Abstract
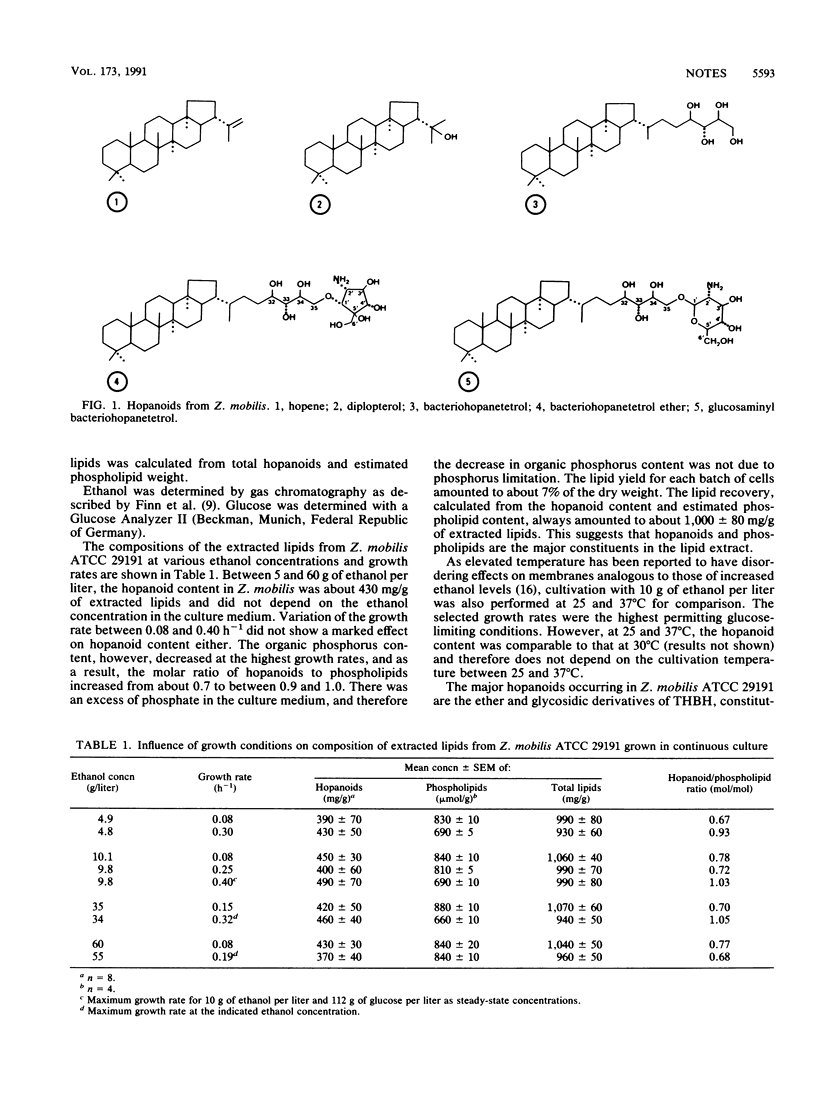
By using a new method for quantification of the different hopanoid derivatives, a total hopanoid content of about 30 mg/g (dry cell weight) was observed in Zymomonas mobilis. This value is the highest reported for bacteria so far. The major hopanoids in Z. mobilis were the ether and glycosidic derivatives of tetrahydroxy-bacteriohopane, constituting about 41 and 49% of the total hopanoids. Tetrahydroxybacteriohopane itself, diplopterol, and hopene made up about 6, 3, and 1%, respectively. Only minor changes in hopanoid composition were observed with changes in growth conditions. Earlier reports on a correlation between hopanoid content and ethanol concentration in the medium could not be confirmed. Over a wide range of ethanol concentrations (5 to 60 g/liter), growth rates (0.08 to 0.25 h-1), and temperatures (25 to 37 degrees C), the molar ratio of hopanoids to phospholipids in the cells amounted to about 0.7. Only at growth rates of greater than 0.30 h-1 did the molar ratio increase to about 1.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson L. R., Johnston A. M., Martin C. E. The effects of temperature acclimation on membrane sterols and phospholipids of Neurospora crassa. Biochim Biophys Acta. 1982 Nov 12;713(2):456–462. doi: 10.1016/0005-2760(82)90265-x. [DOI] [PubMed] [Google Scholar]
- Anderson R. L., Minton K. W., Li G. C., Hahn G. M. Temperature-induced homeoviscous adaptation of Chinese hamster ovary cells. Biochim Biophys Acta. 1981 Mar 6;641(2):334–348. doi: 10.1016/0005-2736(81)90490-9. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Barrow K. D., Chuck J. A. Determination of hopanoid levels in bacteria using high-performance liquid chromatography. Anal Biochem. 1990 Feb 1;184(2):395–399. doi: 10.1016/0003-2697(90)90699-a. [DOI] [PubMed] [Google Scholar]
- Carey V. C., Ingram L. O. Lipid composition of Zymomonas mobilis: effects of ethanol and glucose. J Bacteriol. 1983 Jun;154(3):1291–1300. doi: 10.1128/jb.154.3.1291-1300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demel R. A., De Kruyff B. The function of sterols in membranes. Biochim Biophys Acta. 1976 Oct 26;457(2):109–132. doi: 10.1016/0304-4157(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Flesch G., Rohmer M. Prokaryotic triterpenoids. A novel hopanoid from the