Abstract
Background
Dengue is the most important mosquito borne viral infection in the world. Nearly 90% of infections occur in children. At present, prospective information on clinical and laboratory findings in South Asian children with dengue is generally lacking.
Aim
To describe patterns of clinical disease in a cohort of children hospitalised with dengue during a major dengue epidemic in Sri Lanka.
Results
A total of 104 children were studied during a three month period. Eighteen had dengue fever (DF) and 86 had dengue haemorrhagic fever (DHF). Of those with DHF, 34, 23, 27, and 2 had DHF grade I, II, III, and IV respectively. Based on dengue serology testing, 13 of the DF patients had a primary infection and 5 had secondary dengue infections. In contrast, 68 of the children with DHF had secondary and 18 had primary dengue infections. Oral candidiasis was seen in 19 children. The odds ratio for children with secondary dengue infection to develop DHF was 9.8 (95% CI 3.1 to 31.2).
Conclusion
Studies on patterns of paediatric dengue disease in different regions should help clinicians and health administrators make more informed and evidence based health planning decisions. It should also help towards mapping out dengue trends on a global scale. Oral candidiasis has not been previously documented in children suffering with acute dengue in Sri Lanka or elsewhere. Studying underlying reasons for this manifestation during future dengue epidemics may provide useful leads in understanding overall dengue pathogenesis.
Keywords: candida, Sri Lanka, clinical findings, dengue, laboratory findings
Dengue is epidemic or endemic in virtually every tropical country.1 It is considered the most important viral haemorrhagic fever in the world. Infection may be asymptomatic or give rise to undifferentiated fever, dengue fever (DF), dengue hemorrhagic fever (DHF), or dengue shock syndrome (DSS).1 The dengue virus is a RNA virus of the flaviviridae family and consists of four serotypes (DEN1–4). Generally, infection with one serotype confers future protective immunity against that particular serotype but not against others. Nearly 90% of dengue infections occur in children.2 The risk that a child will die during a secondary dengue infection is nearly 15‐fold higher than that of adults.3 Epidemics of dengue occur regularly in Sri Lanka,4,5 with the largest and most severe one recorded so far occurring between April and August 2004 (H Tissera, Sri Lanka Epidemiology Unit, personal communication, 2004). These epidemics result in significant morbidity and mortality and its control and management requires the allocation of considerable financial and medical resources.
Although children are the main group affected by dengue, little published data are available regarding dengue infections in children living in South Asia.6,7,8 Such information is necessary if we are to put in place evidence based health policy measures to deal with the problem as it applies to this region, in turn ensuring the best use of limited health resources. Although the dynamics of dengue viral infections together with genetic, social, and demographic factors differ between South Asian and South‐East Asian countries, the tendency so far has been to make health policy decisions by blindly postulating from South‐East Asian data, some of which was generated nearly 40 years ago.9 In this study, we studied the patterns of disease in a cohort of Sri Lankan children hospitalised with dengue, seen during a recent major epidemic, and compared these with patterns reported previously from other parts of the world. We also assessed possible risk factors associated with severe disease in this cohort.
Methods
This study was carried out in a general paediatric ward at the Lady Ridgeway Hospital for children in Colombo, Sri Lanka during a three month period (24 April to 31 July 2004). All children with clinical features suggestive of dengue infections admitted to this ward were included after obtaining informed written consent from the parent or guardian. Ethical clearance for the study was obtained from the ethical review committee of the University of Sri Jayawardanapura, Sri Lanka. Basic demographic data were collected from each child included in the study. Clinical and haematological/biochemical findings were recorded serially until discharge. A history of asthma or eczema (both past and present) in each child was ascertained from their parents, and treatment being used at present for these conditions was noted. Heights were measured to the nearest 0.1 cm using a standiometer, and weights measured to the closest 100 g using an electronic weighing scale. Body mass index (BMI) was calculated and plotted on revised NCHS (2000) growth charts in order to obtain a global estimate of nutritional status. A tourniquet test was performed to determine its usefulness in predicting future bleeding manifestations.10
The WHO classification and case definitions were used to classify disease in these children as either DF or DHF.2 Evidence of plasma leakage (pleural effusions, ascites, or rise in packed cell volume (PCV) >45) or shock, even in the absence of bleeding manifestations, was considered as indicating DHF. A lateral decubitus chest radiograph was obtained in all children with clinical suspicion of a pleural effusion; blood groups were assessed in all. DHF was further divided into four grades (I, II, III, IV) as per WHO definitions.2 Dengue viral specific antibodies were detected using the PANBIO Dengue duo IgM and IgG rapid strip test,11 on a serum sample taken at least seven days after onset of the illness.
