, a clustering of cardiovascular risk factors linked to increased morbidity and mortality in adult life.30 Those children with metabolic syndrome without impaired glucose tolerance also had evidence of pancreatic dysfunction manifest as a raised split pro‐insulin in addition to hyper‐insulinaemia, suggesting an increased risk of developing type 2 diabetes at a later date.31 Equally worryingly, the clustering of dyslipidaemia, systolic hypertension, and obesity as seen in many of our children, is associated with an increased generation of fibrous plaques in the coronary arteries of adolescents and children, with cumulative effects being seen for each additional abnormality.32 We were unable to show any association between the presence of the metabolic syndrome and either the degree of obesity, total percentage body fat, central fat, acanthosis nigricans, age, sex, pubertal status, and family history of diabetes, or obesity. However, this may have been due to the relatively small number of children identified with this condition, and future studies in larger groups are warranted to investigate whether such associations exist.
A significant proportion of our childhood obesity clinic has either impaired glucose tolerance or the metabolic syndrome. Our prevalence levels mirror those described by Viner and colleagues4 from a separate UK clinic, suggesting that these levels are probably a true reflection of the situation in the country generally.
Recently, a number of pharmacological agents have undergone trials in adolescents with evidence of benefit in terms of weight loss, improving lipid profiles, insulin sensitivity, and blood pressure,7,8 while other studies have shown benefit to morbidity with weight loss (BMI SDS loss >0.5) or weight maintenance, whatever the intervention.5,6 In order to characterise those most likely to benefit from a more intensive approach to obesity, we need sufficient information on which to base such decisions. The measurement of fasting glucose alone seems inadequate to characterise glucose homoeostasis within childhood and we advocate assessment by oral glucose challenge. Furthermore, we believe acanthosis nigricans is a poor clinical marker of any liability to abnormal glucose tolerance, but that those with a family history of type 2 diabetes warrant special attention and further investigation. We also provide some evidence that obese children with a low birth weight are more liable to have features of the metabolic syndrome and probably also deserve special attention.
What this study adds
The clinical importance of an oral glucose tolerance test to examine glucose metabolism fully is shown
It is suggested that birth weight influences HDL cholesterol independent of obesity
Acknowledgements
MS is a Diabetes UK Clinical Training Fellow (BDA:RD 03/0002642). We would also like to thank Dr Janet Stone in the Clinical Chemistry Dept at the Bristol Royal Infirmary for her help with the determination of biochemical markers, and Professor S O'Rahilly and Dr S Farooqi for the determination of split 32/33 proinsulins as part of their Genetics of Obesity Study.
Abbreviations
IGT - impaired glucose tolerance
OGTT - oral glucose tolerance test
MS - metabolic syndrome
Footnotes
Competing interests: Dr Shield has provided paid consultancy to Roche, Abbott, and NovoNordisk Pharmaceuticals
References
- 1.Weiss R, Dufour S, Taksali S E.et al Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet 2003362951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drake A J, Smith A, Betts P R.et al Type 2 diabetes in obese white children. Arch Dis Child 200286207–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss R, Dziura J, Burgert T S.et al Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 20043502362–2374. [DOI] [PubMed] [Google Scholar]
- 4.Viner R M, Segal T Y, Lichtarowicz‐Krynska E.et al Prevalence of the insulin resistance syndrome in obesity. Arch Dis Child 20059010–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss R, Taksali S E, Tamborlane W V.et al Predictors of changes in glucose tolerance status in obese youth. Diabetes Care 200528902–909. [DOI] [PubMed] [Google Scholar]
- 6.Reinehr T, Kiess W, Kapellen T.et al Insulin sensitivity among obese children and adolescents, according to degree of weight loss. Pediatrics 20041141569–1573. [DOI] [PubMed] [Google Scholar]
- 7.Ozkan B, Bereket A, Turan S.et al Addition of orlistat to conventional treatment in adolescents with severe obesity. Eur J Pediatr 2004163738–741. [DOI] [PubMed] [Google Scholar]
- 8.Godoy‐Matos A, Carraro L, Vieira A.et al Treatment of obese adolescents with sibutramine: a randomized, double‐blind, controlled study. J Clin Endocrinol Metab 2005901460–1465. [DOI] [PubMed] [Google Scholar]