ethanol-producing bacterium Zymomonas mobilis. Biochem J. 1989 Sep 1;262(2):673–675. doi: 10.1042/bj2620673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Pearson R. H., Sundell S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim Biophys Acta. 1981 Jun 16;650(1):21–51. doi: 10.1016/0304-4157(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Hossack J. A., Sharpe V. J., Rose A. H. Stability of the plasma membrane in Saccharomyces cerevisiae enriched with phosphatidylcholine or phosphatidylethanolamine. J Bacteriol. 1977 Feb;129(2):1144–1147. doi: 10.1128/jb.129.2.1144-1147.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter K., Rose A. H. Lipid composition of Saccharomyces cerevisiae as influenced by growth temperature. Biochim Biophys Acta. 1972 Apr 18;260(4):639–653. doi: 10.1016/0005-2760(72)90013-6. [DOI] [PubMed] [Google Scholar]
- Ingram L. O., Buttke T. M. Effects of alcohols on micro-organisms. Adv Microb Physiol. 1984;25:253–300. doi: 10.1016/s0065-2911(08)60294-5. [DOI] [PubMed] [Google Scholar]
- Poralla K., Kannenberg E., Blume A. A glycolipid containing hopane isolated from the acidophilic, thermophilic Bacillus acidocaldarius, has a cholesterol-like function in membranes. FEBS Lett. 1980 Apr 21;113(1):107–110. doi: 10.1016/0014-5793(80)80506-0. [DOI] [PubMed] [Google Scholar]
- Rogers P. L., Goodman A. E., Heyes R. H. Zymomonas ethanol fermentations. Microbiol Sci. 1984 Sep;1(6):133–136. [PubMed] [Google Scholar]
- Rottem S., Yashouv J., Ne'eman Z., Razin S. Cholesterol in mycoplasma membranes. Composition, ultrastructure and biological properties of membranes from Mycoplasma mycoides var. capri cells adapted to grow with low cholesterol concentrations. Biochim Biophys Acta. 1973 Nov 16;323(4):495–508. doi: 10.1016/0005-2736(73)90158-2. [DOI] [PubMed] [Google Scholar]
- Schulenberg-Schell H., Neuss B., Sahm H. Quantitative determination of various hopanoids in microorganisms. Anal Biochem. 1989 Aug 15;181(1):120–124. doi: 10.1016/0003-2697(89)90403-x. [DOI] [PubMed] [Google Scholar]
- Wodtke E. Lipid adaptation in liver mitochondrial membranes of carp acclimated to different environmental temperatures: phospholipid composition, fatty acid pattern and cholesterol content. Biochim Biophys Acta. 1978 May 25;529(2):280–291. doi: 10.1016/0005-2760(78)90071-1. [DOI] [PubMed] [Google Scholar]



