Abstract
Background
The relationship between asthma severity and atopy is complex. Many studies have failed to show significant relationships between clinical severity or lung function and markers of atopic sensitisation.
Aim
To determine whether increasing asthma severity is related to atopic sensitisation in a population of children with asthma.
Methods
A total of 400 children (7–18 years) with asthma were recruited as part of a multicentre study of the genetics of asthma. Detailed phenotypic data were collected on all participants. Associations between measures of asthma severity and atopic sensitisation were sought using multilevel models allowing variation at the individual and family level.
Results
Children recruited to the study had a range of asthma severities, with just over a third having mild persistent asthma. The logarithm of total serum IgE was associated with increased asthma severity score, decreased FEV1, increased airways obstruction, risk of hospital admission, and inhaled steroid use. Increasing skin prick test reactivity to a panel of seven aeroallergens was associated with increased risk of hospital admission, use of an inhaled steroid, and airways obstruction. The results remained highly significant after corrections for age, gender, and birth order.
Conclusions
In children with asthma, increasing atopy is associated with increasing asthma severity. However, the relationships between asthma severity and skin prick tests, and asthma severity and total serum IgE values, appear subtly different.
Keywords: asthma, atopy, skin prick tests, lung function
The relationship between atopy and asthma is not straightforward. Historically “atopy” has been used as a poorly defined term to refer to allergic conditions such as hay fever, asthma, and eczema, which cluster in families. As knowledge about the immunological features of atopic diseases increased, a clearer definition evolved. Since the original description of IgE in 1966, knowledge about its role in the pathogenesis of allergic diseases has proliferated.1 Atopy can be broadly defined as the tendency to generate an IgE response to specific allergens2 or the tendency to generate a wheal of greater than 3 mm in response to skin prick testing.3
Evidence now implicates serum IgE in development and expression of the asthma phenotype. Observational and epidemiological studies4,5 prompted the development of specific monoclonal antibodies to IgE.6 The therapeutic success of anti‐human IgE antibody in adults7,8 and children9 with asthma supports the importance of IgE in disease expression.
The degree of atopy can also be defined as the number and/or size of positive skin prick tests (SPTs) to a standard panel of allergens.10,11,12,13 In children, increasing wheal size to house dust mite Der p 1 has been associated with increasing asthma symptoms and increased risk of hospital admission.14,15 However, relationships between atopy and asthma severity are often weak or fail to achieve statistical significance. Even in large studies results have been mixed. Siroux et al failed to show a relationship between the degree of allergic sensitisation measured by the number of positive SPTs or the size of the wheal reactions and asthma severity.10 They suggested further studies including a sufficiently large number of severe patients to clarify such relationships. We therefore used the data collected as part of the GAIN collaboration (Genetics of Asthma International Network) to address this question in a larger number of children than had been available in previous studies.
Methods
Study subjects
A total of 232 families with at least one asthmatic child (aged 7–18 years) were recruited via an asthmatic proband from the two centres (North Staffordshire and Sheffield) between January 1999 and December 2001. Inclusion criteria for probands included documented episodes of wheezing in the previous 12 calendar months, physician diagnosis of asthma, and agreement to participate by the natural mother and father. All first‐degree relatives of probands were invited to participate in this study, but only those with a previous physician diagnosis of asthma and who were less than 19 years of age were included in the present analysis. Identical twins were not eligible to participate, siblings without a physician diagnosis of asthma were not eligible, and subjects with cardiac, other respiratory, or inflammatory diseases were excluded. The study was approved by the local research ethics committee at each centre and written informed consent was obtained from all participants.
Participants completed a standardised observer administered ISAAC questionnaire16 and underwent baseline spirometry (Morgan Rolling‐seal 232).17 Lung function results in children and adults were expressed as percentage predicted using previously established reference ranges for UK Caucasian children18 and adults.19 Spirometry in both centres was undertaken in a dedicated lung function laboratory in accordance with American Thoracic Society standards.20 Skin prick testing (SPT) and measurement of total serum IgE were performed on all subjects. For measurement of total serum IgE, serum was separated and stored at −80°C within 4 hours of venepuncture. Total serum IgE was measured using a standard Immulite assay (EuroDPC UK Ltd, Gwynedd, Wales). The between‐run coefficient of variation of the IgE assay was 5.1–6.7%. As IgE values in excess of 2000 IU/ml were reported as >2000 IU/ml, these were assigned a value of 2001 IU/ml. Skin prick testing was performed against a panel of seven standardised aeroallergens and a standard histamine positive control supplied from a single source (ALK (UK), Berkshire). The panel of allergens and test protocols were identical at both centres and included: D pteronyssinus; D farinae; Grass mix; Cat hair; Dog hair; Cockroach; and Alternaria. Prior to testing, antihistamines were withheld for 72 hours; no participants were taking oral steroids in excess of 10 mg prednisolone/day.