Data was analysed using the SPSS (version 10.0) statistical package. To calculate odds ratios of possible risk factors for severe dengue infections, DF and DHF grade I were categorised as mild infections, and DHF grade II–IV as severe infections.
Results
During the three month period of this study, 125 children were admitted to a single paediatric ward with clinical features suggestive of dengue infection. Of these, 104 (83.2%) had dengue serology indicative of an acute infection; 61 (59.8%) were female and 43 (41.2%) male, and their ages ranged from 1 month to 12 years (mean 7.9 years, SD 2.9).
Eighteen (17.3%) had DF and 86 (82.7%) had DHF. Of those with DHF, 34 (39.5%), 23 (26.7%), 27 (31.4%), and 2 (2.3%) had DHF grade I, II, III, and IV respectively. Based on dengue serology testing, 13 (72.2%) of the DF patients had a primary infection and 5 (27.7%) had secondary dengue infections. In contrast, 68 (79.1%) of the children with DHF had secondary and 18 (20.9%) had primary dengue infections. The odds ratio (OR) for children with secondary dengue infection to develop DHF was 9.8 (95% CI 3.1 to 31.2).
Clinical findings
The spectrum of clinical findings in children with DF and DHF is shown in table 1. Ninety three (90%) of 104 children had a flushed appearance irrespective of the severity of their illness. A runny nose and pharyngeal congestion (features usually not described during adult dengue infections) were present in 20 (19.2%) and 17 (16.3%) children respectively. Pharyngeal congestion was more prevalent in children with DHF then DF. The mean (SD) duration of hospitalisation in children with DF and DHF was 4.1 (2.4) and 4.8 (4.1) days respectively.
Table 1 Clinical and laboratory findings in paediatric dengue patients.
| Clinical and laboratory findings | DF (n = 18) | DHF (n = 86) | p value* |
|---|---|---|---|
| No. (%) | No. (%) | ||
| Flushed appearance | 17 (94) | 76 (88) | >0.05 |
| Diarrhoea | 02 (11) | 16 (19) | >0.05 |
| Vomiting | 11 (61) | 66 (77) | >0.05 |
| Headache | 12 (66) | 62 (72) | >0.05 |
| Pharyngeal congestion | 06 (33) | 11 (13) | <0.05 |
| Runny nose | 05 (27) | 15 (17) | >0.05 |
| Bleeding manifestations | 04 (22) | 36 (42) | >0.05 |
| Petechiae | 03 (16) | 14 (16) | |
| Ecchymoses | 01 (6) | 04 (5) | |
| Haemetemesis | 01 (6) | 13 (15) | |
| Melaena | 00 (0) | 05 (6) | |
| Bleeding from gums | 01 (6) | 12 (14) | |
| Epistaxis | 01 (6) | 03 (4) | |
| Positive tourniquet test | 04 (22) | 24 (28) | >0.05 |
| Hypotension | 01 (6) | 30 (35) | |
| Prolonged capillary refilling time | 02 (11) | 29 (34) | <0.05 |
| Pleural effusions | 00 (0) | 70 (81) | <0.001 |
| Ascites | 00 (0) | 39 (45) | <0.001 |
| Impaired consciousness | 01 (6) | 06 (7) | |
| Presence of recovery rash | 07 (39) | 17 (20) | >0.05 |
| Platelet count | <0.001 | ||
| >100×109/l | 15 (83) | 16 (19) | |
| 51–100×109/l | 03 (17) | 30 (35) | |
| 21–50×109/l | 00 (0) | 30 (35) | |
| ⩽20×109/l | 00 (0) | 10 (12) | |
| Haemoconcentration (PCV >45) | 02 (11) | 50 (58) | <0.001 |
| Low WBC (<4×109/l) | 03 (17) | 13 (15) | >0.05 |
| Raised ALT | 04 (22) | 47 (55) | >0.05 |
| Raised AST | 05 (28) | 65 (76) | <0.005 |
Percentages rounded to nearest whole number.
