Abstract
Objective
To determine the presenting features of brain tumours in children.
Design
Retrospective case note review.
Setting
Paediatric and neurosurgical services at the Wessex Neurology Centre and Southampton General Hospital, UK.
Patients
200 patients presenting with a CNS tumour between 1988 and 2001.
Results
The commonest first presenting symptoms were headache (41%), vomiting (12%), unsteadiness (11%), visual difficulties (10%), educational or behavioural problems (10%), and seizures (9%). The commonest symptoms occurring at any time were headache (56%), vomiting (51%), educational or behavioural problems (44%), unsteadiness (40%), and visual difficulties (38%). Neurological signs were present at diagnosis in 88%: 38% had papilloedema, 49% cranial nerve abnormalities, 48% cerebellar signs, 27% long tract signs, 11% somatosensory abnormalities, and 12% a reduced level of consciousness. The median symptom interval was 2.5 months (range 1 day to 120 months). A short symptom interval was significantly associated with high grade tumours and patient age of 3 years or younger.
Conclusions
The well known predominance of headache in children with CNS tumours is confirmed. Visual, behavioural, and educational symptoms were also prominent. With the exception of seizures, every initial symptom was accompanied by other symptoms or signs by the time of diagnosis. Questions about visual symptoms and educational or behavioural difficulties, as well as the more widely recognised symptoms of raised intracranial pressure and motor dysfunction, are important in the diagnosis of brain tumours, as are vision assessment and the appropriate plotting of growth and head size.
Keywords: brain tumour, child, presenting features
Brain tumours are the commonest solid tumours in children, affecting about 500 children and adolescents annually in the United Kingdom,1 and are now the commonest cause of cancer deaths in childhood.2 Their diagnosis poses difficulties and this is reflected in their symptom interval, defined as the time period between onset of symptoms and diagnosis. While some brain tumours are diagnosed rapidly,3 the majority of studies report a mean symptom interval of at least 14 weeks.4,5,6,7,8,9 This is in striking contrast to mean symptom intervals of 4.5 and 2.8 weeks for leukaemia and Wilms' tumour, respectively.10
Multiple factors contribute to diagnostic difficulties caused by brain tumours. Many of the initial symptoms and signs of brain tumours are non‐specific and mimic other more common and less serious illnesses. Children typically present with less complete or clear symptomatology than adults,11 although this may partly reflect underrecognition of non‐specific cognitive and personality change in adults.12 Brain imaging of young children often requires anaesthesia and may therefore not be readily available.
Published descriptions of the presenting features of children with brain tumours are limited in number and detail.4,5,13,14,15 Many reports describe cohorts or case series of children with tumours of a specific histological type,3,9,16,17,18,19,20,21,22,23,24,25,26,27 but this information is not available at the time of clinical diagnosis. The present study was undertaken to determine the constellations of presenting clinical features that characterise children with brain tumours, to assist in identifying those for whom further investigation was or was not likely to lead to the diagnosis.
Methods
Information was obtained from the hospital medical records on the clinical features of all children with a brain tumour seen by the paediatric and neurosurgical services at the Wessex Neurology Centre and Southampton General Hospital over a 14 year period up to June 2001. In most cases, general paediatric records, neurosurgical records, and the referral letter, usually from the family practitioner, provided three separate sources of record of the initial clinical presentation. Symptoms were categorised into 11 symptom complexes (headache, vomiting, visual difficulties, unsteadiness, weakness, educational or behavioural problems, seizures, focal weakness, abnormalities of growth including weight loss or gain, increased head circumference, and “other”), and signs into six groups (papilloedema, cranial nerve abnormalities, cerebellar signs, long tract signs, sensory abnormalities, and reduced level of consciousness). The nature of the first symptom and of all symptoms occurring at any stage prior to diagnosis was recorded. Signs were classified according to whether they occurred alone or in combination with other signs.
Statistical analysis
All analyses were undertaken using SPSS 12.0. Subgroup comparison was undertaken using the Mann–Whitney test for two subgroups and the Kruskal–Wallis test for more than two subgroups. Logistic regression analysis was undertaken to explore the relation between symptom interval and tumour location, tumour grade, and patient age.
