Abstract
Aims
To evaluate the prognostic predictive values of cytochrome c, cytokines, and other laboratory measurements in serum collected during neurological onset in acute encephalopathy with multiple organ failure.
Methods
In addition to general laboratory examinations, the concentrations of cytochrome c (apoptosis marker) and cytokines (inflammatory markers) were measured in serum samples collected at the initial phase in 29 patients with acute encephalopathy. The obtained values were evaluated as predictors for the development of severe encephalopathy.
Results
Cytochrome c, tumour necrosis factor α (TNF‐α), interleukin 6 (IL‐6), soluble TNF‐receptor 1 (sTNF‐R1), and aspartate aminotransferase (AST) concentrations at the initial phase were high and correlated well with patient outcome. High concentrations of serum cytochrome c (>45 ng/ml), sTNF‐R1 (>2000 pg/ml), AST (>58 IU/dl), IL‐6 (>60 pg/ml), and TNF‐α (>15 pg/ml) predicted an unfavourable prognosis (sequelae and death) at 93%, 79%, 82%, 77%, and 60%, respectively. The specificity of those markers was 100%, 89%, 83%, 100%, and 100%, respectively.
Conclusions
Serum cytochrome c is the most sensitive and specific predictor for the development of severe encephalopathy at the initial phase. Results suggest that this marker might be used to guide decisions regarding the start of the initial treatment and further intensive care.
Keywords: acute encephalopathy, cytochrome c, multiple organ failure, prognostic predictive marker
Central nervous system (CNS) manifestations follow various viral infections in children. Recently, the high incidence and mortality of acute encephalopathy with multiple organ failure has become a serious health problem in Japan.1,2 This type of encephalopathy is often associated with influenza and occasionally with other viral infections mainly in children age <10 years, and develops either on the day that signs of infection, such as high fever, cough, and fatigue, appear, or on the following day. The major initial neurological signs include abrupt onset of seizure and altered consciousness or loss of consciousness. In most patients with unfavourable prognosis (sequelae and death), their neurological state deteriorates and multiple organ dysfunction (liver, renal, coagulative, and haematopoietic systems) developed. Brain oedema detected by computed tomography (CT) becomes evident on the day after neurological onset. Rates of mortality (31.8%) and disability (27.7%) are high.2 The outcome of the disease varies and the clinical symptoms at neurological onset do not predict the prognosis.2 A number of strategies for the treatment of the encephalopathy are proposed, but an effective therapy has not been established. A prognostic predictor in the illness is desired for deciding when to start the initial treatment and further intensive care, and for evaluation of the therapeutic efficacy.
Serum concentrations of several proinflammatory cytokines and cytokine receptors, such as tumour necrosis factor α (TNF‐α), interleukin 6 (IL‐6), and soluble TNF‐receptor 1 (sTNF‐R1) are raised in the initial phase of acute encephalopathy associated with influenza.3,4,5,6,7 Serum cytochrome c concentrations are also high in patients with an unfavourable prognosis.8 In the present study, we measured serum cytochrome c and cytokine concentrations in addition to general laboratory examinations, and those markers were evaluated as prognostic predictors in acute encephalopathy with multiple organ failure.