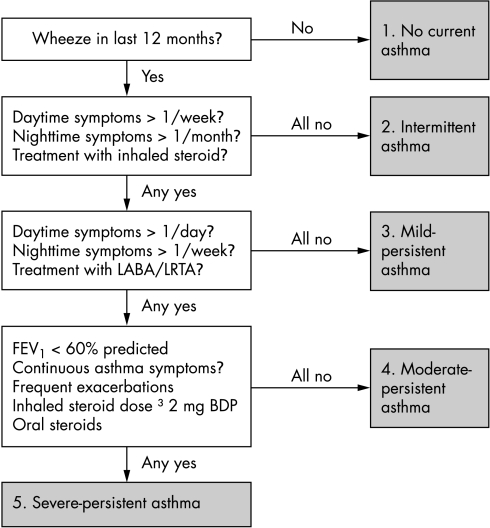
Atopy was defined as a positive response to SPT (at least one wheal ⩾3 mm greater than saline response to a panel of seven aeroallergens) or a total serum IgE ⩾100 IU/ml. Asthma severity was determined based on treatment, lung function, and symptoms (fig 1 and Carroll et al21). Participants from Stoke‐on‐Trent (145 families) completed a supplementary questionnaire which included data on hospital admissions in the previous 12 months.
Figure 1 Algorithm for severity scoring.
Statistical analysis
Relationships between allergy and asthma severity were analysed using successively different markers of allergy: a biological marker (total serum IgE), then the presence of positive SPT to aeroallergens, and finally the degree of sensitisation measured by the wheal sizes of skin prick tests. Total IgE values were log10 transformed and results were expressed as arithmetic (AM) and geometric means (GM).
Analysis of the data had to take into account the correlation of measurements of atopy and asthma between individuals within each family. This precluded the use of standard regression techniques but was countered using multilevel models generated with the software MLwiN (Institute of Education, version 1.10.0007).22 The rationale for this type of approach has been discussed and validated previously,23 but briefly allows data to be nested within two levels, namely at the family and individual levels. Multilevel models provided an estimate of the statistical significance between perceived associations. Data were presented as uncorrected and corrected values following inclusion of potential confounders, including age, gender, and birth order within each model (table 3).
Table 3 Significance of associations determined using multilevel models allowing variation at the individual and family levels.
| Log IgE | Wheal size | |||
|---|---|---|---|---|
| Uncorrected | Corrected* | Uncorrected | Corrected* | |
| Severity score | <0.001 | <0.001 | 0.109 | 0.017 |
| FEV1 < 80% predicted | 0.009 | 0.011 | 0.767 | 0.878 |
| Hospitalisation in last year | <0.001 | <0.001 | <0.001 | <0.001 |
| Inhaled steroid use | 0.001 | 0.002 | 0.011 | <0.001 |
| FEV1 (as % predicted) | <0.001 | <0.001 | 0.082 | 0.071 |
| FVC (as % predicted) | 0.230 | 0.424 | 0.042 | 0.087 |
| FEV1/FVC(as % predicted) | <0.001 | <0.001 | <0.001 | <0.001 |
*Following correction for age, gender, and birth order.
Results
Demographics
The characteristics of the children are summarised in table 1. The mean age was 12.1 years (range 7.0–18.9 years), with more boys than girls. The range of asthma severity was wide, with over one third having mild persistent asthma and with almost equal numbers of children in each of the other four severity categories.