Results
Patient characteristics
In all, 204 patients presented with a CNS tumour during the 14 year period of study, and 200 sets of medical records were available for analysis. The mean age at presentation was 7.4 years (range 15 weeks to 17 years). The male to female ratio was 4:3 (114 male, 86 female). Another medical diagnosis preceded the diagnosis of brain tumour in 16 patients: three shunted hydrocephalus, two severe learning difficulties of unknown aetiology, two tuberous sclerosis, six neurofibromatosis (NF) type 1, one NF type 2, one juvenile chronic arthritis, and one Duchenne's muscular dystrophy.
Symptom complexes
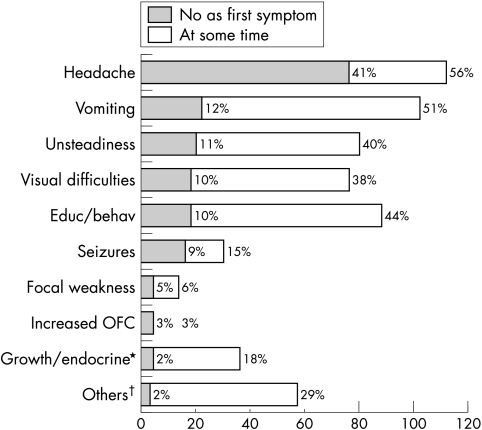
Headache was the commonest first symptom (41%) followed by vomiting (12%), unsteadiness (11%), visual difficulties (10%), educational or behavioural problems (10%), and seizures (9%). Growth and endocrine abnormalities, focal weakness, and excessive head growth occurred as the first symptom in 5% or fewer of cases. Headache was also the commonest symptom, occurring at any time during the symptom interval, affecting 112 children (56%), with the next commonest symptoms being vomiting in 102 (51%), educational or behavioural problems in 88 (44%), unsteadiness in 80 (40%), and visual difficulties in 76 (38%) (fig 1).
Figure 1 Relative frequency of symptoms in 200 children with brain tumours. *Includes symptoms of weight loss. †Other symptoms: developmental regression/delay; abnormal hand use/focal motor signs; abnormal gait; reduced hearing, tinnitus, anorexia, dizziness/vertigo; slurred speech; incontinence; poor coordination; incidental/antenatal diagnosis; head tilt/neck stiffness; clumsiness; flu‐like illness.
In children with headache, the symptom had been present for 4 months or longer in 36% of 106 cases in whom duration of headache was recorded. Irrespective of duration of headache, other symptoms were also present at diagnosis in all cases. The commonest associated symptoms were vomiting, unsteadiness, educational or behavioural problems, visual difficulties, and disturbed sleep (table 1). The pattern of headache, recorded in 71 of the 112 children presenting with headache, was nocturnal or occurred in the early morning in 43 (61%), present continuously in 13 (18%), and occurred more in the daytime or evening in 15 (21%). Despite the presence of additional symptoms, 14 (13%) of the children with headache had been diagnosed with migraine, and eight (7%) with tension headache before the diagnosis of brain tumour.
Table 1 Percentage rates of associated symptoms in children with headache.
| Symptom | Headache<4 months (n = 68) | Headache⩾4 months (n = 38) |
|---|---|---|
| Vomiting | 87 | 76 |
| Vision | 53 | 63 |
| Unsteadiness | 49 | 45 |
| Education /behavioural | 37 | 45 |
| Disturbed sleep | 26 | 31 |
| Growth/fluid balance | 7 | 21 |
| Seizures | 7 | 8 |
| None | 0 | 0 |
New onset of educational or behavioural difficulties was recorded in 88 children. Of these 50 (57%) had behavioural symptoms alone, 20 (23%) had educational problems alone, and 18 (20%) had both educational and behavioural difficulties. Thirty of 68 children with behavioural symptoms (44%) reported lethargy, while less common problems included irritability, personality change, aggression, and labile emotions. Twelve of 38 children with educational problems (32%) had a reported deterioration in reading and writing, other problems included memory difficulty, poor concentration, global deterioration, and decrease in school attendance.
Visual disturbance was reported by 76 children. Additional details were recorded in 69 of these. Double vision in 30 (43%) and blurred vision in 27 (39%) were the most frequent specific symptoms. Less frequent visual complaints included photophobia in four (6%), squint in three (4%), abnormal eye movements in two (3%), primitive visual hallucinations (flashing lights) in two (3%), and tunnel vision in one (1%).
