Abstract
Ovarian hyperstimulation is a recognised complication of longstanding hypothyroidism. A 12 year old girl with atrophic thyroiditis who presented with abdominal pain and distension is reported. She was noted to have bruising in the vicinity of the umbilicus (Cullen's sign). She had pronounced ovarian enlargement on ultrasonography and it was hypothesised that this profound phenotype might reflect an abnormal FSH receptor. However sequencing of the FSH receptor was normal. The ovarian enlargement resolved with thyroxine replacement. Physicians and surgeons should consider longstanding hypothyroidism in patients presenting with Cullen's sign.
Keywords: hypothyroidism, ovarian hyperstimulation, Cullen's sign, FSH receptor
The association between early pubertal development and primary hypothyroidism was first described over 40 years ago,1 with many reports since then. Ovarian stimulation in the hypothyroid child may result in oestrogen production, breast development, endometrial proliferation, and vaginal bleeding.2 It is likely that raised TSH concentrations bind and stimulate the FSH receptor, although a similar overlap phenomenon might occur at the level of the pituitary, with enhanced TRH production stimulating the GnRH receptor with subsequent ovarian enlargement.3 The cystic ovarian enlargement resolves with thyroid hormone replacement.
We report a case of primary hypothyroidism with associated ovarian enlargement presenting as an acute abdomen with bruising in the vicinity of the umbilicus (Cullen's sign). We hypothesised that she may have an abnormality of the FSH receptor with associated hyperstimulation leading to this unusual phenotype.
Case report
A 12 year old girl was referred by the general practitioner to the local hospital because of a four day history of periumbilical pain radiating to her back and loins. She had been vomiting 3–4 times daily for the last three days, and her abdomen was becoming progressively more distended. She had experienced episodes of vaginal bleeding 10 months earlier when 11 years and 4 months old. The last episode occurred four months prior to presentation.
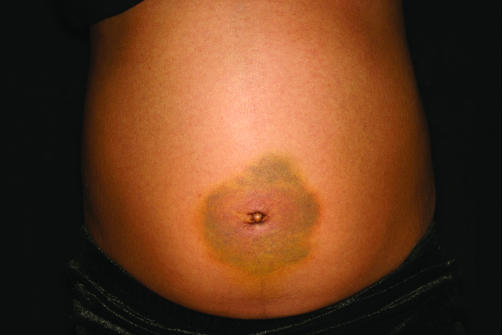
On examination she appeared unwell and in some discomfort. She had a temperature of 37.6°C. Her heart rate was 88 bpm with a blood pressure that was within the normal range (96 mm Hg systolic). Her abdomen was distended with periumbilical bruising (Cullen's sign, fig 1). A mass was also palpable in the periumbilical region. An ultrasound scan showed a large cystic structure in her pelvis. Blood tests revealed a normal alpha‐fetoprotein of 5 kU/l (normal <10) and undetectable HCG levels. She was referred to the paediatric oncology team who felt that a biopsy of the cystic structure was indicated. She was transferred to a surgical ward and underwent a laparoscopy with biopsy and drainage of cystic ovaries. Haemorrhagic fluid was present in the peritoneal cavity. The fluid from the peritoneal cavity was heavily bloodstained but of low cellularity. In the immediate postoperative period she was reviewed by the paediatric endocrine team who elicited a long history of lethargy and constipation. On examination she was Tanner puberty stage B2, PH1. She was noted to have a round face, flat expression, short stature (height –2.4 SD and weight on the 50th centile) with dry skin and slow sustained reflexes. She did not have a goitre.
Figure 1 Cullen's sign. Consent was obtained for publication of this figure.
Thyroid function testing revealed a TSH that was raised at 1310 m IU/l (0.3–4.7 mIU/l) with a free thyroxine of 2 pmol/l (normal range 11–23). Thyroid microsomal antibodies were negative. An LH‐RH test showed a prepubertal response with unrecordable LH levels and FSH levels between 3.6 and 4.3 U/l. Histological examination of the ovarian biopsy showed benign ovarian cysts with extensive haemorrhage, in keeping with that seen in longstanding hypothyroidism.
Our patient was commenced on thyroxine and in the subsequent months her hypothyroid phenotype resolved. An ultrasound 12 weeks after the initiation of thyroxine therapy showed that the multilocular cystic masses were no longer evident. She was biochemically euthyroid five months after presentation; ultrasound examination 10 months after presentation showed that her ovaries were relatively large (left ovary 23.5 ml and right ovary 17.1 ml), but the largest follicle was 7 mm in diameter. The abnormal cystic ovarian enlargement continued to resolve at subsequent ultrasound examinations.
FSH receptor sequencing
Recent case reports raised the possibility that our patient's extreme phenotype might be linked to an underlying abnormality of the FSH receptor.4,5 The rationale behind examination of the FSH receptor was explained to the family, and informed consent was obtained before sequencing was performed.