*χ2 test.
Bleeding manifestations were seen in 40 (38.5%) children (petechiae, gum bleeding, and haematemesis were the most frequent manifestations). Seventeen (16.3%) children were given blood products (platelet concentrates, n = 9; fresh frozen plasma, n = 14). Bleeding manifestations were also present in 4 (22.2%) of 18 children with DF. Platelet levels of ⩽100×109/l were detected in 73 (70.2%) children, of whom 29 (39.7%) had bleeding manifestations. Fourteen (46.7%) of 30 and 5 (50%) of 10 children with platelet counts 20–50×109/l and ⩽20×109/l respectively had bleeding manifestations. There was no significant association (p > 0.05, χ2 test) between the degree of thrombocytopenia and the presence of bleeding manifestations. The tourniquet test was positive in 19 (47.5%) of 40 children having other bleeding manifestations, in 8 (24.2%) children with platelet counts 51–100×109/l, and in 15 (37.%) children with platelet counts ⩽50×109/l.
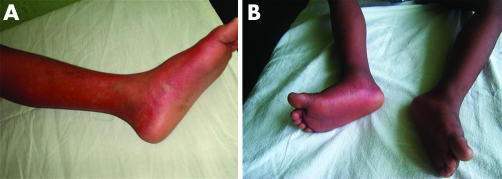
Three types of rash were seen in the children. Rashes were most obvious during the convalescent phase of their illness. These included a “recovery rash” (generalised erythematous rash with islands of pallor; fig 1A) in 24 (23.1%), a generalised macular papular rash (fig 1B) in 9 (8.7%), and a petechial rash in 8 (7.7%) children. The “recovery rash” was present in a higher proportion of those having primary (48.3%) than secondary dengue infections (14.7%). The petechial rash was only seen in those recovering from DHF.
Figure 1 Rashes observed in children with dengue viral infections. (A) “Recovery rash”, a generalised erythematous rash with islands of pallor. (B) Generalised macular popular rash. Consent was obtained for publication of this figure.
An altered level of consciousness (defined as a score of <10 out of a possible 15 points on a modified Glasgow coma scale) was seen in 6 (5.8%) children. Mean alanine transaminase (ALT) and aspartate transaminase (AST) levels in these six children were >8 and >12 times the upper limits of normal. Myocarditis (manifested as tachycardia, triple rhythm, and heart failure) was seen in 3 (2.9%) children, all of whom had DHF. All had low ejection fractions on echocardiography carried out during the acute stage, which returned to normal 7–10 days later. The QT interval on an ECG was prolonged in one of the children with myocarditis. Three (2.9%) children developed prolonged shock and needed ICU admission (one of them had an altered level of consciousness). No deaths were seen in our series. Seven (6.7%) children had secondary bacterial infections, such as lobar pneumonia and septicaemia, complicating their dengue infection. E coli was isolated from blood cultures in two children.