Methods
From January 1997 to December 2002, 29 patients were diagnosed with acute encephalopathy in Fukushima Prefecture, Japan (table 1). In this study, acute encephalopathy was defined as an acute CNS disorder with fever characterised by altered or loss of consciousness more than 24 hours after acute onset and brain oedema detected by CT. Twenty patients had cerebrospinal fluid (CSF) examination; no pleocytosis in CSF was observed. The other nine patients had diffuse brain oedema detected by initial CT examination and did not receive CSF examination for fear of brain herniation caused by lumbar puncture. Neurological, metabolic, endocrine, toxicological, and drug induced disorders were excluded. Diffuse cerebral oedema and localised cerebral oedema were observed on CT examination in 22 and 7 patients, respectively. Of the 22 patients with diffuse cerebral oedema, two had low density areas in the brain stem and both thalami. One patient had low density areas only in both thalami. Virological examinations revealed viral infections in 17 of the 29 patients. Diagnosis of type H3N2 influenza (n = 7) was based on viral isolation from throat swabs. A diagnosis of human herpes virus 6 infection (n = 2) was based on the detection of serum IgM antibody against the virus by enzyme linked immunosorbent assay (ELISA). A diagnosis of influenza A (n = 6) and rotavirus (n = 1) was based on virus antigen detection from throat swabs by ELISA and from faeces by the latex agglutination test, respectively. Enterovirus infection (n = 1) was diagnosed based on viral genome detection from throat swab by polymerase chain reaction. The 29 patients with acute encephalopathy were assigned to three groups according to outcome. Of 29 patients, 11 patients died (group A), 6 patients survived with sequelae (group B), and 12 patients survived without sequelae (group C). Informed consent was obtained from the parents of the patients enrolled in this study. Ethics approval for the study was obtained from our Institutional Review Board.
Table 1 Summary of 29 patients with acute encephalopathy.
| Group | Outcome | Gender | Age | CT findings | Pathogen |
|---|---|---|---|---|---|
| A | Dead | F | 1y 11m | Diffuse CE | Unknown |
| Dead | M | 4y 9m | Diffuse CE | Influenza AH3 | |
| Dead | M | 8y 0m | Diffuse CE | Influenza AH3 | |
| Dead | M | 3y 5m | Diffuse CE | Influenza AH3 | |
| Dead | M | 2y 1m | Diffuse CE + brain stem LD | Unknown | |
| Dead | F | 7y 5m | Diffuse CE | Unknown | |
| Dead | F | 7y 5m | Diffuse CE | Unknown | |
| Dead | F | 3y 4m | Diffuse CE | Unknown | |
| Dead | F | 7y 0m | Diffuse CE + brain stem LD | Influenza AH3 | |
| Dead | M | 3y 10m | Diffuse CE | Influenza A | |
| Dead | M | 3y 5m | Diffuse CE | Influenza A | |
| B | Alive with sequelae | M | 0y 11m | Left CE | HHV‐6 |
| Alive with sequelae | F | 0y 9m | Right CE | HHV‐6 | |
| Alive with sequelae | M | 3y 10m | Diffuse CE | Influenza A | |
| Alive with sequelae | F | 1y 10m | Right CE | Influenza AH3 | |
| Alive with sequelae | M | 1y 4m | Diffuse CE | Unknown | |
| Alive with sequelae | F | 1y 3m | Right CE | Influenza AH3 | |
| C | Alive without sequelae | M | 0y 9m | Diffuse CE | Rotavirus |
| Alive without sequelae | M | 1y 3m | Bilateral talami LD | Unknown | |
| Alive without sequelae | M | 12y 6m | Diffuse CE | Unknown | |
| Alive without sequelae | M | 5y 2m | Diffuse CE | Enterovirus | |
| Alive without sequelae | M | 0y 11m | Right CE | Influenza AH3 | |
| Alive without sequelae | M | 4y 11m | Diffuse CE | Unknown | |
| Alive without sequelae | F | 0y 11m | Diffuse CE | Unknown | |
| Alive without sequelae | F | 2y 4m | Diffuse CE | Unknown | |
| Alive without sequelae | F | 3y 3m | Diffuse CE | Influenza A | |
| Alive without sequelae | M | 9y 11m | Bilateral parietal CE | Influenza A | |
| Alive without sequelae | F | 1y 11m | Right CE | Unknown | |
| Alive without sequelae | F | 4y 0m | Diffuse CE | Influenza A |
CE, cerebral oedema; LD, low density.