Table 1 Descriptive characteristics of asthma and allergy in asthmatic children.
| Probands | Siblings | All | |
|---|---|---|---|
| n | 232 | 168 | 400 |
| Age, mean±SD | 11.9±2.6 | 12.4±3.1 | 12.1±2.8 |
| Sex, % boys | 61.9 | 58.9 | 60.7 |
| Severity score | |||
| No asthma at present, n (%) | 9 (3.9) | 56 (33.3) | 65 (16.3) |
| Intermittent symptoms only, n (%) | 32 (13.8) | 39 (23.2) | 71 (17.8) |
| Mild persistent, n (%) | 98 (42.2) | 45 (26.8) | 143 (35.8) |
| Moderate persistent, n (%) | 54 (23.3) | 13 (7.7) | 67 (16.8) |
| Severe persistent, n (%) | 39 (16.8) | 15 (8.9) | 54 (13.5) |
| Hospitalisation in previous year, n (%)* | 53 (39.8) | 15 (24.1) | 68 (34.9) |
| Current inhaled steroid use, n (%) | 172 (74.1) | 71 (42.3) | 243 (60.8) |
| IgE, IU/ml, AM (95% CI)† | 524 (441–607) | 437 (342–532) | 488 (425–550) |
| IgE, IU/ml, GM (95% CI)† | 204 (165–251) | 131 (101–172) | 170 (144–200) |
| Sensitisation, % ⩾3 mm (mean SPT wheal in mm)‡ | |||
| Der p 1 | 48.1 (2.4) | 41.5 (2.0) | 45.2 (2.2) |
| Der f 1 | 25.1 (1.5) | 25.1 (1.2) | 25.1 (1.4) |
| Grass | 42.1 (2.4) | 35.4 (1.9) | 39.1 (2.2) |
| Cat | 35.5 (2.0) | 27.9 (1.5) | 32.1 (1.8) |
| Dog | 26.8 (1.4) | 15.0 (0.8) | 21.5 (1.2) |
| Alternaria | 0.5 (0.1) | 1.4 (0.1) | 0.9 (0.1) |
| Cockroach | 1.6 (0.2) | 1.4 (0.2) | 1.5 (0.2) |
| Sensitisation to any allergen, n (%) | 126 (68.8) | 81 (55.1) | 207 (62.7) |
| Summative wheal size (mm) (95% CI) | 10.0 (8.8–11.3) | 7.6 (6.5–9.0) | 9.0 (8.1–9.9) |
| Lung function§ | |||
| FEV1 (% predicted)±SD | 98.7±15.7 | 99.6±14.8 | 99.1±15.3 |
| FVC (% predicted)±SD | 101.0±14.3 | 99.5±14.8 | 100.4±14.5 |
| FEV1/FVC (% predicted)±SD | 98.3±9.6 | 101.1±8.9 | 99.5±9.4 |
| FEV1 <80% predicted n, (%) | 27 (11.7) | 15 (9.0) | 42 (10.6) |
*Based on data from 133 probands and 62 siblings.
†Based on data from 229 probands and 166 siblings.
‡Based on data from 183 probands and 147 siblings.
§Based on data from 230 probands and 166 siblings.
IgE, total serum immunoglobulin E; SPT, skin prick test; Der p 1, Dermatophagoides pteronyssinus; Der f 1, Dermatophagoides farinae; Grass, 6 grass mix; Cat, cat dander; Dog, dog dander; Alternaria, Alternaria alternate; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; AM, arithmetic mean; GM, geometric mean.
Relationship between total serum IgE, skin prick tests, and asthma severity
Total SPT wheal size and log transformed serum IgE values were significantly associated with several phenotypic traits reflecting asthma severity. These relationships are shown in table 2. The logarithm of serum IgE was associated with all our measures of asthma severity. Increasing asthma severity score was associated with incremental changes in total serum IgE.