Oral candidiasis was seen in 19 (18.3%) of the 104 children (10 females, 9 males). Mean ages and sex distribution of these children were comparable to those that did not have oral candidiasis. In 15 (78.9%) children the pharynx alone, and in 4 (21.1%) the whole buccal mucosa (palate, cheeks, and tongue) was involved. Careful examination of the oropharynx using a good light source and a tongue depressor was needed to detect the oral candidiasis. It lasted for a mean (SD) of 7.3 (1.2) and 2.3 (0.8) days. None were previously immunosuppressed or taking steroids. Four (21.1%) had primary and 15 (78.9%) secondary dengue infections. Eight (42.1%) children with oral candidiasis had DHF grade III compared with 19 (22.3%) children without this complication. In contrast, 2 (11%) children with and 21(25%) without oral candidiasis had DHF grade II. A comparison of clinical findings in children with and without oral candidiasis is shown in table 2. Two children with oral candidiasis also had a secondary bacterial infection. All were treated successfully with miconozole oral gel.
Table 2 Characteristics of patients with and without oral candidiasis.
| Oral candidiasis present (n = 19) | Oral candidiasis absent (n = 85) | |
|---|---|---|
| No. (%) | No. (%) | |
| Secondary dengue infections | 15 (80) | 58 (68) |
| Primary dengue infections | 04 (21) | 27 (32) |
| Dengue fever | 02 (11) | 16 (19) |
| DHF | ||
| Grade 1 | 07 (37) | 27 (32) |
| Grade 2 | 02 (11) | 21 (25) |
| Grade 3 | 08 (42) | 19 (22) |
| Grade 4 | 00 (0) | 02 (2) |
| Pleural effusion | 14 (74) | 55 (65) |
| Ascites | 11 (58) | 27 (32) |
| Pedal oedema | 03 (16) | 01 (1) |
| Secondary bacterial infections | 02 (11) | 05 (6) |
| BMI for age <5th centile | 14 (74) | 41 (48) |
| ICU admission | 00 (0) | 03 (3) |
Percentages rounded to nearest whole number.
Laboratory findings
Laboratory findings in children with DF and DHF are shown in table 1. Platelet counts were lowest and the haematocrit highest on mean (SD) 5.6 (1.6) and 5.9 (1.5) days respectively. High ALT and AST levels were seen in 51 (49%) and 70 (67.3%) children. AST levels were significantly higher (p < 0.002) among children with DHF compared to DF.
Risk factors for severe dengue infections
Estimates of possible risk factors (as individual odds ratios) for severe dengue infection in this group of children are shown in table 3. Secondary dengue infections and blood group O increased the risk ratio. Age, sex, presence of atopic diseases (such as asthma or eczema), and nutritional status did not appear to alter the risk.
Table 3 Possible risk factors for severe dengue infections.
| Risk factor | Mild infections (DF + DHF 1) (n = 52) | Severe infections (DHF 2, 3, 4) (n = 52) | OR (95% CI) | p value* |
|---|---|---|---|---|
| No. (%) | No. (%) | |||
| Sex (female) | 29 (55.8) | 32 (61.5) | 1.1 (0.5 to 2.5) | >0.05 |
| Blood group | ||||
| O | 16 (30.8) | 29 (55.8) | 2.5 (1.1 to 5.6) | 0.029 |
| B | 16 (30.8) | 12 (23.1) | 0.6 (0.2 to 1.4) | >0.05 |
| A | 8 (15.4) | 10 (19.2) | 0.7 (0.2 to 1.9) | >0.05 |
| Secondary dengue infection | 28 (53.8) | 45 (86.5) | 3.2 (1.3 to 7.9) | 0.008 |
| Asthma | 12 (23.1) | 12 (23.1) | 0.9 (0.5 to 2.3) | >0.05 |
| Eczema | 8 (15.4) | 7 (15.4) | 1.1 (0.5 to 3.2) | >0.05 |
| BMI for age >90th centile | 3 (5.8) | 4 (7.7) | 1.4 (0.3 6.5) | >0.05 |
| BMI for age <5th centile | 28 (53.8) | 27 (51.9) | 0.9 (0.4 to 2.2) | >0.05 |
*χ2 test.