Blood samples were collected on day 1 (within 6 hours after neurological onset) and on day 2 (12–24 hours after the first collection). In the blood samples, we measured the following: aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactic dehydrogenase (LDH), haemoglobin (Hb), and creatinine (Cr) using standard automated techniques. The remaining serum samples were stored at −80°C until cytokine and cytochrome c assays were performed. TNF‐α, IL‐6, and sTNF‐R1 concentrations were measured using commercially available ELISA kits (TNF‐α and IL‐6: Endogen, Inc., MA, USA; sTNF‐R1: R&D Systems Inc., MN, USA). These assays were performed according to the manufacture's instructions. Sample values were determined from a standard curve. Cytochrome c concentrations were measured using an improved sandwich electrochemiluminescence immunoassay.8 Briefly, microbeads (Dynabeads M‐450 Epoxy, DYNAL A.S., Oslo, Norway) coated with anti‐ cytochrome c monoclonal antibody and anti‐ cytochrome c monoclonal antibody conjugated with ruthenium chelate (Ru‐Ab) were used for the cytochrome c immunoassay. We mixed 25 μl of sample, 200 μl of dilution buffer, and 25 μl of coated microbeads. After incubation at 30°C for 9 minutes, the microbeads were washed twice to remove non‐reacted specimens and Ru‐Ab was added. The microbeads were then washed twice to remove non‐reacted Ru‐Ab, and placed into magnet mounted flow‐cell electrodes to measure the quantity of the emission. The cytochrome c concentration of the sample was calculated using human cytochrome c standard solutions (1–2000 ng/ml). All three operations were performed automatically using a Picolumi 8220 (Sanko Junyaku Co., Ltd, Tokyo, Japan), except for the dilution of the sample.
Differences in the laboratory measurements between day 1 and day 2 were assessed using a paired t test and those between the three outcome groups at day 1 were assessed using the Mann‐Whitney rank sum test. A value of 0.05 or less was considered significant. Statistical analysis was performed on a Macintosh computer with a software package for statistical analysis (Stat View, Abacus Concepts, Berkeley, USA). Receiver operating characteristic (ROC) curves9 and their areas under the curve (AUC) were calculated by use of StatFlex, version 5.0, for Windows (Artec Co., Ltd, Osaka, Japan). In the ROC curve, true positive is plotted on the vertical axis and false positive on the horizontal axis at any cut‐off level. Thus, the ROC curve visually represents diagnostic sensitivity and specificity. The advantages of this curve are as follows: diagnostic accuracy can be compared by AUC, the significance of the reference interval in diagnosis can be evaluated, and the most discriminative cut‐off (threshold) level can be determined on the basis of the value in the top left corner of the ROC curve. Using the threshold level, we calculated sensitivity and specificity.
Results
Comparison of laboratory measurements
We compared serum AST, ALT, LDH, Hb, Cr, cytochrome c, TNF‐α, sTNF‐R1, and IL‐6 concentrations between day 1 and day 2. AST, ALT, and LDH levels generally increased on day 2 compared to day 1. Among groups A, B, and C, the levels significantly increased in group A, but not in groups B and C. Changes in Cr, Hb, cytochrome c, TNF‐α, sTNF‐R1, and IL‐6 levels varied between day 1 and day 2. Differences in the laboratory measurements between the three outcome groups at day 1 were assessed. AST, LDH, Cr, Hb, cytochrome c, TNF‐α, sTNF‐R1, and IL‐6 levels at the early stage were significantly higher in group A than in group C.
Prognostic predictive values of laboratory measurements
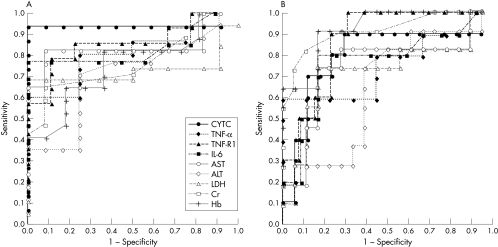
Prognostic predictive values of the nine laboratory measurements at the initial phase (day 1) were compared using AUC determined by ROC curve analysis (see fig 1). Serum cytochrome c, TNF‐α, IL‐6, sTNF‐R1, and AST were better predictive markers for an unfavourable prognosis (sequelae or death) than the others (fig 1A). When we evaluated those markers as predictors for a poor prognosis (death), Hb, Cr, and sTNF‐R1 were better than the others (fig 1B).