Table 2 Relationship between asthma severity scores and measures of atopy.
| n | Total serum IgE IU/ml (95% CI) | Summative wheal size (mm) | ||
|---|---|---|---|---|
| AM | GM | |||
| No asthma at present | 65 | 325 (193–457) | 102 (69–152) | 6.4 (4.3–8.6) |
| Intermittent symptoms | 70 | 446 (295–597) | 150 (101–223) | 8.7 (6.4–11.1) |
| Mild persistent | 142 | 501 (399–602) | 199 (154–258) | 9.9 (8.5–11.3) |
| Moderate persistent | 65 | 556 (384–727) | 155 (95–252) | 8.5 (6.1–11.0) |
| Severe persistent | 53 | 623 (432–814) | 267 (174–411) | 8.5 (6.5–10.5) |
| FEV1 <80% predicted | 40 | 812 (558–1066) | 379 (234–613) | 9.4 (6.3–12.4) |
| FEV1 >80% predicted | 354 | 449 (386–512) | 154 (129–184) | 8.7 (7.7–9.6) |
| Hospitalised in last year | 67 | 726 (550–902) | 329 (227–475) | 12.1 (9.9–14.3) |
| Not hospitalised | 127 | 392 (295–489) | 140 (106–186) | 6.5 (5.3–7.8) |
| Prescribed inhaled steroid | 239 | 540 (456–623) | 201 (153–248) | 9.6 (8.5–10.7) |
| No inhaled steroid | 146 | 404 (309–499) | 127 (96–167) | 7.2 (5.7–8.7) |
IgE, total serum immunoglobulin E; AM, arithmetic mean; GM, geometric mean; FEV1, forced expiratory volume in 1 second.
Mean basal forced expiratory volume in 1 second (FEV1) values below 80% predicted were seen in 10% of the children. They had higher total serum IgE values than those with FEV1 values within the normal range (mean 812 IU/ml versus 449 IU/ml). Small differences were seen in total serum IgE levels between children who were prescribed inhaled corticosteroid treatment compared with those who were not (mean 540 IU/ml versus 404 IU/ml).
Data for almost half of the children (194/400) were available on hospital admissions within the previous 12 months. Sixty seven had been admitted with an asthma exacerbation. Their total serum IgE levels were higher (mean 726 IU/ml) than levels in those not admitted (mean 392 IU/ml).
The significance of these associations was ascertained using multilevel models allowing for variation at the family and individual level. There were significant associations between log transformed IgE values and clinical severity score (p < 0.001), lung function abnormalities (p < 0.001), hospitalisation in the previous year (p < 0.001), and current prescription of an inhaled corticosteroid (p = 0.001). These associations remained highly significant following correction for age, gender, and birth order (summarised in table 3).
In contrast, summative SPT wheal size in millimetres was not significantly associated with the clinical severity score (p = 0.109) or <80% mean basal lung function (p = 0.767). The relationship between total SPT score and asthma severity is seen in table 2. Individuals with mild persistent asthma had the highest summative wheal score (9.9 mm). Those with intermittent asthma, moderate asthma, and severe asthma had very similar scores (8.7 mm, 8.5 mm, and 8.5 mm respectively), while those with no current asthma symptoms had the lowest SPT total scores (6.4 mm). However, total wheal size was a highly significant predictor of hospitalisation in the previous year (p < 0.001) and of prescription of an inhaled corticosteroid (p = 0.011) (table 3). The summative SPT score was almost twice as large in individuals admitted to hospital in the previous 12 months compared to those who were not (12.1 mm versus 6.5 mm).
Relationships between lung function indices and total serum IgE and skin prick tests
As continuous variables, mean basal FEV1 and FEV1/FVC ratios but not FVC values were associated with logarithm serum IgE values. SPT wheal size was significantly associated with evidence of basal airway obstruction (FEV1/FVC ratio) (table 3).
Discussion
We have shown that the type and intensity of atopic sensitisation is positively associated with clinical and spirometric measures of asthma severity in children with asthma. This study is larger than previous reports which may have failed to demonstrate associations because of limited statistical power.10 Differences in the statistical methodology are unlikely to account for the differences in results, as a subsequent analysis of our data using the generalised estimating equation,24 produced almost identical levels of significance for each of the reported associations.
Few published studies have examined the relationships of atopic markers with asthma severity in children. Of these, some showed greater asthma severity with sensitisation to aeroallergens14,25 but others did not.26,27 In children, there are conflicting results regarding the relationships of asthma severity with serum total IgE.10,25,28 Even when relationships have been established, the statistical significance achieved has often been marginal (p > 0.01).