Discussion
In this report we describe clinical and laboratory findings in a sizeable cohort of hospitalised children with dengue infections seen in a paediatric centre in South Asia. Eighteen per cent of these children developed oral candidiasis. To the best of our knowledge this clinical manifestation has not been previously documented in children suffering with acute dengue in Sri Lanka or elsewhere. In 2004, Sri Lanka experienced its largest and most severe dengue epidemic seen so far. By the end of July 2004, over 10 000 cases had been reported (H Tissera, Sri Lanka Epidemiology Unit, personal communication, 2004). The exact reasons for this very severe and large epidemic have not been well defined. A change in the predominant circulating dengue viral serotype has been suggested as a reason for the increased severity of this epidemic. Although DEN 2 has been the predominant serotype during past dengue epidemics in Sri Lanka,12,13 it changed to DEN 3 during the present epidemic.14,15
There was no significant association between platelet counts, bleeding manifestations, and a positive tourniquet test. Some children with DF had mild bleeding manifestations (for example, petechiae) as has been seen in some other studies.16 Two of the children who had a PCV >45 were still classified as having DF and not DHF as they did not have any other criteria for a diagnosis of DHF and their PCV remained >45 even following complete recovery. Over 50% of children had abnormal liver enzymes (high ALT, 49%; high AST, 67%), with AST levels being significantly higher in children with DHF than DF. However, none of the children developed fulminant liver disease. A higher incidence of dengue encephalopathy (5.6%) was seen in this cohort compared to that reported in other series.17,18 Children with this complication had very high AST levels (>10 times the normal upper limit). Some children developed secondary bacterial infections as has been reported previously.19,20
What is already known on this topic
Dengue is the most important mosquito borne viral infection in the world; nearly 90% of dengue infections worldwide occur among children
Secondary dengue infection increases the risk of developing severe forms of disease
Secondary dengue infections increased the risk of severe disease in our cohort. This link is similar to that reported previously in children from South‐East Asia and the Americas.16,20 Of DHF cases in our cohort, 20.9% occurred following primary dengue infections, which is different to the profile normally described. Of children with the severe grades of disease in our cohort, 55.8% had blood group O. Although this value is higher than the prevalence of this blood group in the overall Sri Lankan population (43.4%),21 this difference did not reach statistical significance (p > 0.05). A previous report on adult dengue patients reported that DHF and DSS was seen more commonly in patients with blood group B.22 Although a few previous studies suggest asthma as a risk factor for developing DHF/DSS,23,24 we could not confirm this finding in our children. Similarly, we did not find female children to be more at risk of developing severe disease, despite this been reported in other settings.25
Malnutrition is known to predispose children to acquiring infectious diseases.26 In addition, it is known to increase the severity of some infections such as measles.27 We did not find poor nutritional status to be a risk factor for severe dengue disease, although this has been shown by others.28,29 Although as a group the BMIs were not significantly different in children with severe or mild disease, over 70% of those children who developed oral candidiasis had a BMI for age <5th centile.
A sizable proportion of children hospitalised with dengue infection seen by us developed oral candidiasis. This new manifestation may be due to several reasons. Increased viral virulence (and possibly greater associated immune suppressive effects) in the recent epidemic is a plausible hypothesis. This would need formal testing if seen during future epidemics. This may have played a part in overcoming the normal protective immune responses in a subset of children. Conversely, it may be argued that this feature was only picked up because more detailed patient examination was done in the ongoing study on dengue. However, we think this less likely as the same findings were not observed in any adult dengue patients (with equivalent degrees of disease severity) also studied by us during the same epidemic. The proportion of children in our cohort developing oral candidiasis following dengue infection was nearly 20 times higher than among other hospitalised sick children seen during the same time period. The recognition of this association with dengue should make us look out for it during future dengue epidemics.