Figure 1 Prognostic predictive values at the neurological onset. Prognostic predictive values of the nine laboratory measurements were evaluated using ROC curve analysis. (A) At the initial phase, cytochrome c, TNF‐α, sTNF‐R1, IL‐6, and AST levels were sensitive predictive markers for an unfavourable prognosis (sequelae or death). (B) Hb, Cr, and sTNF‐R1 were sensitive predictive markers for a poor prognosis (death).
Sensitivity and specificity of laboratory measurements
When the threshold was set at 45 ng/ml for cytochrome c, 2000 pg/ml for sTNF‐R1, 58 IU/dl for AST, 60 pg/ml for IL‐6, and 15 pg/ml for TNF‐α, the sensitivity of these measurements as a predictor for an unfavourable prognosis was calculated to be 93%, 79%, 82%, 77%, and 60%, respectively. The specificity of these was 100%, 89%, 83%, 100%, and 100%, respectively. High concentrations of serum Hb (>13.0 g/dl), Cr (>0.65 mg/dl), and sTNF‐R1 (>2000 pg/ml) predicted the patients with a poor prognosis at 91%, 73%, and 90%, respectively. The specificity of Hb, Cr, and sTNF‐R1 was 83%, 94%, and 77%, respectively.
Discussion
In acute encephalopathy associated with influenza, there are raised serum concentrations of several cytokines, such as TNF‐α, sTNF‐R1, IL‐1β, and IL‐6.3,4,5,6,7 Hypercytokinaemia, therefore, is suggested to have an important role in the pathogenesis of acute encephalopathy and multiple organ dysfunction.9 From the suspected pathogenesis, anti‐inflammatory therapies (methylprednisolone pulse therapy, high dose gamma globulin therapy, and plasma exchange therapy) are proposed for the treatment of the illness. We initiated the anti‐inflammatory therapies for the treatment of our patients when brain oedema was detected by CT and multiple organ dysfunction was evident at the exacerbation phase. These therapies, however, seemed to have no effect on the clinical course of the patients with a poor prognosis. We believe that therapy should be initiated at an earlier time point to be effective, and therefore a predictive marker identifying high risk patients is necessary. Serum IL‐6,3,4 sTNF‐R1,5,6 and cytochrome c8 might be predictive for the development of severe illness before massive brain oedema and multiple organ failure develop. We thus compared the values of specific markers for apoptosis (cytochrome c) and inflammation (TNF‐α, sTNF‐R1, and IL‐6) and general measurements for organ dysfunctions (AST, ALT, LDH, Cr, and Hb) as predictors for the prognosis in acute encephalopathy.
ROC analysis indicated that high concentrations of cytochrome c and cytokines on day 1 were predictive for the patients with an unfavourable outcome (sequelae or death); cytochrome c seemed to be the most sensitive and specific predictor for the development of severe encephalopathy. High concentrations of Cr and Hb on day 1 were predictive for the patients with a poor prognosis (death).
Why do high cytochrome c and cytokine levels predict an unfavourable prognosis at an early stage? Cytochrome c is an intramitochondrial protein normally residing in the intermembrane spaces. It triggers the execution phase of apoptosis by massive translocation into the cytoplasm, leading to Apaf‐1 mediated caspase activation.10 Our results suggested that apoptosis as well as inflammation3,4,5,6,7 in vascular vessels was involved in the development of severe encephalopathy. Findings from pathological examination suggest that direct viral invasion and inflammation in CNS are not likely to cause this disease.2,3 Vascular damage with subsequent leakage of plasma and intravascular formation of thrombi is observed in systemic organs as well as in the brain.2,11 TNF‐α is a major apoptosis inducing factor. We hypothesise that inflammatory cytokines might be closely involved in the development of severe encephalopathy and multiple organ dysfunction through the induction of apoptosis in the vascular endothelium2 in CNS and other systemic organs. High blood levels of Hb and Cr at the initial phase might directly represent haemoconcentration and decreased renal flow by massive leakage of plasma, and thus suggest a poor prognosis. High blood levels of AST, ALT, and LDH might represent the results of systemic organ injury, and thus those values increase on day 2.