This analysis was performed in a large population of well characterised children with asthma. It included children recruited from hospital and community sources, although due to the stringent nature of the initial recruitment criteria to the genetic study, these children represent a highly atopic and asthmatic population. This sample may therefore not be entirely representative of typical cases of childhood asthma. However, the wide spectrum of disease severity seen in the group as a whole and the relatively normal values achieved for lung function variables are somewhat reassuring that our results are applicable at a broader level.
The relationship between total serum IgE and risk of asthma is well established in children and adults.4,5,29 The risk of hospital admission has been linked to total serum IgE in several paediatric studies.10,14,25 Although data on hospital admissions were available for slightly less than half of the subjects in our study, we were still able to see a clear association between hospital admission and total serum IgE.
As previous studies have failed to demonstrate associations between asthma symptoms and markers of atopic sensitisation, investigators have concluded that atopy may have a lesser role in determining asthma control than in determining severity.10 This hypothesis is not supported by our data as both the need for hospitalisation and asthma severity scores were significantly associated with total serum IgE. National and international guidelines on asthma clearly define the goals of asthma therapy and identify need for hospital admission and/or persistent symptoms and lung function abnormalities as evidence of poor asthma control.30,31
SPT wheal size is another measure of allergic sensitisation and SPTs have been used widely in epidemiological and clinical studies. However, arguments persist about what constitutes an abnormal response. The formation of a wheal is the result of a complex immunological host response culminating in the release of histamine locally within the skin. It is dependent on many factors including season, age, and treatment of individuals with antihistamine medications.32,33 While it was possible to control for factors such as antihistamine and oral steroid use, data on season of testing were not collected in this study. Despite these potential limitations the use of a summative wheal size as a measure of allergic sensitisation appears to be a useful predictor of morbidity in children with asthma. The discordant nature of the results between total serum IgE and summative wheal size suggests a degree of independence between these two measures. Total serum IgE seems to correlate well with asthma severity; the results of SPTs do not. One explanation for this apparent discordant response may lie in the inclusion of treatment in our asthma severity score (fig 1). There are data to suggest that treatment with leukotriene receptor antagonists may reduce SPT wheal sizes in atopic children.34 This could obscure the perceived relationship between severity and SPTs. SPTs may also be less informative than total serum IgE across a population because they represent the reactions to a chosen panel of only a few allergens. In our study we chose seven aeroallergens, two of which are low prevalence allergens in the UK (Alternaria and Cockroach) and only rarely produced a reaction (table 1). However, from our results we can see that SPTs to even a limited panel are informative and correlate positively with hospitalisation, steroid use, and airways obstruction.
While our results are highly significant, this must be distinguished from clinical significance. The very small p values generated in this study reflect in part its power and the power of the statistical techniques undertaken. The majority of our results remain highly significant even after correction for multiple hypothesis testing. Nonetheless, the coefficients generated by the generalised estimating equation can give some estimates of the relationships between atopy and the asthma phenotype (table 4). A child with a total serum IgE of 200 (log10 = 2.30) will have, on average, an FEV1 of 4.6% less than an asthmatic child with a total serum IgE of 20 (log10 = 1.30).
Table 4 Estimated coefficients and their 95% confidence intervals for significant associations generated using the generalised estimating equation following correction for age, gender, and birth order.
| Log10 IgE | Wheal size | |||
|---|---|---|---|---|
| Coefficient | 95% CI | Coefficient | 95% CI | |
| Severity score | 0.230 | 0.067 to 0.393 | 0.0187 | 0.004 to 0.034 |
| Hospitalisation in last year | 0.180 | 0.086 to 0.273 | 0.0259 | 0.018 to 0.034 |
| FEV1 (as % predicted) | −4.62 | −6.70 to −2.53 | −0.180 | −0.37 to 0.012 |
| FEV1/FVC (as % predicted) | −3.89 | −5.10 to −2.67 | −0.337 | −0.45 to −0.23 |
This study confirms that increasing atopic sensitisation is clearly associated with increasing disease severity in children with asthma. Both total serum IgE and SPTs confer valuable information about the asthma phenotype. These correlate with each other and with other measures of asthma severity including clinical severity, hospital admission, and measures of lung function. Nonetheless, the relationship between different markers of atopic sensitisation and the asthma phenotype is subtly different within our cohort of asthmatic children, and therefore they should probably be considered separately in clinical and epidemiological studies.