- 9.Cole T J, Bellizzi M C, Flegal K M.et al Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 20003201240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sobey W J, Beer S F, Carrington C A.et al Sensitive and specific two‐site immunoradiometric assays for human insulin, proinsulin, 65–66 split and 32–33 split proinsulins. Biochem J 1989260535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pacini G, Mari A. Methods for clinical assessment of insulin sensitivity and beta‐cell function. Best Pract Res Clin Endocrinol Metab 200317305–322. [DOI] [PubMed] [Google Scholar]
- 12.Aguilar‐Salinas C A, Rojas R, Gomez‐Perez F J.et al Analysis of the Agreement Between the World Health Organization Criteria and the National Cholesterol Education Program—III. Definition of the metabolic syndrome: results from a population‐based survey, Diabetes Care 2003261635. [DOI] [PubMed] [Google Scholar]
- 13.Weiss R, Caprio S. The metabolic consequences of childhood obesity. Best Pract Res Clin Endocrinol Metab 200519405–419. [DOI] [PubMed] [Google Scholar]
- 14.de Man S A, Andre J L, Bachmann H.et al Blood pressure in childhood: pooled findings of six European studies. J Hypertens 19919109–114. [DOI] [PubMed] [Google Scholar]
- 15.Dietz W H, Bellizzi M C. Introduction: the use of body mass index to assess obesity in children. Am J Clin Nutr 199970123S–125S. [DOI] [PubMed] [Google Scholar]
- 16.Reilly J J, Wilson M L, Summerbell C D.et al Obesity: diagnosis, prevention, and treatment; evidence based answers to common questions. Arch Dis Child 200286392–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez‐Diaz R, Aguilar‐Salinas C A, Moran‐Villota S.et al Lack of agreement between the revised criteria of impaired fasting glucose and impaired glucose tolerance in children with excess body weight. Diabetes Care 2004272229–2233. [DOI] [PubMed] [Google Scholar]
- 18.Singleton J R, Smith A G, Russell J W.et al Microvascular complications of impaired glucose tolerance. Diabetes 2003522867–2873. [DOI] [PubMed] [Google Scholar]
- 19.Sinha R, Fisch G, Teague B.et al Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med 2002346802–810. [DOI] [PubMed] [Google Scholar]
- 20.Goran M I, Ball G D, Cruz M L. Obesity and risk of type 2 diabetes and cardiovascular disease in children and adolescents. J Clin Endocrinol Metab 2003881417–1427. [DOI] [PubMed] [Google Scholar]
- 21.Invitti C, Guzzaloni G, Gilardini L.et al Prevalence and concomitants of glucose intolerance in European obese children and adolescents. Diabetes Care 200326118–124. [DOI] [PubMed] [Google Scholar]
- 22.Wabitsch M, Hauner H, Hertrampf M.et al Type II diabetes mellitus and impaired glucose regulation in Caucasian children and adolescents with obesity living in Germany. Int J Obes Relat Metab Disord 200428307–313. [DOI] [PubMed] [Google Scholar]
- 23.Shalitin S, Abrahami M, Lilos P.et al Insulin resistance and impaired glucose tolerance in obese children and adolescents referred to a tertiary‐care center in Israel. Int J Obes Relat Metab Disord 200529571–578. [DOI] [PubMed] [Google Scholar]
- 24.Yamazaki H, Ito S, Yoshida H. Acanthosis nigricans is a reliable cutaneous marker of insulin resistance in obese Japanese children. Pediatr Int 200345701–705. [DOI] [PubMed] [Google Scholar]
- 25.Stoddart M L, Blevins K S, Lee E T.et al Association of acanthosis nigricans with hyperinsulinemia compared with other selected risk factors for type 2 diabetes in Cherokee Indians: the Cherokee Diabetes Study. Diabetes Care 2002251009–1014. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen T T, Keil M F, Russell D L.et al Relation of acanthosis nigricans to hyperinsulinemia and insulin sensitivity in overweight African American and white children. J Pediatr 2001138474–480. [DOI] [PubMed] [Google Scholar]
- 27.Srinivasan S R, Frontini M G, Berenson G S. Longitudinal changes in risk variables of insulin resistance syndrome from childhood to young adulthood in offspring of parents with type 2 diabetes: the Bogalusa Heart Study. Metabolism 200352443–453. [DOI] [PubMed] [Google Scholar]
- 28.Goran M I, Coronges K, Bergman R N.et al Influence of family history of type 2 diabetes on insulin sensitivity in prepubertal children. J Clin Endocrinol Metab 200388192–195. [DOI] [PubMed] [Google Scholar]
- 29.RG IJ. Stehouwer C D, Van Weissenbruch M M.et al Evidence for genetic factors explaining the association between birth weight and low‐density lipoprotein cholesterol and possible intrauterine factors influencing the association between birth weight and high‐density lipoprotein cholesterol: analysis in twins. J Clin Endocrinol Metab 2001865479–5484. [DOI] [PubMed] [Google Scholar]
- 30.Reaven G. The metabolic syndrome or the insulin resistance syndrome? Different names, different concepts, and different goals. Endocrinol Metab Clin North Am 200433283–303. [DOI] [PubMed] [Google Scholar]
- 31.Kahn S E, Leonetti D L, Prigeon R L.et al Proinsulin levels predict the development of non‐insulin‐dependent diabetes mellitus (NIDDM) in Japanese‐American men. Diabet Med 199613(9 suppl 6)S63–S66. [PubMed] [Google Scholar]
- 32.Berenson G S, Srinivasan S R, Bao W.et al Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med 19983381650–1656. [DOI] [PubMed] [Google Scholar]