In summary, we have systematically collected and reported clinical and laboratory findings in a cohort of Sri Lankan children with dengue infections and then proceeded to highlight important differences in clinical manifestations seen during the recent severe epidemic. Availability of more studies of this type from different countries should help clinicians and health administrators make more informed and evidence based health planning decisions, and also be able to use pooled data from several countries to study disease trends and variations.
What this study adds
Patterns of dengue disease among a cohort of hospitalised children from Sri Lanka have been described in an attempt to increase the amount of prospectively collected information on paediatric dengue disease from the South Asian region
A group of children who developed oral candidiasis during their acute dengue infection has been described
Acknowledgements
We wish to express our gratitude to Dr John Aaskov, Director of the Arbovirus Reference Centre for providing the Dengue duo IgM and IgG rapid strips for performing dengue virus serology; Dr Vathsala Jayasuriya, Lecturer, Department of Community Medicine, Faculty of Medical Sciences, University of Sri Jayawardanapura for assistance with statistics; Dr Hasitha Tissera, Epidemiology Unit, Sri Lanka for sharing data on the Sri Lankan dengue epidemic in 2004; and the Asian Development Bank for funding the study.
Abbreviations
DF - dengue fever
DHF - dengue haemorrhagic fever
DSS - dengue shock syndrome
Footnotes
Competing interests: none declared
Consent was obtained for publication of figure 1
References
- 1.Guha‐Sapir D, Schimmer B. Dengue fever: new paradigms for a changing epidemiology. Emerg Themes Epidemiol 200521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organisation Prevention and control of dengue and dengue haemorrhagic fever: comprehensive guidelines. WHO regional publication. SEARO, No. 29. WHO 1999
- 3.Guzman M G, Kouri G, Bravo J.et al Effect of age on outcome of secondary dengue 2 infections. Int J Infect Dis 20026118–124. [DOI] [PubMed] [Google Scholar]
- 4.Malavige G N, Fernando S, Fernando D J.et al Dengue viral infections. Postgrad Med J 200480588–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Annual Health Bulletin, Sri Lanka 2000123
- 6.Lucus G N, Amerasinghe A, Sriranganathan S. Dengue haemorrhagic fever in Sri Lanka. Indian J Pediatr 200067503–504. [DOI] [PubMed] [Google Scholar]
- 7.Kabilan L, Balasubramanian S, Keshava S M.et al Dengue disease spectrum among infants in the 2001 dengue epidemic in Chennai, Tamil Nadu, India. J Clin Microbiol 2003413919–3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra B, Ratho R K. Virological interpretations of dengue disease spectrum in infants in Chennai, Tamil Nadu, India, need re‐evaluation. J Clin Microbiol 2004422357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nimmannitiya S, Halstead S B, Cohen S N.et al Dengue and Chikungunya virus infection in man in Thailand, 1962–1964. I. Observations on hospitalized patients with haemorrhagic fever. Am J Trop Med Hyg 196918954–971. [DOI] [PubMed] [Google Scholar]
- 10.Wali J P, Biswas A, Aggarwal P.et al Validity of tourniquet test in dengue haemorrhagic fever. J Assoc Physicians India 199947203–204. [PubMed] [Google Scholar]
- 11.Cuzzubbo A J, Vaughn D W, Nisalak A.et al Comparison of PanBio dengue duo enzyme‐linked immunosorbent assay (ELISA) and MRL dengue fever virus immunoglobulin M capture ELISA for diagnosis of dengue virus infections in Southeast Asia. Clin Diagn Lab Immunol 19996705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Messer W B, Vitarana U T, Sivananthan K.et al Epidemiology of dengue in Sri Lanka before and after the emergence of epidemic dengue hemorrhagic fever. Am J Trop Med Hyg 200266765–773. [DOI] [PubMed] [Google Scholar]