Seizures were the presenting symptom in 30 children, including 19 (63%) that were initially regarded as focal and 11 (37%) as generalised (see below).
Neurological signs
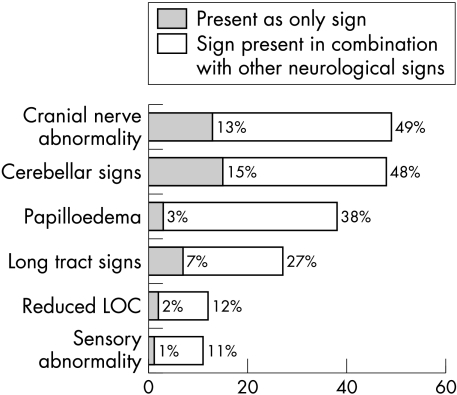
Abnormal neurological signs at diagnosis were recorded in 175 children (88%). Of these, 66 (38%) had papilloedema, 86 (49%) cranial nerve abnormalities, 84 (48%) cerebellar signs, 47 (27%) long tract signs, 19 (11%) somatosensory abnormalities, and 21 (12%) a reduced level of consciousness. Cerebellar signs were the commonest signs seen in isolation, affecting 27 children (15%). The next most common neurological signs seen alone were cranial nerve abnormalities, affecting 22 (13%), and papilloedema, affecting six (3%). A further nine (5%) had only cranial nerve signs plus papilloedema. Thus 37 (21%) of those with abnormal neurological examination had only cranial nerve signs or papilloedema without other signs (fig 2).
Figure 2 Abnormal neurological signs in children with brain tumours (n = 175).
Complete documentation of the findings was available in only 35 of the 97 cases with abnormal cranial nerve examination. Of these, 17 (49%) had a visual abnormality (abnormal fields 23%, abnormal acuity 26%), 13 (37%) had sixth and 10 (28%) seventh nerve palsies. An abnormality of visual field and/or acuity was the only abnormal sign at diagnosis in 11 (31%) of these children.
The absence of any neurological abnormality on examination at diagnosis was recorded in 24 children (12%). Of these, 17 (71%) had seizures, including 14 that were focal and three that were initially regarded as generalised. However, two of those with “generalised” seizures had a persistent focal abnormality on EEG and the remaining child had atypical absence seizures, compatible with the alternative diagnosis of focal seizures. Of the seven children with neither seizures nor abnormal neurological signs, four were of school age and three were aged 2 to 3 years. In the school age group, two had nocturnal or early morning headache and two had headache with vomiting or visual symptoms. Furthermore three of these four had abnormal endocrine function (and underlying craniopharyngioma), and one had “clumsiness” that proved to be secondary to longstanding hydrocephalus (and underlying tectal glioma). In the 2 to 3 year age group, one, with known NF1, presented with vomiting and behaviour change, one had a month's history of unsteadiness in walking, and one was undergoing investigation of malfunction of a pre‐existing shunt; his diagnosis was revised from “aqueduct stenosis” to “tectal glioma”.
Presenting features and age
The 42 children (21%) aged 3 years or less at presentation were less likely to present with headache (12%) or seizures (7%) but were more likely to present with behavioural problems (48%) than older children (68%, 17%, and 30%, respectively). Headache was not reported under the age of 2 years.
Symptom interval
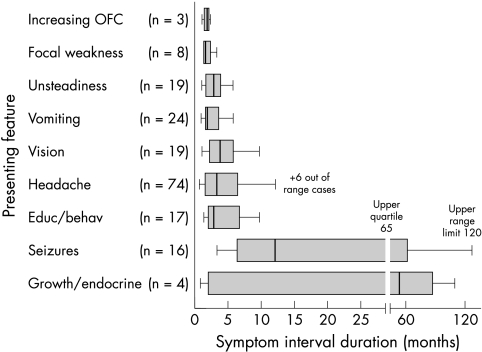
The symptom interval, documented in 175 patients, varied from 1 day to 120 months with a median value of 2.5 months (interquartile range, 1.0 to 6.0). Median duration of symptoms was shorter for headache (2.0 months) than for growth or endocrine problems (4 months) or seizures (6.5 months) (fig 3). Children aged 3 years or less had a significantly shorter median symptom interval than older children (1.0 v 3.0 months).