What is already known on this topic
In children with asthma:
Atopic sensitisation and exposure to specific allergens is associated with increased risk of current asthma
A dose‐response relationship exists between the number of positive skin prick tests and the prevalence of asthma symptoms
What this study adds
In children with asthma:
Increasing total serum IgE is associated with a highly significant increase in asthma severity score, risk of hospital admission, and a reduction in lung function
Increasing skin prick test reactivity is associated with risk of hospital admission and airways obstruction but not asthma severity score
Acknowledgements
The authors gratefully acknowledge the hard work of Sadie Clayton and Siobhan Davies in patient recruitment, and assistance with lung function measurements from Ian Cliffe and Angela Evans in the Respiratory Medicine Department of UHNS.
Footnotes
Funding: Dr Carroll is funded by an educational grant from GlaxoSmithKline Respiratory International. The original genetic study was funded by GlaxoSmithKline. Local genetic analysis was funded by the North Staffordshire “Breath of Life” Campaign.
Competing interests: none declared
References
- 1.Ishizaka K, Ishizaka T, Hornbrook M. Physico‐chemical properties of raginic antibody. V. Correlation of reaginic activity with γE‐globulin antibody. J Immunol 196697840–853.4163008 [Google Scholar]
- 2.Jarvis D, Burney P. The epidemiology of allergic disease. BMJ 1998316607–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oryszczyn M ‐ P, Annesi I, Neukirch F.et al Relationships of total IgE level, skin prick test response, and smoking habits. Ann Allergy 199167355–359. [PubMed] [Google Scholar]
- 4.Sunyer J, Anto J M, Castellsague J.et al Total serum IgE is associated with asthma independently of specific IgE levels. Eur Respir J 199691880–1884. [DOI] [PubMed] [Google Scholar]
- 5.Beeh K M, Ksoll M, Buhl R. Elevation of total serum immunoglobulin E is associated with asthma in nonallergic individuals. Eur Respir J 200016609–614. [DOI] [PubMed] [Google Scholar]
- 6.Stadler B M, Stampfli M R, Miescher S.et al Biological activities of anti‐IgE antibodies. Int Arch Allergy Immunol 1993102121–126. [DOI] [PubMed] [Google Scholar]
- 7.Soler M, Matz J, Townley R.et al The anti‐IgE antibody omalizumab reduces exacerbations and steroid treatment in allergic asthmatics. Eur Respir J 200118254–261. [DOI] [PubMed] [Google Scholar]
- 8.Busse W, Corren J, Lanier B Q.et al Omalizumab, anti‐IgE recombinant humanized monoclonal antibody for the treatment of severe allergic asthma. J Allergy Clin Immunol 2001108184–190. [DOI] [PubMed] [Google Scholar]
- 9.Milgrom H, Berger W, Nayak A.et al Treatment of childhood asthma with anti‐immunoglobulin E antibody (omalizumab). Pediatrics 2001108e36. [DOI] [PubMed] [Google Scholar]
- 10.Siroux V, Oryszczyn M ‐ P, Paty E.et al Relationships of allergic sensitisation, total immunoglobulin E and blood eosinophils to asthma severity in children of the EGEA study. Clin Exp Allergy 200333746–751. [DOI] [PubMed] [Google Scholar]
- 11.Sandford A J, Weir T D, Pare P D. Phenotypic heterogeneity in asthma and allergy. Clin Exp Allergy 19982826–31. [DOI] [PubMed] [Google Scholar]
- 12.Sears M R, Herbison G P, Holdaway M D.et al The relative risks of sensitivity to grass pollen, house dust mite and cat dander in the development of childhood asthma. Clin Exp Allergy 198919419–424. [DOI] [PubMed] [Google Scholar]
- 13.Burrows B, Sears M R, Flannery E M.et al Relation of the course of bronchial responsiveness from age 9 to age 15 to allergy. Am J Respir Crit Care Med 19951521302–1308. [DOI] [PubMed] [Google Scholar]
- 14.Ponsonby A L, Gatenby P, Glasgow N.et al Which clinical subgroups within the spectrum of child asthma are attributable to atopy? Chest 2002121135–142. [DOI] [PubMed] [Google Scholar]