Genomic DNA was extracted from peripheral blood mononuclear cells and was used as a template in the subsequent PCR amplifications and analyses of follicle stimulating hormone receptor (FSHR). Primers were designed in the introns flanking the first nine exons of the FSHR gene. Exons 7 and 8 were amplified as a single product and exon 10 (1454 bp) was amplified as three overlapping products. DNA sequence analysis was performed on both strands by semi‐automated cycle sequencing using DYEnamic ET Terminator kit (Amersham). The sequencing results were compared to the known FSHR sequence6 and with a healthy control using Sequencher. No differences in DNA sequence in the 10 exons were observed between the wild type FSHR gene and those obtained from the patient.
Discussion
A diverse range of clinical signs are associated with longstanding hypothyroidism. These include short stature, visual field defects, and slipped capital femoral epiphysis. Ovarian stimulation with enlargement and cyst formation is also well recognised in primary hypothyroidism. There may be associated ovarian oestrogen production with “pseudo‐precocious puberty” and vaginal bleeding. Cullen's sign, abdominal peri‐umbilical ecchymosis, is usually associated with acute pancreatitis. However, any process causing haemoperitoneum can potentially result in this physical sign and it has also been reported in splenic rupture, metastatic disease, and ectopic pregnancy.7 Cullen's sign as a manifestation of primary hypothyroidism has not been reported before and we suspect that this was a direct consequence of haemorrhage from the massively enlarged ovaries seen on ultrasonography, at the time of surgery, and confirmed on histological examination. Although thyroid microsomal antibodies were negative, we feel that the clinical picture leaves little doubt that the primary hypothyroidism was secondary to antibody mediated thyroid gland destruction. Our patient had a normal TSH in the neonatal period (neonatal screening programme), and the negative autoantibody titre is likely to reflect the suboptimal sensitivity of antibody testing, although it is also possible that antibody titres fell when no antigen within thyroid tissue remained.
The sequence of events that results in ovarian hyperstimulation is unclear. This may simply be the consequence of increased TSH levels interacting and stimulating the FSH receptor. However, it may also be due to enhanced TRH production stimulating gonadotrophin release. It is of note that FSH levels are not particularly high in longstanding primary hypothyroidism despite the degree of gonadal stimulation.3,8
A hyperstimulation phenomenon in patients with an abnormal FSH receptor was described in two recent case reports.4,5 The two women developed ovarian hyperstimulation syndrome during pregnancy and were found to be heterozygous for a mutation in the FSH receptor gene that resulted in an amino acid substitution in the FSH receptor. The abnormal receptor had altered (enhanced) affinity for HCG which resulted in increased signal transduction. In view of the severity of the clinical picture in our patient, we hypothesised that our patient might also have an abnormal FSH receptor. However FSH receptor sequencing was normal.
In summary, primary hypothyroidism should be considered in the differential diagnosis when a child or adolescent presents with Cullen's sign; rare presentations of common diseases are much more common than rare diseases.
Acknowledgements
We would like to than Simon Pearce for his help with FSH receptor sequencing.
Footnotes
Competing interests: none
References
- 1.Van Wyk J J, Grumbach M M. Syndrome of precocious menstruation and galactorrhea in juvenile hypothyroidism. J Pediatr 196057416–435. [Google Scholar]
- 2.Chemaitilly W, Thalassinos C, Emond S.et al Metrorrhagia and precocious puberty revealing primary hypothyroidism in a child with Down's syndrome. Arch Dis Child 200388330–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anasti J N, Flack M R, Froehlich J.et al A potential novel mechanism for precocious puberty in juvenile hypothyroidism. J Clin Endocrinol Metab 199580276–279. [DOI] [PubMed] [Google Scholar]
- 4.Vasseur C, Patrice R, Isabella B.et al A chronic gonadotropin sensitive mutation in the follicle stimulating hormone receptor as a cause of familial gestational spontaneous ovarian hyperstimulation syndrome. N Engl J Med 2003349735–739. [DOI] [PubMed] [Google Scholar]
- 5.Guillaume S, Olatunbosim O, Delbaere A.et al Ovarian hyperstimulation syndrome due to a mutation in the follicle stimulating hormone receptor. N Engl J Med 2003349760–766. [DOI] [PubMed] [Google Scholar]
- 6. www.ensembl.org
- 7.Marinella M A. Cullen's sign associated with metastatic thyroid cancer. N Engl J Med 1999340149–150. [DOI] [PubMed] [Google Scholar]
- 8.Bruder J M, Samuels M H, Bremner W J.et al Hypothyroidism‐induced macroorchidism: use of a gonadotropin‐releasing hormone agonist to understand its mechanism and augment adult stature. J Clin Endocrinol Metab 19958011–16. [DOI] [PubMed] [Google Scholar]