Figure 3 Box and whisker plot of relation between symptom interval and presenting symptom or sign in children with brain tumours (n = 175).
In 164 children whose tumours could be categorised as either high or low grade, high grade tumours presented more rapidly than low grade tumours (median 1.5 v 4.0 months).
Children with infratentorial tumours had a significantly shorter symptom interval than those with supratentorial tumours (median 2.0 v 4.0 months). The symptom intervals for cortical hemispheric tumours were longer than for deep hemispheric tumours (lateral ventricles, corpus callosum, and thalamus), central tumours (third ventricle, tectum, pineal, pituitary, hypothalamus, optic pathway and basal ganglia tumours), and posterior fossa tumours (table 2).
Table 2 Comparison of symptom interval between groups of differing age, tumour location, and histological grade.
| n | Median symptom interval (months) | Range (months) | SD | p Value | ||
|---|---|---|---|---|---|---|
| Interquartile | ||||||
| range | ||||||
| (months) | ||||||
| Age ⩽3 years | 45 | 1.0 | 0.3 to 96.0 | 0.5 to 2.4 | 16.8 | <0.000 |
| Age > 3 years | 130 | 3.0 | 0.03 to 120.0 | 1.0 to 9.0 | 22.5 | |
| High grade | 73 | 1.5 | 0.03 to 60.0 | 0.75 to 3.0 | 7.6 | <0.000 |
| Low grade | 91 | 4.0 | 0.03 to 120.0 | 1.5 to 12.0 | 28.1 | |
| Supratentorial | 80 | 4.0 | 0.03 to 120.0 | 1.1 to 11.5 | 29.7 | 0.002 |
| Infratentorial | 94 | 2.0 | 0.03 to 60.0 | 0.9 to 4.0 | 8.5 | |
| Cortical hemispheric tumours | 25 | 5.0 | 0.03 to 120.0 | 1.7 to 24.0 | 40.4 | 0.003 |
| Deep hemispheric tumours | 16 | 2.0 | 0.5 to 20.0 | 0.6 to 8.3 | 5.6 | |
| Central tumours | 39 | 4.0 | 0.1 to 96.0 | 1.0 to 12.0 | 26.1 | |
| Posterior fossa tumours | 94 | 2.0 | 0.03 to 60.0 | 0.9 to 4.0 | 8.5 |
In a binary univariate logistic regression model of 164 children whose tumours could be classified as high grade or low grade, being aged 3 years or younger, having a high grade of tumour, or having infratentorial or posterior fossa tumour location were significantly associated with a symptom interval of less than the median duration. However, in stepwise multivariate regression only younger patient age and higher tumour grade were significantly associated with a shorter symptom interval (table 3).
Table 3 Factors associated with a symptom interval of less than the median duration.
| Variable | n | OR less than median symptom interval | 95% CI for OR | p Value |
|---|---|---|---|---|
| Univariate analysis | ||||
| Infratentorial location | 94 | 2.33 | 1.24 to 4.37 | 0.009 |
| Posterior fossa tumour | 94 | 3.67 | 1.39 to 9.71 | 0.009 |
| High grade tumour | 73 | 5.13 | 2.62 to 10.00 | <0.001 |
| Age ⩽ 3 years | 45 | 3.94 | 1.58 to 9.8 | 0.003 |
| Multivariate analysis | ||||
| High grade tumour | 73 | 4.83 | 2.43 to 9.62 | <0.001 |
| Age ⩽ 3 years | 45 | 3.46 | 1.32 to 9.09 | 0.012 |
CI, confidence interval; OR, odds ratio.