- 15.Peat J, Tovey E, Gray E.et al Asthma severity and morbidity in a population sample of Sydney schoolchildren. Part II. Importance of house dust mite and allergens. Aust N Z J Med 199424270–276. [DOI] [PubMed] [Google Scholar]
- 16.Asher M I, Kell U, Anderson H R.et al International study of asthma and allergies in childhood (ISAAC): rationale and methods. Eur Respir J 19958485–491. [DOI] [PubMed] [Google Scholar]
- 17.The British Thoracic Society and the Association of Respiratory Technicians and Physiologists Guidelines for the measurement of respiratory function. Respir Med 199488165–194. [PubMed] [Google Scholar]
- 18.Rosenthal M, Bain S H, Cramer D.et al Lung function in white children aged 4 to 19 years. I. Spirometry. Thorax 199348794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quanjer P H, Tammeling G H, Cotes J E.et al Lung volumes and forced ventilatory flows. Report of the working party standardisation of lung function tests. European community for steel and coal. Official statement of the European Respiratory Society. Eur Respir J 1993(suppl 16)5–40. [PubMed]
- 20.American Thoracic Society Standardisation of spirometry. 1994 update. Am J Respir Crit Care Med 19951521107–1136. [DOI] [PubMed] [Google Scholar]
- 21.Carroll W D, Lenney W, Child F.et al Regional variation of airway hyperresponsiveness in children with asthma. Respir Med 200599403–407. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein H.Multilevel statistical models, 2nd edn. London: Arnold 1995
- 23.Carroll W D, Lenney W, Jones P W.et al Effects of glutathione S‐transferase M1, T1 and P1 on lung function in asthmatic families. Clin Exp Allergy. (in press) [DOI] [PubMed]
- 24.Liang K Y, Zeger S L. Regression analysis for correlated data. Annu Rev Public Health 19931443–68. [DOI] [PubMed] [Google Scholar]
- 25.Wever‐Hess J, Kouwenberg J M, Duiverman E J.et al Risk factors for exacerbations and hospital admissions in asthma of early childhood. Pediatr Pulmonol 200029250–256. [DOI] [PubMed] [Google Scholar]
- 26.Rosenstreich D L, Eggleston P, Kattan M.et al The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner‐city children with asthma. N Engl J Med 19973361356–1363. [DOI] [PubMed] [Google Scholar]
- 27.Sarpong S B, Karrison T. Skin test reactivity to indoor allergens as a marker of asthma severity in children with asthma. Ann Asthma Immunol 199880303–308. [DOI] [PubMed] [Google Scholar]
- 28.Takeda K, Shibasaki M, Takita H. Relation between bronchial responsiveness to methacholine and levels of IgE antibody against Dermatophagoides farinae and serum IgE in asthmatic children. Clin Exp Allergy 199323450–454. [DOI] [PubMed] [Google Scholar]
- 29.Sears M R, Burrows B, Flannery E M.et al Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N Engl J Med 19913251067–1071. [DOI] [PubMed] [Google Scholar]
- 30.National Institutes of Health and National Heart, Lung, and Blood Institute Global Initiative for Asthma, Global Strategy for Asthma Management and Prevention. NIH Publication 02‐3659. Bethesda, MD: National Institutes of Health, 2002
- 31.British Guideline on the Management of Asthma Thorax. 2003;58 (suppl 1):i1–i94. doi: 10.1136/thorax.58.suppl_1.1i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hahhtela T. Skin tests used for epidemiologic studies. In: Dreborg S, Frew A, eds. Position paper: Allergen standardisation and skin tests. Proceedings of the annual meeting of the Executive Committee of the European Academy of Allergology and Clinical Immunology (EACCI), Glasgow 199076–80.
- 33.Pastorello E A. Skin tests for diagnosis of IgE‐mediated allergy. In: Dreborg S, Frew A, eds. Position paper: Allergen standardisation and skin tests. Proceedings of the annual meeting of the Executive Committee of the European Academy of Allergology and Clinical Immunology (EACCI), Glasgow, 1990;57–62
- 34.Sekerel B E, Akpinarli A. The effect of montelukast on allergen‐induced cutaneous responses in house dust mite allergic children. Pediatr Allergy Immunol 200314212–215. [DOI] [PubMed] [Google Scholar]