- 13.Velathanthiri N S, Fernando R, Fernando S.et al Development of a polymerase chain reaction (PCR) for the detection of Dengue virus and its sero types [abstract]. Presented at the Sri Lanka College of Microbiologists annual sessions 2002
- 14.Velathanthiri N S, Malavige G N, Ranatunga P.et al Serological, virological and molecular biological investigation of the dengue epidemic in 2004 [abstract]. Presented at the Annual Scientific Sessions of the Sri Lanka College of Microbiologists 2004
- 15.Baranage G, Seneviratne D, Gamage P.et al Screening of febrile cases for early diagnosis of dengue and identification of dengue virus type using in‐house diagnostic kits based on polymerase chain reaction [abstract]. Presented at the Annual Scientific Sessions of the Sri Lanka College of Microbiologists 2004
- 16.Wichmann O, Hongsiriwon S, Bowonwatanuwong C.et al Risk factors and clinical features associated with severe dengue infection in adults and children during the 2001 epidemic in Chonburi, Thailand. Trop Med Int Health 200491022–1029. [DOI] [PubMed] [Google Scholar]
- 17.Cam B V, Fonsmark L, Hue N B.et al Prospective case‐control study of encephalopathy in children with dengue hemorrhagic fever. Am J Trop Med Hyg 200165848–851. [DOI] [PubMed] [Google Scholar]
- 18.Lee I K, Liu J W, Yang K D. Clinical characteristics and risk factors for concurrent bacteremia in adults with dengue hemorrhagic fever. Am J Trop Med Hyg 200572221–226. [PubMed] [Google Scholar]
- 19.Kalayanarooj S, Chansiriwongs V, Nimmannitya S. Dengue patients at the Children's Hospital, Bangkok: 1995–1999. Dengue Bulletin 20022633–43. [Google Scholar]
- 20.Guzman M G, Kouri G P, Bravo J.et al Dengue hemorrhagic fever in Cuba, 1981: a retrospective seroepidemiologic study. Am J Trop Med Hyg 199042179–184. [DOI] [PubMed] [Google Scholar]
- 21.de Zoysa N S. Prevalence of Rhesus blood groups in Sri Lanka. Ceylon Med J 199338129–130. [PubMed] [Google Scholar]
- 22.Bulugahapitiya D U, Satarasinghe R L. Preponderance of blood group B among dengue fever patients with serious complications in a tertiary care hospital. Ceylon Med J 20034895–96. [DOI] [PubMed] [Google Scholar]
- 23.Guzman M G, Kouri G, Soler M. Dengue 2 virus enhancement in asthmatic and non asthmatic individual. Mem Inst Oswaldo Cruz 199287559–564. [DOI] [PubMed] [Google Scholar]
- 24.Cunha R V, Schatzmayr H G, Miagostovich M P.et al Dengue epidemic in the State of Rio Grande do Norte, Brazil, in 1997. Trans R Soc Trop Med Hyg 199993247–249. [DOI] [PubMed] [Google Scholar]
- 25.Nimmannitya S. Dengue haemorrhagic fever: current issues and future research. Asian‐Oceanian Journal of Paediatrics and Child Health 200211–21. [Google Scholar]
- 26.Cegielski J P, McMurray D N. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis 20048286–298. [PubMed] [Google Scholar]
- 27.Phillips R S, Enwonwu C O, Okolo S.et al Metabolic effects of acute measles in chronically malnourished Nigerian children. J Nutr Biochem 200415281–288. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen T H, Nguyen T L, Lei H Y. Association between sex, nutritional status, severity of dengue hemorrhagic fever, and immune status in infants with dengue hemorrhagic fever. Am J Trop Med Hyg 200572370–374. [PubMed] [Google Scholar]
- 29.Kalayanarooj S, Nimmannitya S. Is dengue severity related to nutritional status? Southeast Asian J Trop Med Public Health 200536378–384. [PubMed] [Google Scholar]