Discussion
This study of a large series of children with brain tumours confirms the well known predominance of headache, although the classical diurnal pattern of headache was not reported in a large minority of affected children. Less well recognised, however, is the fact that visual symptoms or educational or behavioural difficulties together accounted for one fifth of all initial symptoms and occurred at some point before diagnosis in four fifths of all children. Lethargy was a common behavioural change and has been identified in other studies.4,5 In a series of children who died as a result of acute hydrocephalus, lethargy was a prominent feature and the authors suggest that more emphasis should be placed on it as a specific neurological symptom rather than as a non‐specific symptom of systemic illness.28 Other investigators have drawn attention to the fact that parents are experts on the behaviour of their own child but parental concern about behaviour could lead to the investigation of large numbers of children.29 Every initial symptom other than seizures was subsequently accompanied by other symptoms or signs at diagnosis. This multiplicity of clinical features was an important aid to diagnosis. One fifth of all children had cranial nerve signs or papilloedema without other signs, underlining the importance of cranial nerve examination including fundoscopy. Only 12% of children with brain tumours had a completely normal neurological examination at diagnosis, the majority of them presenting with seizures. Cranial nerve signs were as common as cerebellar signs, each occurring in about half of all children, and were more common than long tract signs. The median symptom interval for this cohort is similar to that in other studies.4,6,7,30 Symptom intervals were longer in older children, in children with lower grade tumours, and in those in whom the presenting symptoms were seizures or problems with growth and endocrine function.
A careful history may be required to distinguish generalised from focal seizures, particularly those with secondary generalisation. Focal seizures suggest an underlying structural cause, and the recent NICE (National Institute for Clinical Excellence) guidelines recommend that magnetic resonance imaging (MRI) is particularly important in children or in those who have any suggestion of a focal onset on history, examination, or EEG (unless there is clear evidence of benign focal epilepsy). MRI within four weeks is also recommended for children who develop epilepsy before the age of 2 years or in whom seizures continue in spite of first line drug treatment.31 Application of this policy would have led to tumour diagnosis in all children with focal seizures in the present report. The likelihood of detecting an underlying structural cause in a child with generalised seizures and otherwise normal history and examination is low, and imaging in idiopathic generalised epilepsy was discouraged in the NICE guidelines.
Just under a third of children had an abnormality of visual field or acuity, and in the majority this was their only cranial nerve deficit underlining the importance of assessment of vision in any child suspected of having a brain tumour. Specialist ophthalmological assessment in selected cases will assist in the diagnosis.32 New onset of paralytic squint always requires investigation, and Bell's palsy should be a diagnosis of exclusion in children.33,34
The study population all presented to the only paediatric neurosurgical centre in their geographical region and the series is, in that sense, unselected. The non‐recording of a symptom was taken to mean that it was not present. Although this is clearly not true in every case, the history recorded in a paediatric neuroscience centre will reflect the history taken there and at the time of any previous presentation to health professionals. Medical decisions will always be based on such histories rather than on the underlying full facts to which they relate. We cannot know, however, whether any symptom or sign, seen alone or in combination at the time of diagnosis of the brain tumour, was also present at an earlier stage in the history, although it is reasonable to assume that this was often the case. Any inference from this study about how to achieve earlier diagnosis of childhood brain tumour rests on that assumption.
What is already known on this topic
Headaches are a common presenting symptom in children with brain tumours
Brain tumours have a long symptom interval in comparison with other childhood malignancies
What this study adds
Visual symptoms and educational and behavioural problems are common in children with brain tumours
At diagnosis the majority will have abnormal neurological findings
A prolonged symptom interval is associated with low grade tumours, seizures, growth and endocrine symptoms, and age over 3 years
This study addresses the issue of sensitivity but not that of specificity of symptoms and signs to the presence of an underlying brain tumour. Non‐specific headache, for example, affects 20% of 5 year olds,35 and 10% of school age children suffer from migraine.36,37 Studies of unselected children with headache do, however, suggest that the presence of headache with abnormal neurological signs is also rather specific to the presence of an underlying lesion such as a brain tumour.38 The chance of a feature being indicative of a brain tumour (positive predictive value of the feature) will increase with the prevalence of brain tumours in the population in question. Our observations may therefore be of more value in guiding decisions in children already selected by referral to secondary or tertiary care than in unselected children.
This study suggests that a careful history with questions about visual symptoms and educational or behavioural difficulties, as well as about the more widely recognised symptoms of raised intracranial pressure or motor dysfunction, along with a thorough examination including, if possible, the assessment of visual acuity, visual fields, and the remainder of the cranial nerves, and appropriate plotting of growth and head size are important in the diagnosis of brain tumours. Multiple symptomatology should also raise the clinician's index of suspicion as it is common in these children and is more likely to be detected when patients are followed up after an interval. Neuroimaging should be undertaken routinely for epilepsy where seizures had their onset in the first 2 years after birth or have focal features. Among children who present with a single symptom other than seizures and have a normal neurological examination and normal growth, very few will have an underlying brain tumour. The exceptions will usually be preschool children who are uncooperative for examination.