What is already known on this topic
In the initial phase of acute encephalopathy associated with influenza, serum concentrations of several proinflammatory cytokines and cytokine receptors, such as TNF‐α, IL‐6, and sTNF‐R1 are raised
Serum cytochrome c concentrations are also high in patients with an unfavourable prognosis
What this study adds
Serum cytochrome c is the most sensitive and specific predictor for the development of severe encephalopathy at the initial phase
This marker might be used to guide decisions regarding the start of the initial treatment and further intensive care in acute encephalopathy with multiple organ failure
In conclusion, serum cytochrome c level was the most sensitive and specific predictor for an unfavourable prognosis at the initial phase, as intravascular apoptosis might have a significant role in the development of severe encephalopathy. Real time monitoring of serum cytochrome c might be useful for deciding when to start intensive care in acute encephalopathy. This needs confirmation in another large patient cohort.
Footnotes
Competing interests: none declared
References
- 1.Kasai T, Togashi T, Morishima T. Encephalopathy associated with influenza epidemics. Lancet 20003551558–1559. [DOI] [PubMed] [Google Scholar]
- 2.Morishima T, Togashi T, Yokota S.et al Encephalitis and encephalopathy associated with an influenza epidemic in Japan. Clin Infect Dis 200235512–517. [DOI] [PubMed] [Google Scholar]
- 3.Ito Y, Ichiyama T, Kimura H.et al Detection of influenza virus RNA by reverse transcription‐PCR and proinflammatory cytokines in influenza‐virus‐associated encephalopathy. J Med Virol 199958420–425. [DOI] [PubMed] [Google Scholar]
- 4.Aiba H, Mochizuki M, Kimura M.et al Predictive value of serum interleukin‐6 level in influenza virus‐associated encephalopathy. Neurology 200157295–299. [DOI] [PubMed] [Google Scholar]
- 5.Ichiyama T, Nishikawa M, Yoshitomi T.et al Tumor necrosis factor‐α, interleukin‐1β, and interleukin‐6 in cerebrospinal fluid from children with prolonged febrile seizures: comparison with acute encephalitis/encephalopathy. Neurology 199850407–411. [DOI] [PubMed] [Google Scholar]
- 6.Ichiyama T, Morishima T, Isumi H.et al Analysis of cytokine levels and NF‐kappaB activation in peripheral blood mononuclear cells in influenza virus‐associated encephalopathy. Cytokine 20042731–37. [DOI] [PubMed] [Google Scholar]
- 7.Kawada J, Kimura H, Ito et al Systemic cytokine responses in patients with influenza‐associated encephalopathy. J Infect Dis 2003188690–698. [DOI] [PubMed] [Google Scholar]
- 8.Hosoya M, Nunoi H, Aoyama M.et al Cytochrome c and TNF‐α levels in serum and CSF of patients with influenza‐associated encephalopathy. Pediatr Infect Dis J 200524467–470. [DOI] [PubMed] [Google Scholar]
- 9.Swets J A. Measuring the accuracy of diagnostic systems. Science 19882401285–1293. [DOI] [PubMed] [Google Scholar]
- 10.Li P, Najhawan D, Budihardjo I.et al Cytochrome c and dATP‐dependent formation of Apaf‐1/caspase‐9 complex initiates an apoptotic protease cascade. Cell 199791479–489. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi M, Yamada T, Nakashita Y.et al Influenza virus‐induced encephalopathy: clinicopathologic study of an autopsied case. Pediatr Int 200042204–214. [DOI] [PubMed] [Google Scholar]