Acknowledgements
We are extremely grateful to the following: Dr Paul Davies, Trust Statistical Advisor, Birmingham Children's Hospital NHS Trust; Eleanor Lutman, Research assistant, University of Southampton; and Shona Mackie, Specialist Nurse in Paediatric Neurology, Southampton General Hospital.
Footnotes
Competing interests: none declared
References
- 1.Stiller C. Childhood cancer. The health of children and young people. London: Office for National Statistics, 2004
- 2.ONS Deaths by age, sex and underlying cause, 2003 registrations. London: Office for National Statistics, 2004
- 3.Comi A M, Backstrom J W, Burger P C.et al Clinical and neuroradiologic findings in infants with intracranial ependymomas. Pediatric Oncology Group. Pediatr Neurol 19981823–29. [DOI] [PubMed] [Google Scholar]
- 4.Edgeworth J, Bullock P, Bailey A.et al Why are brain tumours still being missed? Arch Dis Child 199674148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta V, Chapman A, McNeely P D.et al Latency between symptom onset and diagnosis of pediatric brain tumors: an Eastern Canadian geographic study. Neurosurgery 200251365–372. [PubMed] [Google Scholar]
- 6.Haimi M, Nahum M P, Arush M W B. Delay in diagnosis of children with cancer. A retrospective study of 315 children. Pediatr Hematol Oncol 20042137–48. [PubMed] [Google Scholar]
- 7.Furuta T, Tabuchi A, Adachi Y.et al Primary brain tumors in children under age 3 years. Brain Tumor Pathol 1998157–12. [DOI] [PubMed] [Google Scholar]
- 8.Halperin E C, Friedman H S. Is there a correlation between duration of presenting symptoms and stage of medulloblastoma at the time of diagnosis? Cancer 199678874–880. [DOI] [PubMed] [Google Scholar]
- 9.Zuccaro G, Sosa F, Cuccia V.et al Lateral ventricle tumors in children: a series of 54 cases. Childs Nerv Syst 199915774–785. [DOI] [PubMed] [Google Scholar]
- 10.Flores L E, Williams D L, Bell B A.et al Delay in the diagnosis of pediatric brain tumors. Am J Dis Child 1986140684–686. [DOI] [PubMed] [Google Scholar]
- 11.Punt J. Clinical syndromes. In: Walker D, Perilongo G, Punt J, et al, editors. Brain and spinal tumours of childhood. London: Arnold, 200499–106.
- 12.Davies E, Clarke C. Early symptoms of brain tumours. J Neurol Neurosurg Psychiatry 2004751205–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mucic‐Pucic B, Cvitanovic‐Sojat L, Hajnzic T.et al Neurologic symptoms as first signs of brain tumour in children. Acta Clinica Croatica 20014027–30. [Google Scholar]
- 14.Dobrovoljac M, Hengartner H, Boltshauser E.et al Delay in the diagnosis of paediatric brain tumours. Eur J Pediatr 2002161663–667. [DOI] [PubMed] [Google Scholar]
- 15.Keene D L, Hsu E, Ventureyra E. Brain tumors in childhood and adolescence. Pediatr Neurol 199920198–203. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan A M, Albright A L, Zimmerman R A.et al Brainstem gliomas in children. A Children's Cancer Group review of 119 cases. Pediatr Neurosurg 199624185–192. [DOI] [PubMed] [Google Scholar]
- 17.Chitnavis B, Phipps K, Harkness W.et al Intrinsic brainstem tumours in childhood: a report of 35 children followed for a minimum of 5 years. Br J Neurosurg 199711206–209. [DOI] [PubMed] [Google Scholar]
- 18.Behnke J, Christen H J, Bruck W.et al Intra‐axial endophytic tumors in the pons and/or medulla oblongata. I. Symptoms, neuroradiological findings, and histopathology in 30 children. Childs Nerv Syst 199713122–134. [DOI] [PubMed] [Google Scholar]
- 19.Johnson J H, Hariharan S, Berman J.et al Clinical outcome of pediatric gangliogliomas: ninety‐nine cases over 20 years. Pediatr Neurosurg 199727203–207. [DOI] [PubMed] [Google Scholar]
- 20.Young‐Poussaint T, Kowal J R, Barnes P D.et al Tectal tumours of childhood: clinical and imaging follow‐up. Am J Neuroradiol 199819977–983. [PMC free article] [PubMed] [Google Scholar]
- 21.Drummond K J, Rosenfeld J V. Pineal region tumours in childhood. A 30‐year experience. Childs Nerv Syst 199915119–126. [DOI] [PubMed] [Google Scholar]
- 22.Yang H J, Nam D H, Wang K C.et al Supratentorial primitive neuroectodermal tumor in children: clinical features, treatment outcome and prognostic factors. Childs Nerv Syst 199915377–383. [DOI] [PubMed] [Google Scholar]
- 23.Dirks P B, Harris L, Hoffman H J.et al Supratentorial primitive neuroectodermal tumours in children. J Neurooncol 19962975–84. [DOI] [PubMed] [Google Scholar]
- 24.Akyuz C, Emir S, Akalan N.et al Intracranial ependymomas in childhood – a retrospective review of sixty‐two children. Acta Oncologica 20003997–100. [DOI] [PubMed] [Google Scholar]
- 25.Erdincler P, Lena G, Sarioglu A C.et al Intracranial meningiomas in children: Review of 29 cases. Surg Neurol 199749136–141. [DOI] [PubMed] [Google Scholar]
- 26.Halperin E C, Watson D M, George S L. Duration of symptoms prior to diagnosis is related inversely to presenting disease stage in children with medulloblastoma. Cancer 2001911444–1450. [DOI] [PubMed] [Google Scholar]
- 27.Alston R D, Newton R, Kelsey A.et al Childhood medulloblastoma in northwest England 1954 to 1997: incidence and survival. Dev Med Child Neurol 200345308–314. [DOI] [PubMed] [Google Scholar]
- 28.Shemie S, Jay V, Rutka J.et al Acute obstructive hydrocephalus and sudden death in children. Ann Emerg Med 199729524–528. [DOI] [PubMed] [Google Scholar]
- 29.Dixon‐Woods M, Findlay M, Young B.et al Parents' accounts of obtaining a diagnosis of childhood cancer. Lancet 2001357670–674. [DOI] [PubMed] [Google Scholar]
- 30.Mehta V, Chapman A, McNeely P D.et al Latency between symptom onset and diagnosis of pediatric brain tumors: an Eastern Canadian geographic study. Neurosurgery 200251365–372. [PubMed] [Google Scholar]
- 31.NICE The epilepsies: The diagnosis and management of the epilepsies in adults and children in primary and secondary care. National Institute for Clinical Excellence 2004
- 32.Ottar W L. The ABCs of visual acuity assessment. Insight 1997381–87. [DOI] [PubMed] [Google Scholar]
- 33.Rowlands S, Hooper R, Hughes R.et al The epidemiology and treatment of Bell's palsy in the UK. Eur J Neurol 2002963–67. [DOI] [PubMed] [Google Scholar]
- 34.Brown J K. Disorders of the peripheral nervous system. In: Campbell AGM, McIntosh N, eds. Forfar and Arneil's textbook of pediatrics, 5th edition. Edinburgh: Churchill Livingstone, 1998725
- 35.Sillanpaa M, Piekkala P, Kero P. Prevalence of headache at preschool age in an unselected child population. Cephalalgia 199111239–242. [DOI] [PubMed] [Google Scholar]
- 36.Abu‐Arefeh I, Russell G. Prevalence of headache and migraine in schoolchildren. BMJ 1994309765–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mortimer M J, Kay J, Jaron A. Epidemiology of headache and childhood migraine in an urban general practice using ad hoc, Vahlquist and IHS criteria. Dev Med Child Neurol 1992341095–1101. [DOI] [PubMed] [Google Scholar]
- 38.Lewis D W, Dorbad D. The utility of neuroimaging in the evaluation of children with migraine or chronic daily headache who have normal neurological examinations. Headache 200040629–632. [DOI] [PubMed] [Google Scholar]